Transfer of Self-Fruitfulness to Cultivated Almond from Peach and Wild Almond
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Controlled Crosses
2.3. Self-Compatibility Assessment
2.4. Advanced Selection Yield Evaluation
3. Results and Discussion
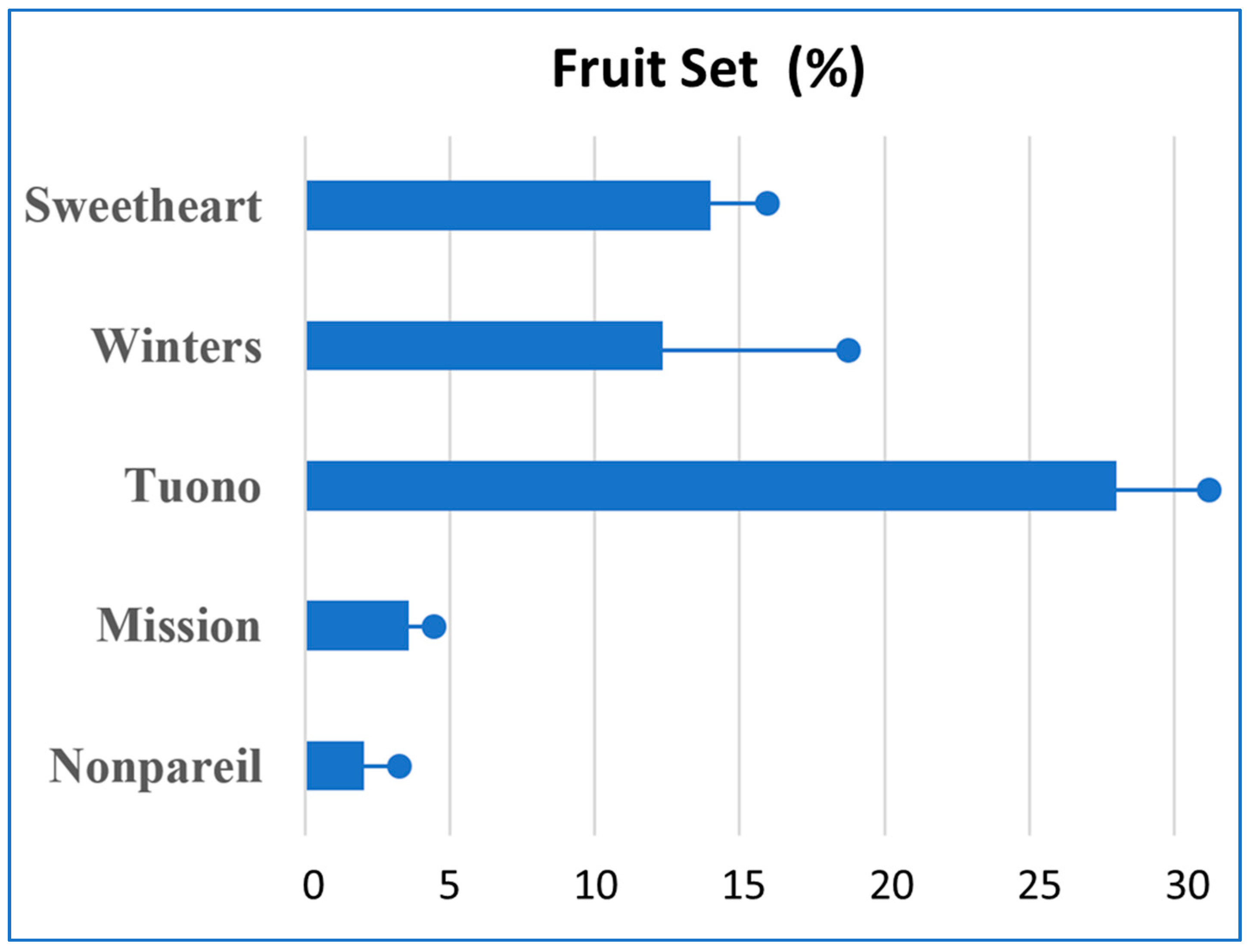
3.1. Self-Compatibility
3.1.1. Almond Cultivars
3.1.2. Related Almond Species
3.1.3. Related Peach Species
3.2. Autogamy
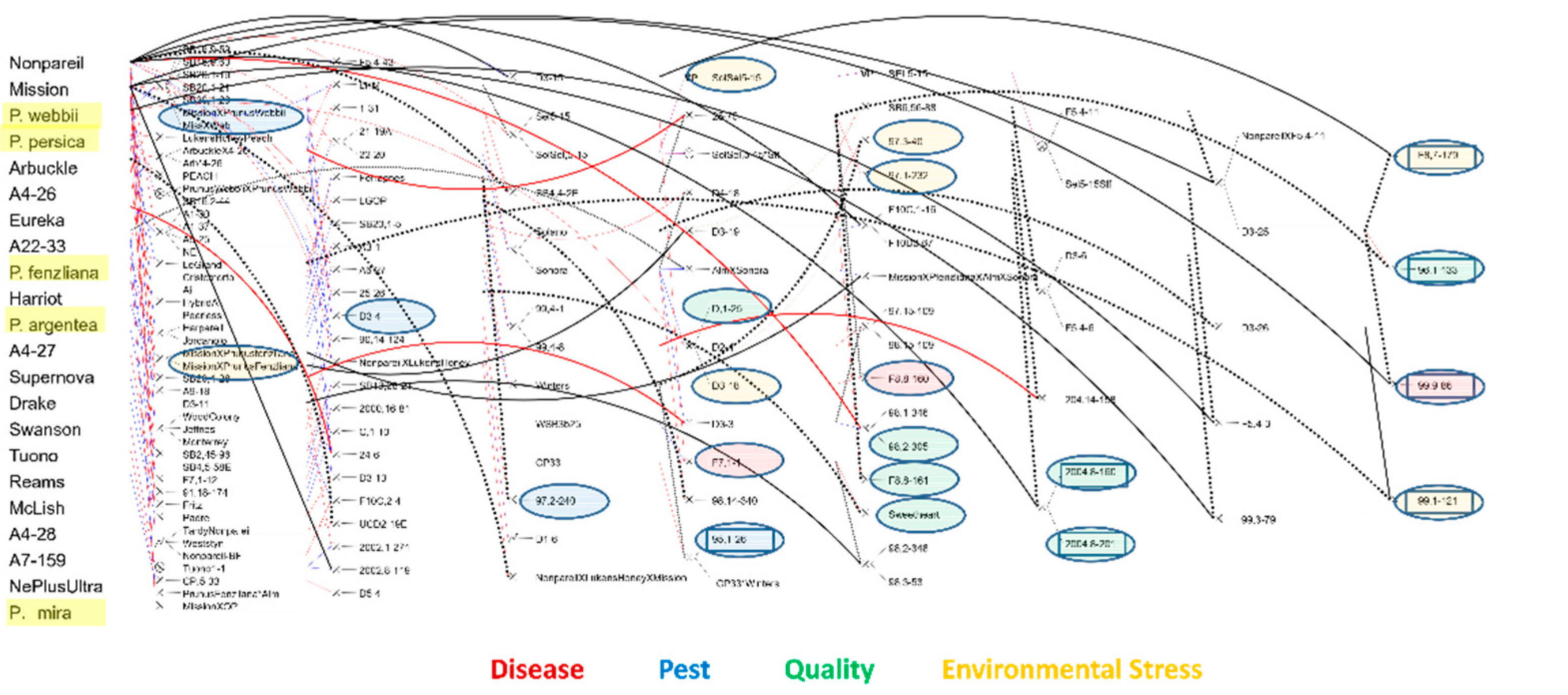
3.3. Breeding Strategy
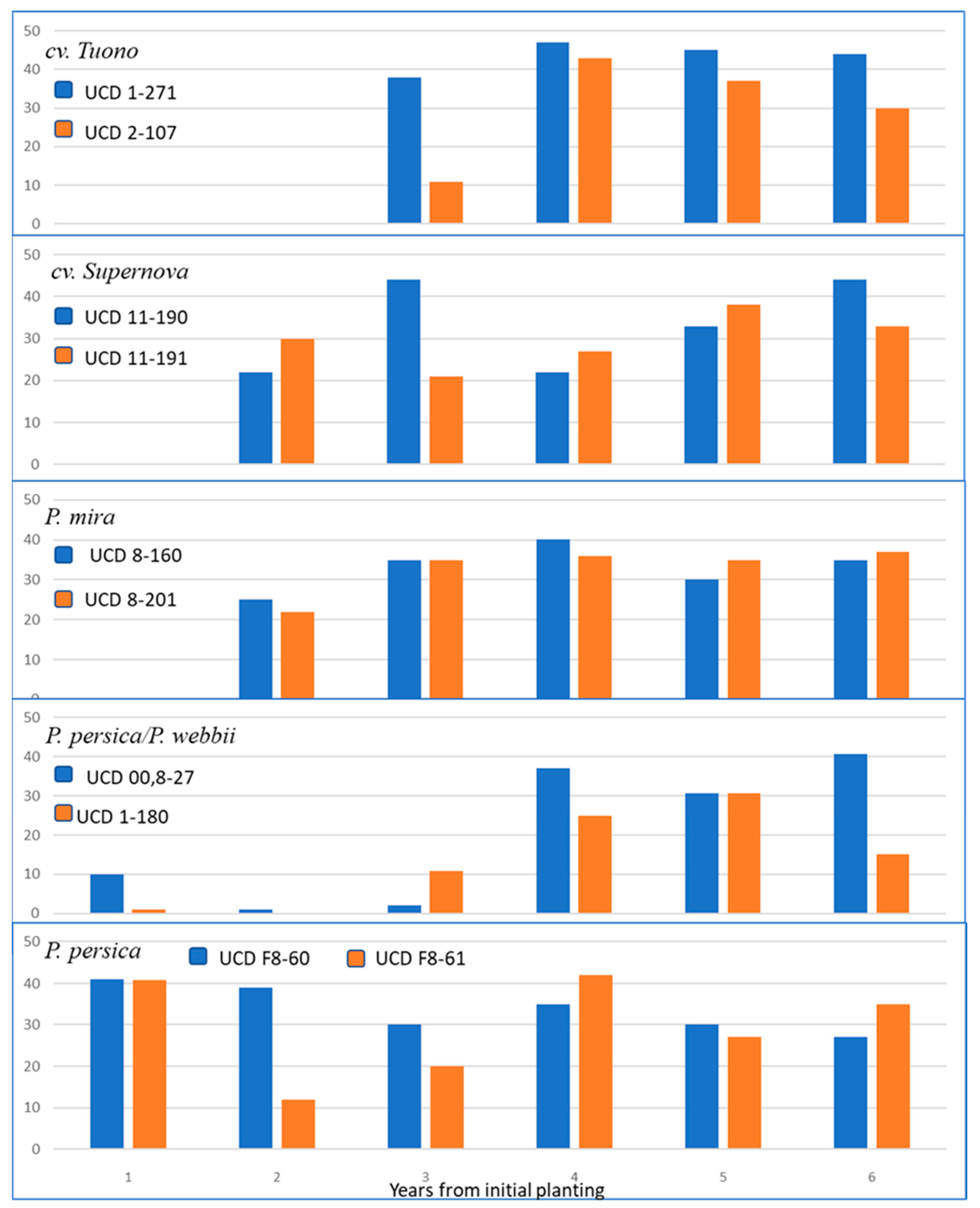
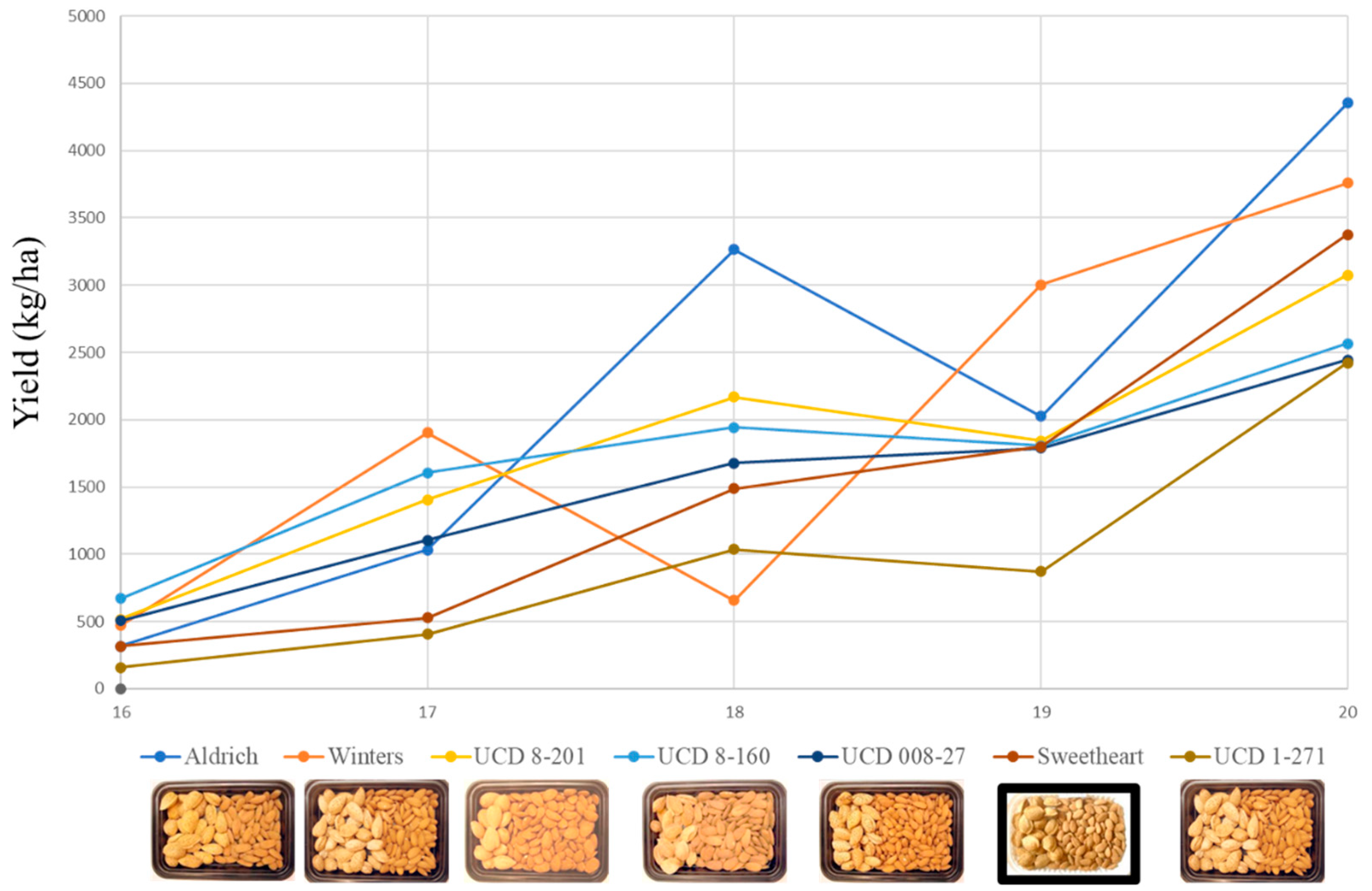
3.4. Field Testing
4. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almond Board of California. Almond Almanac. 2020. Available online: https://www.almonds.com/sites/default/files/2020-12/2020%20Almond%20Almanac.pdf (accessed on 10 October 2021).
- Arquero, O.; Jarvis-Shean, K. Orchard Management. In Almonds: Botany, Production and Uses; Socias i Company, Gradziel, T., Eds.; CABI Press: Boston, MA, USA, 2017; pp. 228–240. [Google Scholar]
- Connell, J.; Gradziel, T.; Lampinen, B.; Micke, W.; Verdegaal, P. Bloom timing of almond cultivars in California. Acta Hortic. 2018, 1219, 25–30. [Google Scholar] [CrossRef]
- USDA Agricultural Statistics Service, Pacific Region. Acreage Report. 2020. Available online: https://www.nass.usda.gov/ (accessed on 10 October 2021).
- Socias i Company; Ansón, J.M.; Espiaus, M.T. Taxonomy, Botany and Physiology. In Almonds: Botany, Production and Uses; Socias i Company, Gradziel, T., Eds.; CABI Press: Boston, MA, USA, 2017; pp. 1–43. [Google Scholar]
- Cobos, F.P.D.L.; Martínez-García, P.J.; Romero, A.; Miarnau, X.; Eduardo, I.; Howad, W.; Mnejja, M.; Dicenta, F.; Rubio-Cabetas, M.J.; Gradziel, T.M.; et al. Pedigree analysis of 220 almond genotypes reveals two world mainstream breeding lines based on only three different cultivars. Hortic. Res. 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Socias i Company; Kodad, O.; Fernandez i Martí, A.; Alonso, J.M. Mutations conferring self-compatibility in Prunus species: From deletions and insertions to epigenetic alterations. Sci. Hortic. 2015, 192, 125–131. [Google Scholar] [CrossRef]
- Gradziel, T.; Martinez-Gomez, P.; Dandekar, A.; Uratsu, S.; Ortega, E. Multiple genetic factors control self-fertility in almond. Acta Hortic. 2001, 591, 221–227. [Google Scholar] [CrossRef]
- Muñoz-Sanz, J.V.; Zuriaga, E.; López, I.; Badenes, M.L.; Romero, C. Self-(in)compatibility in apricot germplasm is controlled by two major loci, S and M. BMC Plant Biol. 2017, 17, 82. [Google Scholar] [CrossRef]
- Gradziel, T.M.; Kester, D.E. Breeding for self-fertility in california almond cultivars. Acta Hortic. 1997, 470, 109–117. [Google Scholar] [CrossRef]
- Gradziel, T.M. Almond origin and domestication. Hortic. Rev. 2011, 38, 23–82. [Google Scholar]
- Socias i Company; Kester, D.E.; Bradley, M.V. Effects of Temperature and Genotype on Pollen Tube Growth in Some Self-incompatible and Self-compatible Almond Cultivars. J. Am. Soc. Hortic. Sci. 1976, 101, 490–493. [Google Scholar] [CrossRef]
- Gradziel, T.; Weinbaum, S.A. High Relative Humidity Reduces Anther Dehiscence in Apricot, Peach, and Almond. HortScience 1999, 34, 322–325. [Google Scholar] [CrossRef]
- Sánchez-Pérez, R.; Del Cueto, J.; Dicenta, F.; Martínez-Gómez, P. Recent advancements to study flowering time in almond and other Prunus species. Front. Plant. Sci. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- Donoso, J.; Picañol, R.; Serra, O.; Howad, W.; Alegre, S.; Arús, P.; Eduardo, I. Exploring almond genetic variability useful for peach improvement: Mapping major genes and QTLs in two interspecific almond × peach populations. Mol. Breed. 2016, 36, 1–17. [Google Scholar] [CrossRef]
- Alonso, J.M. Environmental Requirements. In Almonds: Botany, Production and Uses; Socias i Company, Gradziel, T., Eds.; CABI Press: Boston, MA, USA, 2017; pp. 254–278. [Google Scholar]
- Brooks, R.M.; Olmo, H.P. Register of new fruit, nut varieties: Nemaguard peach. Proc. Am. Soc. Hort. Sci. 1961, 78, 634–635. [Google Scholar]
- Kester, D.E.; Gradziel, T.M. Almonds. In Fruit Breeding; Janick, J., Moore, J.N., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1996; Volume III: Nuts, pp. 1–97. [Google Scholar]
- Gradziel, T.M.; Martínez-Gómez, P.; Dicenta, F.; Kester, D.E. The utilization of related almond species for almond variety improvement. J. Am. Pomol. Soc. 2001, 55, 100–108. [Google Scholar]
- Lampinen, B.D.; Gradziel, T.M.; Yeager, J.T.; Thorpe, M.A.; Micke, W.C.; Connell, J.H.; Verdegaal, S.P.; Viveros, M. Regional almond variety trials for cultivar evaluation in California. Acta Hort. 2002, 591, 457–464. [Google Scholar] [CrossRef]
- Socias i Company. Pollen–Style (In)compatibility: Development of Autogamous Cultivars. In Almonds: Botany, Production and Uses; Socias i Company, Gradziel, T., Eds.; CABI Press: Boston, MA, USA, 2017; pp. 188–208. [Google Scholar]
- Gradziel, T.; Lampinen, B.; Connell, J.; Viveros, M. ‘Winters’ Almond: An Early-Blooming, Productive and High Quality Pollenizer for ‘Nonpareil’. HortScience 2007, 42, 1725–1727. [Google Scholar] [CrossRef]
- Gradziel, T.; Lampinen, B.; Niederholzer, F.; Viveros, M. ‘Sweetheart’ Almond: A Fully Cross-compatible Pollenizer for the Early ‘Nonpareil’ Bloom that Exhibits Very High ‘Marcona’-type Kernel Quality. HortScience 2013, 48, 1320–1322. [Google Scholar] [CrossRef]
- Kodad, O.; Sánchez, A.; Oliveira, M.M. The Expression of Self-compatibility in Almond May Not Only Be Due to the Presence of the Sf Allele. J. Am. Soc. Hortic. Sci. 2009, 134, 221–227. [Google Scholar] [CrossRef]
- Fernandez i Marti, A.; Hanada, T.; Alonso, J.M.; Yamane, H.; Tao, R.; Socias i Company. A modifier locus af-fecting the expression of the S-RNase gene could be the cause of breakdown of self-incompatibility in almond. Sex. Plant Repro. 2009, 22, 179–186. [Google Scholar] [CrossRef]
- Barckley, K.K.; Uratsu, S.L.; Gradziel, T.; Dandekar, A. Multidimensional Analysis of S-alleles from Cross-incompatible Groups of California Almond Cultivars. J. Am. Soc. Hortic. Sci. 2006, 131, 632–636. [Google Scholar] [CrossRef]
- Grasselly, C.; Olivier, G. Mise en evidence de quelques types autocompatibles parmi les cultivars d’amandier (P. amygdalus Batsch) de la population des Pouilles. Ann. Amélioration Plantes 1976, 26, 71–75. [Google Scholar]
- Ortega, E.; Dicenta, F. Suitability of four different methods to identify self-compatible seedlings in an almond breeding programme. J. Hortic. Sci. Biotechnol. 2004, 79, 747–753. [Google Scholar] [CrossRef]
- Godini, A. Ipotesi sulla comparsa dell’autocompatibilita nel mandorlo. Riv. Sci. Tec. Agrar. 1979, 19, 3–10. [Google Scholar]
- Monastra, F.; Della Strada, G.; Fideghelli, C.; Quarta, R. Supernova, une nouvelle variete d’amandier obtenue par mutagenese. Rapp. EUR 1988, 11557, 3–7. [Google Scholar]
- Felipe, A.J.; Socias i Company, R. ‘Aylés’, ‘Guara’, and ‘Moncayo’ almonds. HortSci 1987, 22, 961–962. [Google Scholar] [CrossRef]
- Marchese, A.; Boškovic’, R.; Martinez-Garcia, P.J.; Tobutt, K.R. The origin of the self-compatible almond ‘Supernova’. Plant Breed. 2008, 127, 105–107. [Google Scholar] [CrossRef]
- Dicenta, F.; Sánchez-Pérez, R.; Rubio, M.; Egea, J.; Batlle, I.; Miarnau, X.; Palasciano, M.; Lipari, E.; Confolent, C.; Martínez-Gómez, P.; et al. The origin of the self-compatible almond ‘Guara’. Sci. Hortic. 2015, 197, 1–4. [Google Scholar] [CrossRef]
- Rahemi, A.; Fatahi, R.; Ebadi, A.; Taghavi, T.; Hassani, D.; Gradziel, T.; Chaparro, J. Genetic variation of S-alleles in wild almonds and their related Prunus species. Austral. J. Crop Sci. 2010, 4, 648–659. [Google Scholar]
- Martinez-Gomez, P.; Arulsekar, S.; Potter, D.; Gradziel, T. Relationships among Peach, Almond, and Related Species as Detected by Simple Sequence Repeat Markers. J. Am. Soc. Hortic. Sci. 2003, 128, 667–671. [Google Scholar] [CrossRef]
- Ushijima, K.; Sassa, H.; Dandekar, A.M.; Gradziel, T.M.; Tao, R.; Hirano, H. Structural and transcriptional analysis of the self-incompatibility locus of almond: Identification of a pollen-expressed F-Box Gene with Haplotype- specific poly-morphism. Plant Cell 2003, 15, 771–781. [Google Scholar] [CrossRef]
- Hanada, T.; Watari, A.; Kibe, T.; Yamane, H.; Wunsch, A.; Gradziel, T.; Sasabe, Y.; Yaegaki, H.; Yamaguchi, M.; Tao, R. Two Novel Self-compatible S Haplotypes in Peach (Prunus persica). J. Jpn. Soc. Hortic. Sci. 2014, 83, 203–213. [Google Scholar] [CrossRef]
- Kevan, P.; Ebert, T. Can almond nectar & pollen poison honey bees? Am. Bee J. 2005, 145, 507–509. [Google Scholar]
- Gradziel, T.M.; Martínez-Gómez, P. Almond Breeding. Plant Breed. Rev. 2013, 37, 207–258. [Google Scholar]
- Serra, O.; Donoso, J.; Picañol, R.; Batlle, I.; Howad, W.; Eduardo, I.; Arús, P. Marker-assisted introgression (MAI) of almond genes into the peach background: A fast method to mine and integrate novel variation from exotic sources in long intergeneration species. Tree Genet. Genom. 2016, 12, 96. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Prudencio, A.S.; Gradziel, T.M.; Dicenta, F. The delay of flowering time in almond: A review of the combined effect of adaptation, mutation and breeding. Euphytica 2017, 213, 1–10. [Google Scholar] [CrossRef]
- Bartolozzi, F.; Warburton, M.; Arulsekar, S.; Gradziel, T. Genetic Characterization and Relatedness among California Almond Cultivars and Breeding Lines Detected by Randomly Amplified Polymorphic DNA (RAPD) Analysis. J. Am. Soc. Hortic. Sci. 1998, 123, 381–387. [Google Scholar] [CrossRef]
- Prudencio, A.S.; Sánchez-Pérez, R.; Martínez-García, P.J.; Dicenta, F.; Gradziel, T.M.; Martínez-Gómez, P. Genomic Designing for New Climate-Resilient Almond Varieties. Genom. Des. Climate-Smart Fruit Crops 2020, 1, 1–21. [Google Scholar] [CrossRef]
- Martinez-Gomez, P.; Arulsekar, S.; Potter, D.; Gradziel, T. An extended interspecific gene pool available to peach and almond breeding as characterized using simple sequence repeat (SSR) markers. Euphytica 2003, 131, 313–322. [Google Scholar] [CrossRef]
- Dangl, G.S.; Yang, J.; Golino, D.A.; Gradziel, T. A practical method for almond cultivar identification and parental analysis using simple sequence repeat markers. Euphytica 2009, 168, 41–48. [Google Scholar] [CrossRef]
- Felipe, A.; Gómez-Aparisi, J.; Socias, R.; Carrera, M. The almond x peach hybrid rootstocks breeding program at Zaragoza (Spain). Acta Hortic. 1997, 451, 259–262. [Google Scholar] [CrossRef]
- Arús, P.; Yamamoto, T.; Dirlewanger, E.; Abbott, A.G. Synteny in the Rosaceae. Plant Breed. Rev. 2006, 27, 175–211. [Google Scholar] [CrossRef]
- Gradziel, T.M. Redomesticating Almond to Meet Emerging Food Safety Needs. Front. Plant Sci. 2020, 11, 778. [Google Scholar] [CrossRef] [PubMed]
- Gradziel, T.M.; Lampinen, B.D. Defining the limits of almond productivity to facilitate marker assisted selection and orchard management. Acta Hortic. 2013, 912, 33–39. [Google Scholar] [CrossRef]
- Socias i Company, R.; Fernandez i Marti, A.; Kodad, O.; Alonso, J.M. Self-compatibility evaluation in almond: Strategies, achievements and failures. HortScience 2010, 45, 1155–1159. [Google Scholar] [CrossRef]
- Gradziel, T.M. Classical genetics and traditional breeding. In Genetics, Genomics and Breeding of Stone Fruits; Abbott, A.G., Kole, C., Eds.; Science Publishers: Plymouth, UK, 2012; pp. 22–53. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradziel, T.M. Transfer of Self-Fruitfulness to Cultivated Almond from Peach and Wild Almond. Horticulturae 2022, 8, 965. https://doi.org/10.3390/horticulturae8100965
Gradziel TM. Transfer of Self-Fruitfulness to Cultivated Almond from Peach and Wild Almond. Horticulturae. 2022; 8(10):965. https://doi.org/10.3390/horticulturae8100965
Chicago/Turabian StyleGradziel, Thomas M. 2022. "Transfer of Self-Fruitfulness to Cultivated Almond from Peach and Wild Almond" Horticulturae 8, no. 10: 965. https://doi.org/10.3390/horticulturae8100965
APA StyleGradziel, T. M. (2022). Transfer of Self-Fruitfulness to Cultivated Almond from Peach and Wild Almond. Horticulturae, 8(10), 965. https://doi.org/10.3390/horticulturae8100965




