Abstract
Moringa (Moringa oleifera Lam.) is a fast-growing tree that can reach a height of 3 m when left to grow naturally. Cutting-back management can enhance lateral branching, water use efficiency, regrowth, and leaf biomass production. Although M. oleifera can thrive in diverse ecological environments, including areas with high temperatures and drought where most crops cannot survive, the prevailing agro-ecological conditions can influence the tree’s response to cut-back and nutritional composition. An observation trial on the re-sprouting of M. oleifera after cutting back was conducted in three agro-ecological zones, including arid, semi-arid, and dry sub-humid regions. The soil analysis from the three agro-ecological zones showed variations, with the soil collected from the arid area recording the highest clay content (24.4%), nitrogen (8.14%), and phosphorus (168.2 mg/kg). The nutritional composition of the M. oleifera leaves in response to the cutting back was assessed. The trees planted in the arid region responded well to the cutting back by producing considerable new vegetative growth in the spring (13.4 sprouts averaging 21.50 cm in length) compared to semi-arid and dry sub-humid regions. The region’s climatic conditions favoured M. oleifera re-sprouting, and the stems did not die after cutting back. During the winter, the stems die back, sprout from the root collar area during more favourable weather conditions, and take longer to reach the harvestable stage. The leaves of M. oleifera harvested from the arid and dry sub-humid regions exhibited increased total fat, magnesium, sodium, phosphorus, potassium, and zinc content when compared to the semi-arid agro-ecological zone. In contrast, the calcium content of the leaves was higher in the semi-arid region than in the other agro-ecological zones. The influence of cutting back M. oleifera trees on biomass production and quality in varying agro-ecological zones requires further investigation to ensure that smallholder farmers use appropriate crop management practices in those regions for long-term, economically viable tree production.
1. Introduction
Moringa oleifera Lam. is categorized as a fast-growing tropical deciduous plant of the Moringaceae family, with all its parts, including the leaves, flowers, seeds, and roots, used in traditional medicine [1]. Moringa oleifera is native to the southern foothills of the Himalayans in Northern India [2], especially in the southwestern region, which experiences hot steppe, hot subtropical summers, dry-summer subtropical, and subtropical-dry winters [3]. Due to its economic and medicinal properties, M. oleifera has been introduced into other parts of the world, including Africa, Asia Minor, and Arabia [1]. The tree has historically been used in traditional medicine and for ornamental purposes in different parts of the world [4]. Its use in traditional and alternative medicine in the treatment/management of several ailments, including skin diseases, asthma, and fever [4], is attributed to its antioxidant activity, phytochemicals, and nutritional composition [5].
Moringa oleifera can grow in various rainfall conditions and withstand long periods of drought, growing well in arid and semi-arid areas [6]. The tree can tolerate various soil pH conditions and grow in soils with a pH of between 4.5 and 8 [2]. Moringa oleifera is capable of growing and living for up to 20 years and reaching 3 m in height [7]. The tree is also known as the horseradish tree due to the flavour of its roots and as the drumstick tree due to the form of its pods [8]. Moringa oleifera is a multipurpose tree with every plant part having beneficial properties [9] for humans and animals [6]. The tree is a versatile plant because it can provide edible food from the leaves and pod shells. The leaves can be ingested freshly cooked or as a powder that can be reserved for an extended period without depleting their nutritional status [10]. The leaves are used for vegetable soups, salads, and spices [11]. The Moringa oleifera leaf is a rich source of minerals, such as calcium, potassium, zinc, magnesium, iron, phosphorus, and copper [12]. Moringa oleifera leaves contain a high protein content and can be considered a source of protein in food systems [12]. The leaves, a magnificent source of trace elements, carotenoids, and antioxidant substances, are consumed for nutritional purposes. The seed oil consists of calcium, sodium, manganese, magnesium, potassium, iron, and zinc (204, 155, 3, 220, 479, 31, and 8 mg/kg, respectively) with a protein of 18.92 g/100 g and oil of 2.74 g/100 g [13]. The seeds can also be used in hair cosmetics and preparation products such as conditioners and moisturizers [14]. Moringa oleifera is an alley crop contributing to decreased soil acidity [15,16]. Integrating shade-loving crops with M. oleifera in intercropping or agroforestry can contribute to properly utilizing natural resources and benefit farmers [17].Other benefits of M. oleifera include seed oil of low cost, the ability to purify water, and being the most readily available source of biodiesel l [18,19].
The practice of cutting back trees, such as M. oleifera, has numerous advantages. Cutting back can increase water use efficiency and improve quality, growth, and leaf nutrients [20]. However, cutting back seems to affect the growth and performance of M. oleifera [2]. Although M. oleifera can thrive in diverse ecological regions, including in areas with high temperatures and drought where most crops cannot survive, the prevailing agro-ecological conditions can influence the tree’s response to cutting back [21,22]. Previous research documented the importance of cutting back on M. oleifera as a strategy to manipulate and foster the vegetative and reproductive growth of the tree [23]. The tree re-sprouts vigorously, particularly after a cutting back, resulting in a dense shape, shaded canopies, and excessive tree size, particularly in hot climates. Cutting back is often recommended to enhance lateral branching, give the tree a bushy shape, and facilitate harvesting [23].
Research has been conducted on cutting back various tree species [23]. However, there is a dearth of reported research on M. oleifera cutting back and the nutritional composition. This study evaluated the influence of cutting-back practice and the nutritional composition of M. oleifera from different agro-ecological zones in South Africa.
2. Materials and Methods
2.1. Study Sites
An observation trial on the re-sprouting of M. oleifera after cutting back was conducted at three study sites: the Agriculture Research Council (ARC)–Roodeplaat Research farm (Gauteng Province), Tooseng in Capricorn District Municipality (Limpopo Province), and Makonde in Vhembe District Municipality (Limpopo Province). These three sites fall into three agro-ecological zones in South Africa, which are semi-arid (ARC-Roodeplaat Research farm), dry sub-humid (Capricorn District), and arid (Vhembe District) regions (Figure 1). The data on the number of sprouts and sprout length were collected from September 2020 (the period after dormancy) until the beginning of the first sprouts in November–December 2021.
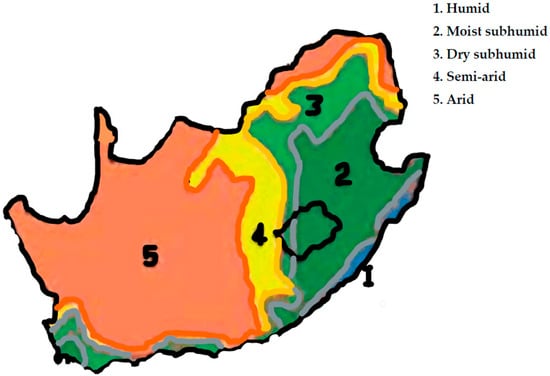
Figure 1.
Map of South Africa showing the different agro-ecologies. The Vhembe and Capricorn districts are in the Limpopo Province, in the arid (5) and dry sub-humid (3) regions, respectively. The ARC’s Roodeplaat Research Farm is located in the Gauteng Province, within the semi-arid (4) region (adopted from FAO [24]).
The ARC’s Roodeplaat Research Farm is located at latitude 25°59′ S, longitude 28°35′ E, and an altitude of 1200 m above sea level. The ARC regional weather station (Campbell Scientific, Logan, UT, USA) provided climatic data for the experiment during the 2019/2020 growing seasons. The two regions (Vhembe and Capricorn Districts) in Limpopo Province are located in the northernmost part of South Africa, neighbouring Mozambique, Botswana, and Zimbabwe. The Province has five districts: Vhembe, Mopani, Great Sekhukhune, Capricorn, and Waterberg (Figure 2). The weather data (Figure 3) for the Vhembe and Capricorn districts were collected from an automatic weather station near the study site.
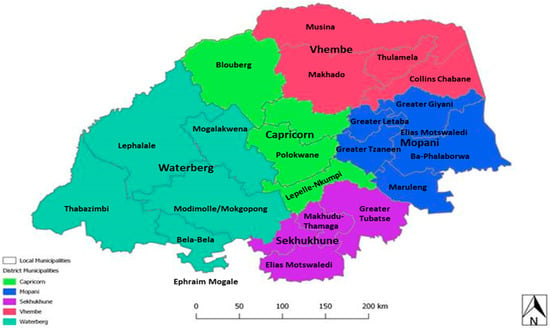
Figure 2.
Map of the Limpopo Province showing the districts within the Province [25].
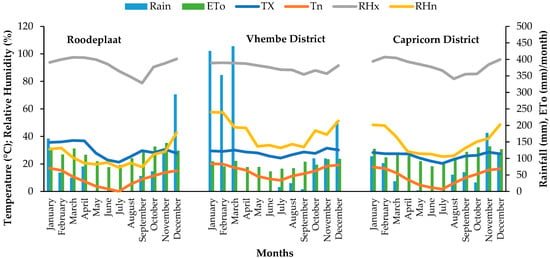
Figure 3.
Weather conditions of Moringa oleifera plantations in three agro-ecological zones in South Africa (Roodeplaat, Vhembe District, and Capricorn District) from January to December 2020. Tx = maximum temperature (°C), Tn = minimum temperature (°C), RHx = maximum relative humidity, RHn = minimum relative humidity, ETo = reference evapotranspiration.
2.2. Growing Degree Days (GDD)
The phenomenon of calculating the Growing Degree Days (GDD) plays a vital role in the description and prediction of phenological events such as crop development stage and crop phenology. The GDD is a temperature-derived index that determines the amount of heat available for the growth of plants such as M. oleifera. Growing Degree Days (GDD) were calculated by taking the integral of warmth above a base temperature of 8 °C (Tb) and below an upper limit maximum temperature for crop growth of 48 °C (Tcutoff) [26,27], following the equation below [26,27]:
where integration is over the period with Tcutoff > T(t) > Tb.
2.3. Measurement of the Number of Sprouts and Sprout Length of M. oleifera Plantation in Three Growing Areas
The experimental design used in this study was a randomized complete block design (RCBD) with five replicates. Three treatments (M. oleifera growing regions) were investigated, as follows: (1) ARC-Roodeplaat; (2) Vhembe district, and (3) Capricorn district. The entire M. oleifera plantation was divided into five blocks (replicates) in each growing region. In each block, five representative trees were tagged and labelled as the sample trees for data collection of the number and length of sprouts.
2.4. Soil Collection and Analysis
Soil samples were collected from the three sites and analyzed at different depths (0–30 cm and 30–60 cm)(Table 1). Soils were analyzed for nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and pH (H2O) using standard analytical methods.

Table 1.
Chemical characteristics of soils collected from Moringa oleifera plantations in three growing areas in South Africa during the 2020 growing season.
2.5. Leaf Nutritional Composition
To evaluate the nutritional composition, leaves from trees planted in each agro-ecological site were harvested during the cropping season, freeze-dried, and ground for analysis. Crude protein was determined using the Kjeldahl method. The minerals Ca, Mg, Na, P, K, Fe, and Zn were determined in triplicates using inductively coupled plasma optical emission spectrometry (ICP-OES), while β-carotene and vitamin C were quantified using a high-performance liquid chromatography (HPLC)(Shimadzu, Kyoto, Japan) method [28].
2.6. Data Analysis
Data were analyzed using GenStat for Windows 18th Edition (VSN International, Hemel Hempstead, UK). Analysis of variance (ANOVA) was performed for the three growing regions combined after testing the homogeneity of the experimental error variances using Bartlett’s test. Fisher’s least significant difference (LSD) was calculated at the 5% level to compare means for significant effects. The coefficient of variation was also calculated to determine the reliability of the data.
3. Results
3.1. Soil Analysis from Three Agro-Ecological Zones
The chemical properties of the soil varied greatly. The ARC-Roodeplaat site had the highest K (287.7 mg/kg)(Table 1). The clay content of the soil collected from the ARC-Roodeplaat is relatively comparable to that of the soil from the Vhembe District. The Vhembe District soil contained the highest nitrogen (8.14%), followed by the Capricorn District, and the least amount recorded was with the soil collected from the ARC-Roodeplaat (Table 1). The Ca composition of the soils from ARC-Roodeplaat, and the Vhembe District was half of what was recorded in the soil from the Capricorn district. The ARC-Roodeplaat soil had the highest pH (7.06), followed by that of the Capricorn district (6.50), while the soil from the Vhembe district was slightly acidic.
3.2. Number and Length of Sprouts of Trees from the Three Agro-Ecological Zones
The number and length of sprouts of trees from the three agro-ecological zones are displayed in Table 2 and Table 3. The analysis of variance values in Table 2 showed significant differences between the sum of squares, mean square, and variation ratio for the number and length of sprouts from three agro-ecological zones.

Table 2.
ANOVA values and significant tests for the number of sprouts and length of Moringa oleifera from three agro-ecological zones.

Table 3.
Mean number and length of sprouts of Moringa oleifera trees in three growing areas in South Africa during the 2020 growing season.
The trees in the Vhembe and Capricorn districts showed new sprouts in response to the cut-back by producing a considerable amount of new vegetative growth in the spring (Figure 4A,B) compared to that observed at ARC-Roodeplaat (Figure 4C). Trees in the Vhembe District had the highest number and length of sprouts when compared to trees in the Capricorn District and ARC-Roodeplaat (Table 3), with the latter having the lowest number and length of sprouts. The observed variations in the number and length of sprouts could be attributed to relatively high minimum ambient temperatures in the Vhembe and Capricorn regions (daily values varying from 3.2 to 24.5 °C in Makonde, Vhembe District; and from −4.2 to 21.7 °C in Tooseng, Capricorn District) compared to those observed at ARC-Roodeplaat (−7.2 to 20.6 °C).
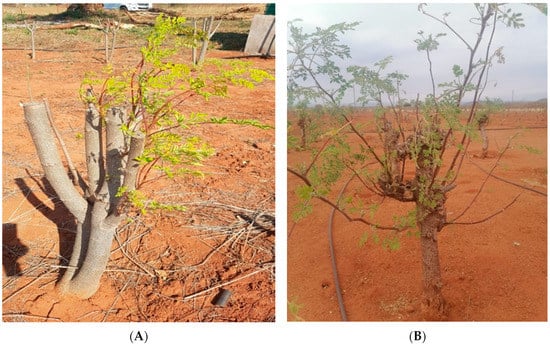

Figure 4.
Pruning of Moringa oleifera at Vhembe District (A), Capricorn District (B), and ARC-Roodeplaat (C).
3.3. Thermal Time Accumulation of Moringa oleifera across the Three Agro-Ecological Zones of South Africa
The variability of ambient temperature across the three study regions was further evaluated by computing Growing Degree Days (GDD) or heat units to explain variations in the growth and development rates of M. oleifera during the experimental period (Figure 5). The weather conditions in the Vhembe District resulted in faster heat accumulation throughout the growing season of M. oleifera. In contrast, the ARC-Roodeplaat and the Capricorn District showed unnoticeable thermal time accumulation differences, which is explained by the relatively lower maximum ambient temperatures in the Capricorn district (11.9 to 36.5 °C) compared to the ARC-Roodeplaat (15.2 to 43.8 °C).
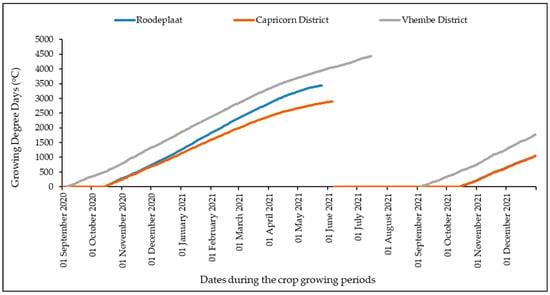
Figure 5.
Thermal time accumulation (expressed in Growing Degree Days–GDD) of Moringa oleifera across the three study regions of South Africa, from September 2020 (period after dormancy) to the beginning of the first sprouts in November–December 2021.
In general, the plants required more days under cold conditions to reach maturity compared to summer conditions. The Vhembe District is located in an area with predominantly mild winters that are mostly frost-free and very hot. The district receives an average annual rainfall of 455 mm, which falls between November and March (Figure 3). The rainfall data showed that the Capricorn District was drier (655.58 mm) but with better rainfall distribution than the other two regions. In contrast, the Vhembe District was exceptionally wet during the 2020 growing season (Figure 3).
The regions’ climatic conditions favoured M. oleifera re-sprouting, and the stems did not die back after the cut-back (Figure 4A). Contrarily to the Vhembe District, the ARC-Roodeplaat region is close to a river (Pienaarsrivier, South Africa), and temperatures can go below 4 °C during the winter months. During winter, the cold weather conditions are unfavourable for M. oleifera growth. The stems die back during winter and sprout from the root collar area during more favourable weather conditions (Figure 4C).
3.4. Nutritional Composition of Moringa oleifera Leaves across the Study Three Agro-Ecological Zones of South Africa
The total fat, magnesium, sodium, phosphorus, potassium, and zinc contents of leaves from the Capricorn and Vhembe districts were significantly higher than those from the ARC-Roodeplaat (Table 4). The zinc content ranged from 17.5 mg/kg for Roodeplaat to 47.2 mg/kg for the Vhembe district. Thus, the zinc content recorded from the Vhembe district was two-fold compared to the content reported for the Roodeplaat. The protein, β-carotene, and vitamin C contents did not vary significantly across the three agro-ecological zones (Table 4).

Table 4.
Nutritional composition of Moringa oleifera leaves from ARC-Roodeplaat, Capricorn, and Vhembe Districts in South Africa.
4. Discussion
Moringa oleifera can adapt to low temperatures, but temperatures below −5 °C, even for one night, can significantly affect the tree [29]. For many reasons, frost can negatively affect plants, resulting in freezing-induced cavitation [30], as an example. The weather conditions in the three agro-ecological zones might have contributed to optimum growth (shown by the number and length of sprouts). Cavitation happens when there is a loss of cohesion between water molecules in the xylem conduits and when there is a loss of adhesion between water and the conduit walls of the xylem [30]. Thus, the water columns’ integrity is disrupted, the sap ascent mechanism is interrupted, and the accumulation of cavitation events leads to plant death [31]. Wilson and Jackson [31] found that more significant cavitation occurs when drought is followed by a freeze–thaw cycle, which could be the case in the ARC-Roodeplaat M. oleifera trees where winters are dry and cold. Under such unfavourable conditions, the underground tuberous root system could be a survival strategy for the M. oleifera trees.
The re-sprouting rate of M. oleifera trees was highly influenced by the prevailing ambient temperatures, with minimum daily temperatures being the primary factor affecting the productivity of the crop. Moringa oleifera is a drought-tolerant crop that tolerates annual rainfall from 250 to 3000 mm/annum, with optimum ambient temperatures fluctuating between 25 and 35 °C. The tree grows well in warm, semi-arid tropical conditions [29].
Frost-free regions, such as the Vhembe district, are more promising for increased M. oleifera productivity, as the environmental conditions are more conducive to a faster accumulation of thermal time and increased development rates than cooler regions, such as ARC-Roodeplaat. The sprouting observed in this study agrees with the report by Del Tredici [32], stipulating that the increase can be attributed to natural environmental conditions, hence the difference between the three agro-ecological zones. Previous reports showed that the sprouting ability could differ with the trees’ age (or size); the younger trees can sprout more, but the ability can be lost with age [33,34]. The re-sprouting from dormant buds has been reported in previous studies for different tree species, and most re-sprout continuously after stress [35,36]. In contrast, M. oleifera becomes dormant in winter due to decreased temperatures, while sprouting occurs from the root collar area during more favourable weather conditions and takes longer to reach the harvestable stage.
Further research is, however, needed to optimize and better understand the cut-back of M. oleifera in varying agro-ecological zones. On-station research trials are currently being conducted at ARC-Roodeplaat to obtain more insight into M. oleifera crop responses to varying field management practices. This will ensure increased market competitiveness and the long-term viability of M. oleifera production amongst smallholder farmers in such regions.
The high protein content across the three regions is particularly of nutritional significance for individuals suffering from protein-calorie malnutrition. Protein-rich plants such as M. oleifera can be a primary option for many resource-poor smallholder farmers to combat food insecurity. The leaves of M. oleifera contain essential amino acids and are rich in protein and minerals, highly valued for treating digestive ailments and colon cancer [37,38]. These essential micronutrients can act as a carrier or participate in the breakdown of macronutrients. Vitamins are essential and play a significant role in the body’s energy processing [38]. The nutritional composition reported in this study was from the leaf sprouts collected from the arid and dry sub-humid and semi-arid regions (Table 4; Figure 1). Calcium is considered one of the essential minerals, whereas dried M. oleifera powder is an excellent source of that element [12]. The iron and zinc content reported in this study are well enough to fulfil the daily requirement in the diet and agree with previous studies [12,38]. While the current results were obtained from young sprouts after the dormant stage of M. oleifera, further research on matured leaves following crop management practices such as pruning is essential. The exploration of crop management practices can set standards for best pruning strategies, cutting back, fertilizer applications, intercropping, and understanding the climatic conditions (e.g., temperature and rainfall) for producing M. oleifera. Such standards and guidelines, directed explicitly to crop management of M. oleifera, can influence the nutritional quality of products and their derivative products. Hence, standardisation of crop management practices such as cut-back techniques in each agro-ecological zone may be necessary for improved productivity and quality assurance.
5. Conclusions
Although M. oleifera trees become dormant in the winter as temperatures drop, the cut-back practice has the potential to improve growth and yield depending on agro-ecological climatic conditions. During the winter, the stems die back and sprout from the root collar area during warmer weather, taking longer to reach the harvestable stage. Crop management strategies such as pruning can impact the nutritional quality of products and their derivatives. As a result, crop management practices in each agro-ecological zone may be required for increased productivity and guidance to smallholder farmers cultivating M. oleifera.
Author Contributions
S.M., N.A. and H.A. conceptualized the study and drafted the manuscript. S.M., N.A. and H.A. analyzed and interpreted the data. S.M., N.A., H.A., S.A., C.d.P., M.M (Motiki Mofokeng), K.M. and M.M. (Manaka Makgato) contributed to the reviewing and writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agricultural Research Council, the Department of Science and Innovation (DST/CON 0026/2019) and The Water Research Commission (Project No. C2020/2021-00484).
Data Availability Statement
Not applicable.
Acknowledgments
The Water Research Commission (Project No. C2020/2021-00484) and the Agricultural Research Council are gratefully acknowledged for funding and institutional support. The ARC–Corporate Office, Biometry Department (particularly Ms Liesl Morey) is gratefully acknowledged for assistance with growth data statistical analysis.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Patil, S.V.; Mohite, B.V.; Marathe, K.R.; Salunkhe, N.S.; Marathe, V.; Patil, V.S. Moringa Tree, Gift of Nature: A Review on Nutritional and Industrial Potential. Curr. Pharmacol. Rep. 2022, 8, 262–280. [Google Scholar] [CrossRef] [PubMed]
- Korsor, M.; Ntahonshikira, C.; Bello, H.M.; Kwaambwa, H.M. Growth Performance of Moringa oleifera and Moringa ovalifolia in Central Namibia Semi-Arid Rangeland Environment. Agric. Sci. 2019, 10, 131–141. [Google Scholar] [CrossRef]
- Godino, M.; Arias, C.; Izquierdo, M. Moringa oleifera: Potential areas of cultivation on the Iberian Peninsula. Acta Hortic. 2017, 1158, 405–412. [Google Scholar] [CrossRef]
- Milla, P.; Peñalver, R.; Nieto, G. Health Benefits of Uses and Applications of Moringa oleifera in Bakery Products. Plants 2021, 10, 318. [Google Scholar] [CrossRef]
- Saini, R.K.; Sivanesan, I.; Keum, Y.-S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016, 6, 203. [Google Scholar] [CrossRef]
- Daba, M. Miracle Tree: A Review on Multi-purposes of Moringa oleifera and Its Implication for Climate Change Mitigation. J. Earth Sci. Clim. Chang. 2016, 7, 8. [Google Scholar] [CrossRef]
- Seifu, E.; Teketay, D. Introduction and expansion of Moringa oleifera Lam. in Botswana: Current status and potential for commercialization. S. Afr. J. Bot. 2020, 129, 471–479. [Google Scholar] [CrossRef]
- Tshabalala, T.; Ndhlala, A.; Ncube, B.; Abdelgadir, H.; Van Staden, J. Potential substitution of the root with the leaf in the use of Moringa oleifera for antimicrobial, antidiabetic and antioxidant properties. S. Afr. J. Bot. 2019, 129, 106–112. [Google Scholar] [CrossRef]
- Sarkar, S.; Panda, S. Moringa oleifera Lam.—A multipurpose and great therapeutic tree: A review. In Medicinal Plants: Herbal Wealth of India; Sharma, I.R., Ed.; Agrobios Research: Jodhpur, India, 2021; pp. 103–119. [Google Scholar]
- Glover-Amengor, M.; Aryeetey, R.; Afari, E.; Nyarko, A. Micronutrient composition and acceptability of Moringa oleifera leaf-fortified dishes by children in Ada-East district, Ghana. Food Sci. Nutr. 2016, 5, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Popoola, J.O.; Obembe, O.O. Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria. J. Ethnopharmacol. 2013, 150, 682–691. [Google Scholar] [CrossRef]
- Islam, Z.; Islam, S.M.R.; Hossen, F.; Mahtab-Ul-Islam, K.; Hasan, R.; Karim, R. Moringa oleifera is a Prominent Source of Nutrients with Potential Health Benefits. Int. J. Food Sci. 2021, 2021, 6627265. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Ogunsina, B.S.; Indira, T.N.; Bhatnagar, A.S.; Radha, C.; Debnath, S.; Krishna, A.G.G. Quality characteristics and stability of Moringa oleifera seed oil of Indian origin. J. Food Sci. Technol. 2011, 51, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Boukandoul, S.; Casal, S.; Zaidi, F. The Potential of Some Moringa Species for Seed Oil Production. Agriculture 2018, 8, 150. [Google Scholar] [CrossRef]
- Abdullahi, I.N.; Ozobia, A.P. The performance of Soybean using Moringa as an alley to improve soil productivity in North-Central Nigeria. Afr. J. Agric. Res. 2017, 12, 1182–1189. [Google Scholar]
- Reppin, S.; Kuyah, S.; de Neergaard, A.; Oelofse, M.; Rosenstock, T.S. Contribution of agroforestry to climate change mitigation and livelihoods in Western Kenya. Agrofor. Syst. 2019, 94, 203–220. [Google Scholar] [CrossRef]
- Fahey, J. Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Trees Life J. 2005, 1, 5. [Google Scholar]
- Delelegn, A.; Sahile, S.; Husen, A. Water purification and antibacterial efficacy of Moringa oleifera Lam. Agric. Food Secur. 2018, 7, 25. [Google Scholar] [CrossRef]
- Mabapa, M.P.; Ayisi, K.K.; Mariga, I.K. Effect of Planting Density and Harvest Interval on the Leaf Yield and Quality of Moringa (Moringa oleifera) under Diverse Agroecological Conditions of Northern South Africa. Int. J. Agron. 2017, 2017, 2941432. [Google Scholar] [CrossRef]
- Bopape-Mabapa, M.P. Yield Characteristics, Carbon Capture and Chemical Composition of Moringa Oleifera under Diverse Planting Populations and Agro-Ecological Conditions of the Limpopo Province. Ph.D. Thesis, University of Limpopo, Mankweng, Africa, 2019. [Google Scholar]
- Pinkard, E.A.; Beadle, C.L. A physiological approach to pruning. Int. For. Rev. 2000, 2, 295–305. [Google Scholar]
- Du Toit, E.S.; Sithole, J.; Vorster, J. Pruning intensity influences growth, flower and fruit development of Moringa oleifera Lam. under sub-optimal growing conditions in Gauteng, South Africa. S. Afr. J. Bot. 2019, 129, 448–456. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organisation). A Perspective on Water Control in Southern Africa. 2003. Available online: http://www.fao.org/docrep/006/y5096e/y5096e00.htm#Contents (accessed on 17 April 2013).
- Serote, B.; Mokgehle, S.; Du Plooy, C.; Mpandeli, S.; Nhamo, L.; Senyolo, G. Factors Influencing the Adoption of Climate-Smart Irrigation Technologies for Sustainable Crop Productivity by Smallholder Farmers in Arid Areas of South Africa. Agriculture 2021, 11, 1222. [Google Scholar] [CrossRef]
- Trigo, C.; Castelló, M.L.; Ortolá, M.D.; García-Mares, F.J.; Soriano, M.D. Moringa oleifera: An unknown crop in developed countries with great potential for industry and adapted to climate change. Foods 2021, 10, 31. [Google Scholar] [CrossRef]
- Ibraimo, N.A.; Taylor, N.J.; Steyn, J.M.; Gush, M.B.; Annandale, J.G. Estimating water use of mature pecan orchards: A six stage crop growth curve approach. Agric. Water Manag. 2016, 177, 359–368. [Google Scholar] [CrossRef]
- Moyo, M.; Amoo, S.O.; Aremu, A.O.; Gruz, J.; Šubrtová, M.; Jarošová, M.; Tarkowski, P.; Doležal, K. Determination of Mineral Constituents, Phytochemicals and Antioxidant Qualities of Cleome gynandra, Compared to Brassica oleracea and Beta vulgaris. Front. Chem. 2018, 5, 128. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Sass-Klaassen, U.; Sterck, F.; Goudzwaard, L.; Akhmetzyanov, L.; Poorter, L. Growth of 19 conifer species is highly sensitive to winter warming, spring frost and summer drought. Ann. Bot. 2021, 128, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Cochard, H.; Badel, E.; Herbette, S.; Delzon, S.; Choat, B.; Jansen, S. Methods for measuring plant vulnerability to cavitation: A critical review. J. Exp. Bot. 2013, 64, 4779–4791. [Google Scholar] [CrossRef]
- Wilson, C.J.; Jackson, R.B. Xylem cavitation caused by drought and freezing stress in four co-occurring Juniperus species. Physiol. Plant. 2006, 127, 374–382. [Google Scholar] [CrossRef]
- Del Tredici, P. Sprouting in temperate trees: A morphological and ecological review. Bot. Rev. 2001, 67, 121–140. [Google Scholar] [CrossRef]
- Bond, W.J.; Midgley, J.J. The Evolutionary Ecology of Sprouting in Woody Plants. Bot. Gaz. 2003, 164, S103–S114. [Google Scholar] [CrossRef]
- Vesk, P.A.; Westoby, M. Sprouting ability across diverse disturbances and vegetation types worldwide. J. Ecol. 2004, 92, 310–320. [Google Scholar] [CrossRef]
- Kennard, D.; Gould, K.; Putz, F.; Fredericksen, T.; Morales, F. Effect of disturbance intensity on regeneration mechanisms in a tropical dry forest. For. Ecol. Manag. 2002, 162, 197–208. [Google Scholar] [CrossRef]
- Pauw, A.; Van Bael, S.A.; Peters, H.A.; Allison, S.D.; Camargo, J.L.C.; Cifuentes-Jara, M.; Conserva, A.; Restom, T.G.; Heartsiii-Scalley, T.; Mangan, S.A.; et al. Physical damage in relation to carbon allocation strategies of tropical forest tree saplings. Biotropica 2004, 36, 410–413. [Google Scholar] [CrossRef]
- Rani, N.Z.A.; Husain, K.; Kumolosasi, E. Moringa Genus: A Review of Phytochemistry and Pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Mbikay, M. Therapeutic Potential of Moringa oleifera Leaves in Chronic Hyperglycemia and Dyslipidemia: A Review. Front. Pharmacol. 2012, 3, 24. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).