Abstract
The application of organic fertilizer has been one of the most important agricultural practices to increase tea plant productivity. However, a single organic fertilizer alone could not match the nutrient requirements of tea plant. According to the nutrient demand of tea plant, tea-specific fertilizer with an appropriate nutrient ratio in combination with organic fertilizer would be an appropriate way. A four-year field experiment was conducted to characterize the sensory quality of green tea subjected to four different fertilization treatments, i.e., tea-specific fertilizer (N-P2O5-K2O: 18-8-12) + urea + colza cake, tea-specific fertilizer + urea + livestock waste compost, and tea-specific fertilizer + urea, combined tea-specific fertilizer (referred to as M1, M2, and M3, respectively); nonfertilizing (CK) served as control. The fertilizer formulated specifically for tea could enhance the taste qualities of green tea, such as water extract, caffeine, tea polyphenol, EGCG, and ECG. However, the effect of this fertilizer on enhancing the aroma level of green tea was weaker than that of the application of tea-specific fertilizer combined with organic fertilizer, whereby this combined fertilization significantly improved the concentration of green tea aroma compounds, such as D-limonene, cis-jasmone, nonanal, linalool, cis-3-hexenyl hexanoate, and cis-3-hexenyl benzoate. This study provides a theoretical basis for judicious fertilization by combining tea-specific fertilizer and organic fertilizer in tea orchards.
1. Introduction
Tea plant (Camellia sinensis) is a valuable and popular cash crop and is widely planted in the tropical and subtropical areas of China. The popularity of tea has been increasing steadily in the past decades because it contains beneficial antioxidants, vitamins, and amino acids [1]. Apart from the beneficial effects on human health, green tea has received much attention due to its unique flavor. The flavor can be divided into the sensations of taste and aroma. Taste is mainly composed of nonvolatile compounds perceived by the oral cavity. Amino acids, polyphenols, and caffeine are critical ingredients in determining the umami, astringency, and typical bitterness of a green tea infusion, respectively [2,3]. Aroma consists of volatile compounds perceived by the olfactory epithelium [2]. There are about 700 volatile compounds in tea but only almost 300 with content of about 0.005–0.02% were detected in green tea [4].
The quality of green tea is closely related to agronomic practices and harvest time. The application of fertilizer has been one of the most important agronomic measures to increase crop productivity. However, with an increasing market demand for tea production, chemical fertilizers have been applied progressively by farmers [5]. Numerous researchers have reported that the over-application of chemical fertilizers in long-term tea cultivation resulted in land degradation problems, including soil acidification, structural damage, nutrient loss, and deficiency of beneficial bacteria [6,7,8].
Organic fertilizer is an important nutrient resource. Cavagnaro [9] reported that organic fertilizer could increase nutrient-use efficiency for supporting plant growth and development. Wang et al. [10] noted significant differences in the effects of inorganic and organic fertilization treatments on soil physicochemical properties. In tea orchards, previous studies also indicate that cow manure treatment significantly changes the enrichment of pathways related to amino acids, sugars, and fatty acids in green tea [11]. However, organic fertilizers have low total nutrient contents, low N availability, and ratios of N/P and N/K that do not match the nutrient requirements of tea plants. Furthermore, organic fertilizers need to be applied in large volumes, resulting in high transportation and application costs. Thus, organic fertilizers should be applied together with mineral fertilizer from the perspectives of nutrient availability, application convenience, and economy. Based on soil testing and fertilizer field experiments, crop nutrient requirements, soil fertilizer performance, and fertilizer effect, specific fertilizers have been formulated to be suitable for specific areas and specific crops. In view of the large range of tea areas and the complex changes of soil fertility in tea plantations in China, a special fertilizer for tea plants with the grade of tea-specific fertilizer 18-8-12 (N-P2O5-K2O) was proposed [12,13]. In this study, a field experiment was conducted with four different fertilization treatments, including a combined tea-specific fertilizer plus a fixed rate of urea with or without an organic fertilizer. The differences in sensory quality of green tea under different fertilization treatments and key quality compounds were revealed to provide a theoretical reference for rational fertilization in tea orchards.
2. Materials and Methods
2.1. Experimental Design
The experimental tea garden is located in Jinjiapu Town (N 30°53′3″, E 115°38′15″), Huanggang City, Hubei Province. The tea variety, Camellia sinensis (L.) cv. “Mingshan 131”, bred from a Mingshan mountain population (Sichuan, China) and suitable for processing green tea, had grown for five years. The experiment was conducted in 2016–2020, and the four fertilizer treatments are shown in Table 1. Nonfertilized plots (CK) served as the control, the grade was 18-8-12 (N-P2O5-K2O) in tea-specific fertilizer, 4.6-2.48-1.4 in the colza cake, and 1.68-2.38-1.3 in the livestock waste compost. The plot area was set as 30 m2, and the plots were randomly grouped and repeated three times. The tea garden was managed uniformly across the whole area, with tillage weeding and deep fertilizer placement. The base fertilizer was applied in early November, and topdressing was done in early March every year in 2016–2020. The tea-specific fertilizer, colza cake, and livestock waste compost were supplied as the base fertilizer, and urea was supplied as topdressing. In April 2020, 1 kg of one bud and two leaves of spring shoots were picked by hand randomly in the three repeated plots of each treatment and processed by the traditional green tea processing method, which includes withering, fixing, rolling, and drying.

Table 1.
The type and amount of fertilizer in different treatments.
2.2. Sensory Evaluation
According to the green tea evaluation method specified in GB/T 23776-2018 “Tea Sensory Evaluation Method”, briefly, 3.0 g of green tea was infused with 150 mL of boiling distilled water, and the tea infusion was poured after brewing for 5.0 min. Five professionals conducted sensory evaluation of the appearance, color, aroma, taste, infused leaves, using the 100-score green tea quality in which 25% was awarded for dry tea color, 10% for infusion color, 25% for aroma, 30% for taste, and 10% for infused leaves.
2.3. Analysis of Main Components
The content of the water extract of tea was detected by using the 120 ℃-drying method (GBT 8305-2013). Tea powder (2 g) was extracted with 300 mL of boiling water at 100 ℃ for 45 min (shaken every 10 min) and filtered by a suction filtration device (BSH-3A, Yiheng Scientific LLC., Shanghai, China). The tea powder was baked at 120 ℃ for 1 h, cooled for 30 min, baked for another hour, and weighed. The filtrate was transferred into a 500 mL volumetric flask, water was added to the volume, and the solution was used for amino acid detection by the ninhydrin colorimetric method (GB/T 8314-2013). One milliliter of test liquid, 0.5 mL of phosphate buffer (pH 8.0) and 0.5 mL of 2% w/v ninhydrin (AR, Luoen, Wuhan, China) solution were added to a 25 mL colorimetric tube, heated in a boiling water bath for 15 min, supplemented with 25 mL of water, and measured colorimetrically at 570 nm.
The content of total tea polyphenols was analyzed by the colorimetric method with the Folin–Ciocalteu reagent (GB/T 8314-2013). The procedures were as follows: tea powder (0.2 g) was extracted twice with 5 mL of 70% v/v methanol (AR, Sinopharm, Beijing, China) at 70 °C for 10 min and centrifuged at 3500 rpm for 10 min; then, the extracts were merged and made up to 10 mL with 70% v/v methanol. One milliliter of the solution was diluted to 100 mL with distilled water and served as the test liquid. For tea polyphenol measurements, 1 mL of the test liquid, 5 mL of 10% v/v Folin–Ciocalteu (BR, Sinopharm, Beijing, China) reagent, and 4 mL of 7.5% w/v Na2CO3 were mixed and allowed to react for 60 min. The absorbance was measured at a wavelength of 765 nm. Separately, 2.0 mL of the sample extract was transferred to a 10 mL volumetric flask, diluted with a stabilizing solution (containing 10 mg/mL EDTA-2Na, 10 mg/mL ascorbic acid, and 10% v/v acetonitrile) to volume, and filtered through a 0.45 μm membrane. Catechins and caffeine were analyzed by a high-performance liquid chromatography (HPLC) system (Waters Alliance e2695, Waters Technologies Inc., WWLP, Milford, MA, USA). HPLC was carried out on a Waters C18 column (4.6 mm × 150 mm) at 40 °C. The samples were eluted with a gradient of solvent A (100 mL of water with 2 mL of acetic acid (AR Sinopharm, Beijing, China)) and B acetonitrile (99.9%, Sigma, St. Louis, MO, USA) as follows: 0–10 min, 100% A; 10–20 min, 68% A, and 32% B; 20–30 min, 100% A. Catechins were detected using an ultraviolet detector at a wavelength of 280 nm.
2.4. Analysis of Tea Volatile Compounds
The tea volatile compounds’ assessment method was performed according to Liu et al. [14]. The SPME fiber was exposed for 10 min in the injection port of the GC instrument at 280 °C to remove any remaining volatile compounds from the fiber before each extraction. A total of 3.0 g of the dry tea sample was added to a 100 mL vial sealed with silicone septa and infused with 30 mL of boiling water; then, 20 μL of an internal standard solution (ethyl caprate) was immediately added. The vial was kept in a water bath at 50 °C for 10 min to equilibrate, and the SPME fiber was exposed to the headspace for 50 min while the sample was maintained at 50 °C. The fiber was then placed in the GC injector port and thermally desorbed at 240 °C for 3 min. An Agilent 7890A GC interfaced with an Agilent 5975C MSD ion Trap MS was used to perform the analysis. The detection limit signal-to-noise ratio was 3. The concentration of the volatile compounds was calculated in µg/L based on the internal standard solution.
2.5. Statistical Analysis
The results were expressed as the mean of three tea samples collected from the repeated plots. Significant differences between means were determined by one-way ANOVA (Duncan’s multiple range tests) using SAS JMP version 9.4. A threshold of p < 0.05 was used to assess statistical significance. A principal component analysis (PCA) was conducted by GraphPad Prism 9.0 software. The figures were drawn by Origin 9.0 (Demo version, Northampton, MA, USA). The heat map was generated by TBtools.
3. Results
3.1. Sensory Quality Analysis
As shown in Table 2. The appearance and quality characteristics of M1 and M2 were “fine, tight, bent, slightly tippy, green bloom”, scoring 88 points. The unfertilized (CK) samples, whose dry tea color was described as “dull green”, scored the lowest at 83 points. The description of brew color was mainly “green, bright”. The comments about aroma were “high aroma” in M1 and M2; the total score of M1 was the highest, and the comment about the taste was “heavy, mellow, and brisk”. By contrast, the unfertilized (CK) samples had the lowest score on taste and were rated as relatively fresh and brisk. The overall scores of the four samples ranged from 83.50 to 88.80. In general, green tea brewed from M1 had the highest comprehensive quality and score, which was above 88 points, followed by M2 and M3.

Table 2.
Sensory evaluation of tea samples under different fertilization treatments.
3.2. Main Flavor Quality Components
The contents of main flavor quality components, including tea water extract, free amino acids, caffeine, tea polyphenols, and total catechins, are presented in Table 3. Tea water extract is the sum of all soluble substances, including polyphenols, soluble proteins, pectin, sugars, alkaloids, and so on [15]. The contents of water extracts in M1 (52.92%) and M3 (53.52%) were significantly higher than those in M2 (49.93%) and CK (49.90). Amino acids, one of the most important chemical components in green tea and one of the main components of a fresh and brisk taste of the brew, are the basis for the formation of flavor [16]. The content of free amino acids was in the order of M1 (3.97%) > M3 (3.83%) > M2 (3.66%) > CK (3.10%), without a significant difference between M3 and the other two fertilization treatments (M1, M2). The content of caffeine differed significantly between fertilization and nonfertilization treatments, but no significant difference was noted among the three fertilization treatments M1, M2, and M3.

Table 3.
Effect of different fertilization treatments on the contents of water extract, free amino acids, polyphenols, caffeine, tea polyphenols, and total catechins in green tea (%).
Tea polyphenols are closely related to the quality of tea. They are the main components of tea content; they are functional components and also the representative substances of the bitter taste of tea [17,18]. Statistical analysis showed that there was no significant difference in the content of tea polyphenols in the fertilization treatments M1 and M3, as well as M1 and M2, but the content of tea polyphenols with the three treatments was significantly higher than that of tea grown without fertilization (CK).
Catechins are the main components of tea polyphenols in tea plants, accounting for about 70% to 80% of the total tea polyphenols. The composition and content of catechins were also influenced remarkably by the fertilization treatments. Together, the content of total catechins was in the order of M3 (15.17%) > M1 (13.89%) > M2 (13.81%) > CK (12.57%), with significant difference between M3 and the other treatments (p < 0.05), but no significant difference between M1 and M2. The fertilization treatments significantly influenced catechin composition. The results (Figure 1) demonstrated that the contents of most catechins showed no significant differences among M1, M2, and M3, including (−)-epigallocatechin gallate (EGCG), (−)-gallocatechin 3-O-gallate (GCG), (−)-epigallocatechin (EGC), (−)-(−)-epicatechin (EC), (−)-gallocatechin, (GC), and (+)-catechin (C). However, EGCG, (−)-epicatechin gallate (ECG), GCG, and EC in fertilization treatments M1, M2, and M3 were all significantly higher than in CK. For ECG, the contents revealed no significant differences in M1 and M2, but it was significantly lower than in M3. Furthermore, the contents of (−)-catechin gallate (CG) in fertilization treatment M1 were significantly lower than in the other three treatments.
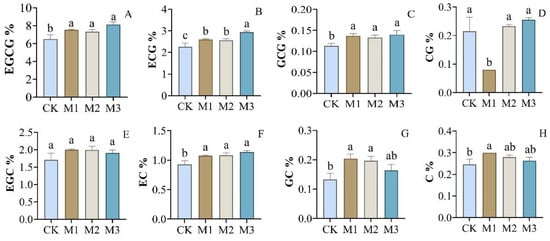
Figure 1.
Effect of different fertilization treatments on the contents of catechins. (A) (−)-Epigallocatechin gallate (EGCG); (B) (−)-epicatechin gallate (ECG); (C) (−)-gallocatechin-3-O-gallate (GCG); (D) (−)-catechin gallate (CG); (E) (−)-epigallocatechin (EGC); (F) (−)-(−)-epicatechin (EC); (G) (−)-gallocatechin, (GC); (H) (+)-catechin (C). All values are the mean ± SD of three tea samples. Values with different roman letters (a–c) above the bars are significantly different according to Duncan’s test (p < 0.05).
3.3. Effect of Fertilization Treatments on the Volatile Compounds of Green Tea
3.3.1. Effect of Fertilization Treatments on Volatile Components
Aroma plays an important role in tea flavor, quality rating, and mass consumption. HS-SPME-GC-MS was used to analyze and identify the aroma components of four tea samples from different fertilization treatments (Table 4). A comparison analysis revealed that all the green teas contained the same 37 constituents. The major volatile substances were D--limonene (7.22~30.97 μg/L), cis-jasmone (2.18~21.10 μg/L), nonanal (3.82–13.04 μg/L), linalool (3.37–11.62 μg/L), cis-3-hexenyl hexanoate (2.26–8.47 μg/L), δ-cadinene (3.67–7.78 μg/L), indole (0.79–6.91 μg/L), cis-3-hexenyl benzoate (1.78–6.70 μg/L), eremophilene (1.40–6.34 μg/L), cis-geraniol (1.89–5.76 μg/L), methyl salicylate (1.47–4.75 μg/L), nerolidol (2.36–4.55 μg/L), and 2,4-di-tert-butylphenol (0.96–4.31 μg/L). Total contents of volatile compounds followed the order of M1 (172.06 μg/L) > M2 (152.90 μg/L) > M3 (79.55 μg/L) > CK (44.00 μg/L). The total aroma of each fertilization treatment was significantly higher than that of the no-fertilization treatment, especially in M1 and M2, which were about 3.9 and 3.5 times that of CK, respectively. Furthermore, compared with CK, some new volatile substances were found in M1, M2, and M3, including β-pinene, dimethyl sulfide, and α-terpineol. By contrast, 1-octen-3-ol, cis-linalool oxide, 2-octenal, trans-β-ocimene, acetic acid, phenylmethyl ester were not found in M1 and M2, whereas 2, 3-octanedione, copaene, 2-octen-1-ol, 2-heptenal, and 2-octenal were not detected in M3.

Table 4.
Effect of different fertilization treatments on the contents of aroma compounds (μg/L).
3.3.2. Effect of Fertilization Treatments on Categories of Volatile Compounds
The volatile compounds were further classified and are presented in Table 5 and Figure 2. Among the 52 aroma components detected in the tested samples, there were 10 types of alcohols, 13 aldehydes, five ketones, nine alkenes, seven esters, and eight others. The contents of all the classes of volatile compounds followed the order of M1 > M2 > M3 > CK (Table 5), but the proportions of different types of aroma accounted for in the contents of total volatile compounds differed (Figure 2). Alkenes accounted for a higher proportion, followed by alcohols and esters.

Table 5.
The total contents of different kinds of aroma in green tea samples under four fertilizer treatments (μg/L).
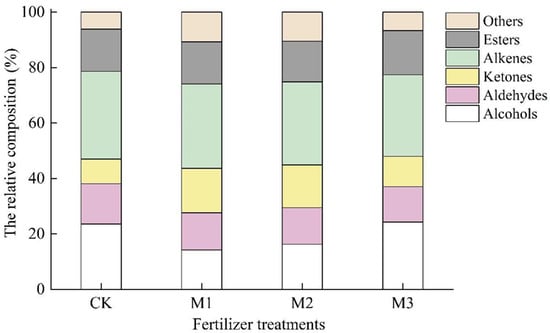
Figure 2.
The relative composition of different classes of volatile compounds accounted for in the total volatile compounds’ contents of tea samples under four fertilizer treatments. All values are the mean ± SD of three tea samples.
Monoterpene alcohols (geraniol, linalool, and their oxides, etc.) in tea are produced by the hydrolysis of glycosides during their processing, and generally contribute to a fresh and pleasant flavor with floral, fruity, and wine aromas. Linalool and geraniol are regarded as important indices for evaluating the aroma quality of tea [19]. Alcohol compounds had the largest proportion (24.24%) in M3 and the smallest (14.19%) in M1.
Aldehydes are closely related to the formation of food aroma and various specific aroma styles. Aliphatic aldehydes mostly have a strong fruit flavor and some bread aroma. Aromatic aldehydes such as phenylacetaldehyde are mainly fragrant and floral [20,21]. The contents of aldehydes followed the sequence of CK > M1 > M2 > M3.
The proportion of ketone aroma components was significantly higher in M1 and M2 (>15%) than in M3 and CK. The content of cis-jasmonone with a floral fragrance was the highest in all tea samples, especially in M1 and M2, the second was geranylacetone with fragrance and then β-ionone with a floral aroma.
The proportion of alkene compounds in the total aroma of tea samples under conventional fertilization was the highest, reaching 30.39%, but there was no significant difference among the other fertilization treatments. The concentration of limonene with lemon aroma was the highest, followed by δ-juniper, phorocolene, α-bocacolene, α-cubedine, and so on.
Most ester compounds have a slight fruity aroma, but the cis-hexanoate-3-hexene ester commonly found in green tea has a strong pear aroma, which has a significant contribution to the formation of green tea aroma [22]. The proportion of ester compounds in M3 was highest among the four treatments.
The other compounds, such as indole, dimethyl sulfide, caffeine, and so on, accounted for the lowest content and the smallest proportion of all volatile compounds detected.
3.4. PCA of Key Quality Components in Tea Samples under Different Fertilization Treatments
Principal component analysis (PCA) is a nontargeted statistical method that can express an entire dataset in a global and qualitative visual pattern, thus highlighting similarities and differences between and within the samples. Principal component analysis of tea key quality components was conducted (Figure 3). The results showed that the variance contribution of the first principal component (PC1) was 73.81%, and that of the second principal component (PC2) was 16.04%; the first two principal components together explained 89.85% of the variance. The four tea samples were obviously divided into three groups (Figure 3A). The treatments M1 and M2 formed one group with lower PC1 scores which were both lower than zero. M3 was grouped separately, with PC1 and PC2 scores higher than those of M1, M2, and CK. According to the loading plot (Figure 3B), the positive portion of the PC1 axis was related mainly to the aroma compounds (1–40). The positive portion of the PC2 axis was related to the water extract (53), tea polyphenols (54), EGCG (60), GCG (62), ECG (63), and CG (64). Fifteen quality parameters with a large contribution rate (the absolute value of the feature vector greater than 0.98) were extracted from the first principal component. In addition, three quality components with large contribution rates (the eigenvector absolute value greater than 0.90) were extracted from the second principal component.
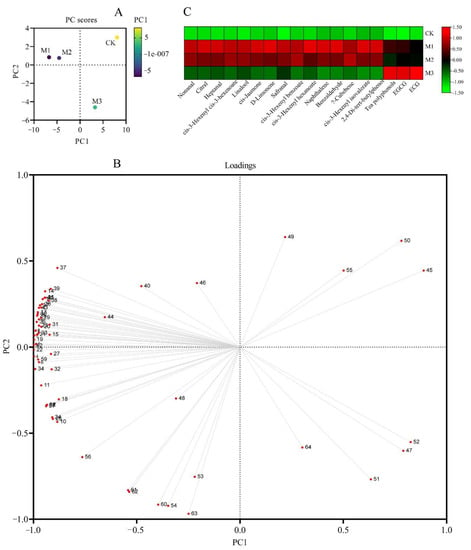
Figure 3.
The PCA score plot of components 1 and 2 (A), loading plot of PC1 versus PC2 (B), and heat map of key quality components (C) of green tea samples under different fertilization treatments. In (B), red dots 1–52 represent aroma substances, consistent with Table 4, and red dots 53–64 represent in an ascending sequence the water extract, tea polyphenols, free amino acids, caffeine, GC, EGC, C, EGCG, EC, GCG, ECG, and CG.
In order to visually display the distribution of 18 key quality components among the four fertilization treatments, a heat map was created (Figure 3C). The common key quality components in M1 and M2 were D-limonene, cis-jasmone, nonanal, linalool, cis-3-hexenyl hexanoate, cis-3-hexenyl benzoate, 2,4-di-tert-butylphenol, cis-3-hexenyl cis-3-hexenoate, α-cubebene, heptanal, cis-3-hexenyl isovalerate, safranal, naphthalene, benzaldehyde, and citral; they were all aroma components and higher in M1 and M2 than in the other two treatments. On the contrary, tea polyphenols, EGCG, and ECG were the highest in M3. The last group was CK that had the lowest all 18 key quality components of all treatments.
4. Discussion
Soil acidity has become a serious agricultural problem in China for the past few years [23,24]. The major reasons of soil acidification are increased application of acidifying nitrogen fertilizers or incomplete cycling of nitrogen compounds in the soil. Under the influence of excessive amounts of nitrogen fertilizer, the nutrient balance in soil is disturbed, accompanied by a significant yield reduction [25,26]. Therefore, fertilization must be optimized. Judicious fertilization is conducive to the maintenance of soil fertility and biological activity, yield stability, and quality improvement of tea, and is of great importance to the sustainable development of tea industry. This has highlighted the potential benefits of organic fertilizer application. Organic fertilizers come from natural sources, such as livestock and poultry waste, plant residues, and subsidiary agricultural products [27,28]. Studies have documented that organic fertilizers significantly improve the soil fungi-to-bacteria ratio as well as soil enzyme activities, thus contributing to higher seedling biomass [29,30]. In addition, compared to tea cultivated with chemical fertilizers, tea treated with organic fertilizers has superior color and taste [30,31]. However, organic fertilizers have a lower nutrient content than chemical fertilizers do. In order to meet the demand for various nutrient elements in the process of crop growth and development, in our study, we used a specific fertilizer which was formulated to be suitable for tea. Xie [32] reported that combinations of 20% or 50% organic fertilizer replacement to chemical fertilizer are appropriate for both mitigating nutrient loss and balancing tea yield and quality, especially the combination of 50% organic fertilizer replacement, which produced the best results. Our study also demonstrated that the combination of a specific fertilizer with an organic fertilizer had the best effect on improving the overall quality of green tea.
Recently, the effects of fertilization practices on soil biological properties have attracted attention, but most studies primarily focus on the composition of rhizosphere bacterial community and the content of heavy metals, or variation in soil nutrient contents [5,10,33]. Moreover, the effects of fertilizers on sensory quality have not been investigated fully and carefully. Sun [11] determined the metabolite profiles of tea shoots applied with cow manure, urea, or no fertilizer, and obtained a total of 74 metabolites, including amino acids, organic acids, fatty acids, and sugars. Liu [34] unraveled the effect of nitrogen fertilization on lipid metabolism. However, these studies were both focused on the nonvolatile variations caused by different fertilizer usage. Sensory quality attributes contain aroma, taste, infusion color, and dry tea and infused leaf appearance, among which tea infusion taste and aroma are determining factors. In the present study, we used a special fertilizer for tea tree with a grade of 18-8-12 (N-P2O5:K2O). We designed three different types of tea fertilizer treatments. In M1 and M2, it was tea-specific fertilizer + organic fertilizer + urea. In M3, we increased the amount of tea-specific fertilizer and combined it with urea but without organic fertilizer. We found that total catechins were higher in M3 than in M1 and M2. There was no statistical difference between M3 and M1, M2 in terms of the water extract, caffeine, or tea polyphenols. However, the concentrations of aroma compounds were much lower in M3 than in M1 and M2. The combination of tea-specific fertilizer and organic fertilizer greatly increased the level of total aroma and important aroma components such as D-limonene, cis-jasmone, nonanal, and linalool in green tea. For example, limonene has an aroma of fresh orange and lemon, which may contribute to the fruity flavor of green tea; cis-jasmone has a natural jasmine fragrance; nonanal has the fragrance of green grass and fat; linalool shows floral, woody, and lavender aroma characteristics; cis-3-hexenyl hexanoate represents a fruity scent; cis-3-hexenyl benzoate has a pleasant jasmine scent [13,35,36,37,38,39]. These volatile compounds contributed greatly to the aroma and flavor in green tea. Furthermore, the aroma components with relatively low content (but still playing a key role in the formation of green tea aroma) also showed differences among the green teas from different fertilization treatments [38]. Β-cyclocitral has a cool, fruity, and fragrant smell [40], and the highest content of 0.90 µg/L was in M2. Excessively fertilized green tea had high levels of high-boiling-point volatile compounds, such as β-ionone and indole, resulting in a strong heavy aroma lacking briskness [41]. As one of carotenoid-derived fragrance compounds, β-ionone significantly contributes to the aroma and quality of tea with its low odor threshold (0.007 µg/L) [42,43,44]. β-ionone is known for a violet aroma and as a complex woody and fruity scent [45]. The content of β-ionone was higher in M2 and M1 than in CK. The indole content of M2 (7.06 μg/L) was 8.9 times higher than that in CK (0.79 µg/L) and 5.5 higher than that in M3 (1.29 µg/L). In summary, the replenishment of organic fertilizers for a specific fertilizer can not only provide a variety of nutrients but also supply the soil organic carbon pool, which may improve the soil’s physical and chemical properties and stimulate soil microbial communities, thus improving the quality and fertility of the soil. The increased diversity of soil microbial communities is thought to have a “priming effect” during the decomposition of soil organic matter, thus positively affecting the aroma of tea plants [46,47]. This kind of fertilization structure was recommended in tea plantations.
5. Conclusions
In this study, the results showed that the application of a tea-specific fertilizer enhanced the taste qualities such as water extract, caffeine, tea polyphenol, EGCG, and ECG. When the tea-specific fertilizer was combined with an organic fertilizer, the concentrations of important green tea aroma compounds, such as D-limonene, cis-jasmone, nonanal, linalool, cis-3-hexenyl hexanoate, and cis-3-hexenyl benzoate, were significantly improved. The results showed that the application of a tea-specific fertilizer combined with an organic fertilizer in soils in tea plantations was favorable in improving the quality of green teas.
Author Contributions
Conceptualization, D.H. and Y.M.; sample collection, preparation, and analysis, J.W., X.C. and H.W.; data curation, D.H. and L.J.; writing—original draft preparation, D.H.; review and editing, Y.W. and R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Key Research and Development Plan (2021YFD1601100), the China Agriculture Research System of MOF and MARA (CARS-019), and the Innovation Center Fund for Agricultural Science and Technology in Hubei Province of China (2019-620-000-001-24).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lin, W.; Lin, M.; Zhou, H.; Wu, H.; Li, Z.; Lin, W. The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PLoS ONE 2019, 14, e0217018. [Google Scholar] [CrossRef]
- de Godoy, R.C.B.; Deliza, R.; Gheno, L.B.; Licodiedoff, S.; Frizon, C.N.T.; Ribani, R.H.; dos Santos, G.G. Consumer perceptions, attitudes and acceptance of new and traditional mate tea products. Food Res. Int. 2013, 53, 801–807. [Google Scholar] [CrossRef]
- Wang, B.; Qu, F.; Wang, P.; Zhao, L.; Wang, Z.; Han, Y.; Zhang, X. Characterization analysis of flavor compounds in green teas at different drying temperature. LWT 2022, 161, 113394. [Google Scholar] [CrossRef]
- Ho, C.-T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Ji, L.F.; Wu, Z.D.; You, Z.M.; Yi, X.Y.; Ni, K.; Guo, S.W.; Ruan, J.Y. Effects of organic substitution for synthetic N fertilizer on soil bacterial diversity and community composition: A 10-year field trial in a tea plantation. Agric. Ecosyst. Environ. 2018, 268, 124–132. [Google Scholar] [CrossRef]
- Gu, S.S.; Hu, Q.L.; Cheng, Y.Q.; Bai, L.Y.; Liu, Z.H.; Xiao, W.J.; Gong, Z.H.; Wu, Y.N.; Feng, K.; Deng, Y.; et al. Application of organic fertilizer improves microbial community diversity and alters microbial network structure in tea (Camellia sinensis) plantation soils. Soil Till. Res. 2019, 195, 104356. [Google Scholar] [CrossRef]
- Lal, R. Soil degradation as a reason for inadequate human nutrition. Food Secur. 2009, 1, 45–57. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Z.; Li, Z.W.; Jiang, Y.H.; Weng, B.Q.; Lin, W.X. Variations of rhizosphere bacterial communities in tea (Camellia sinensis L.) continuous cropping soil by high-throughput pyrosequencing approach. J. Appl. Microbiol. 2016, 121, 787–799. [Google Scholar] [CrossRef]
- Cavagnaro, T.R. Impacts of compost application on the formation and functioning of arbuscular mycorrhizas. Soil Boil. Biochem. 2014, 78, 38–44. [Google Scholar] [CrossRef]
- Wang, J.C.; Song, Y.; Ma, T.F.; Raza, W.; Li, J.; Howland, J.G.; Huang, Q.W.; Shen, Q.R. Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil. Appl. Soil Ecol. 2017, 112, 42–50. [Google Scholar] [CrossRef]
- Sun, L.; Fan, K.; Wang, L.; Ma, D.; Wang, Y.; Kong, X.; Li, H.; Ren, Y.; Ding, Z. Correlation among Metabolic Changes in Tea Plant Camellia sinensis (L.) Shoots, Green Tea Quality and the Application of Cow Manure to Tea Plantation Soils. Molecules 2021, 26, 6180. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.Y.; Ma, L.F.; Yi, X.Y.; Shi, Y.Z.; Ni, K.; Liu, M.Y.; Zhang, Q.F. Integrated nutrient management in tea plantation to reduce chemical fertilizer and increase nutrient use efficiency. J. Tea Sci. 2020, 40, 85–95. [Google Scholar] [CrossRef]
- Yi, X.Y.; Ma, L.F.; Shi, Y.Z.; Ruan, J.Y. Study on the effect of special tea fertilizer on reducing weight and increasing income. Chin. Tea 2017, 39, 2. [Google Scholar]
- Liu, P.P.; Zheng, P.C.; Gong, Z.M.; Feng, L.; Gao, S.W.; Wang, X.P.; Teng, J.; Zheng, L.; Liu, Z.H. Comparing characteristic aroma components of bead-shaped green teas from different regions using headspace solid-phase microextraction and gas chromatography–mass spectrometry/olfactometry combined with chemometrics. Eur. Food Res. Technol. 2020, 246, 1703–1714. [Google Scholar] [CrossRef]
- Fei, T.Y.; Fei, J.; Huang, F.; Xie, T.P.; Xu, J.F.; Zhou, Y.; Yang, P. The anti-aging and anti-oxidation effects of tea water extract in Caenorhabditis elegans. Exp. Gerontol. 2017, 97, 89–96. [Google Scholar] [CrossRef]
- Horanni, R.; Engelhardt, U.H. Determination of amino acids in white, green, black, oolong, pu-erh teas and tea products. J. Food Compos. Anal. 2013, 31, 94–100. [Google Scholar] [CrossRef]
- Kanwar, J. Recent advances on tea polyphenols. Front. Biosci. 2012, 4, 111. [Google Scholar] [CrossRef]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef]
- Xu, X.Q.; Mo, H.Z.; Yan, M.C.; Zhu, Y. Analysis of characteristic aroma of fungal fermented Fuzhuan brick-tea by gas chromatography/mass spectrophotometry. J. Sci. Food. Agric. 2007, 87, 1502–1504. [Google Scholar] [CrossRef]
- Baba, R.; Amano, Y.; Wada, Y.; Kumazawa, K. Characterization of the Potent Odorants Contributing to the Characteristic Aroma of Matcha by Gas Chromatography–Olfactometry Techniques. J. Agric. Food Chem. 2017, 65, 2984–2989. [Google Scholar] [CrossRef]
- Long, L.; Song, S.; Cao, X. Discriminant analysis and similarity evaluation of gas chromatography mass spectrometry fingerprints of aroma components in green tea grading. Chin. J. Chroma. 2019, 37, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.W.D.; Zhang, L.; Zhao, J.H. Aromatic components analysis of green tea in LaoMountain by HS-SPME and GC-MS. Food Mach. 2012, 25, 96–101. [Google Scholar]
- Yan, P.; Shen, C.; Fan, L.C.; Li, X.; Zhang, L.P.; Zhang, L.; Han, W.Y. Tea planting affects soil acidification and nitrogen and phosphorus distribution in soil. Agric. Ecosyst. Environ. 2018, 254, 20–25. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Hedley, M.J.; White, R.E. Processes of soil acidification during nitrogen cycling with emphasis on legume based pastures. In Plant-Soil Interactions at Low pH, Proceedings of the Second International Symposium on Plant-Soil Interactions at Low pH, Beckley West Virginia, WV, USA, 24–29 June 1990; Wright, R.J., Baligar, V.C., Murrmann, R.P., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 169–179. [Google Scholar]
- Zhang, Q.C.; Shamsi, I.H.; Xu, D.T.; Wang, G.H.; Lin, X.Y.; Jilani, G.; Hussain, N.; Chaudhry, A.N. Chemical fertilizer and organic manure inputs in soil exhibit a vice versa pattern of microbial community structure. Appl. Soil Ecol. 2012, 57, 1–8. [Google Scholar] [CrossRef]
- Chang, K.H.; Wu, R.Y.; Chuang, K.C.; Hsieh, T.F.; Chung, R.S. Effects of chemical and organic fertilizers on the growth, flower quality and nutrient uptake of Anthurium andreanum, cultivated for cut flower production. Sci. Hortic. 2010, 125, 434–441. [Google Scholar] [CrossRef]
- Sun, Q.; Yan, X.; Li, X.; Wang, G.; Xiang, S.; Chen, X.; Yin, C.; Mao, Z. Effects of a Mixture of Bacterial Manure and Biochar on Soil Environment and Physiological Characteristics of Malus hupehensis Seedlings. J. Chin. Agric. Sci. Bull. 2017, 33, 52–59. [Google Scholar]
- Xu, H.Q.; Xiao, R.L.; Xiang, Z.X.; Huang, Y.; Luo, W.; Qin, Z. Effects of Different Ecological Management on the Soil Microbial Biomass and Microbial Population of Tea Plantation in Hilly Red Soil Biomass and Microbial Population of Tea Plantation in Hilly Red Soil Region. J. Soil Sci. 2010, 2010, 1355–1359. [Google Scholar]
- Lin, B.; Luo, G.H.; Xu, Q.X.; Wang, Q.S.; Guan, X.F. Effects of biogas residue on yield and quality of tea. Fujian J. Agric. Sci. 2010, 25, 90–95. [Google Scholar]
- Zhang, Q.; Wei, C.X. Effects of Different Organic Fertilizers on Chief Qualities of Tea. Guizhou Agric. Sci. 2012, 40, 65–67. [Google Scholar]
- Xie, S.; Feng, H.; Yang, F.; Zhao, Z.; Hu, X.; Wei, C.; Liang, T.; Li, H.; Geng, Y. Does dual reduction in chemical fertilizer and pesticides improve nutrient loss and tea yield and quality? A pilot study in a green tea garden in Shaoxing, Zhejiang Province, China. Environ. Sci. Pollut. Res. Int. 2019, 26, 2464–2476. [Google Scholar] [CrossRef]
- Ji, L.; Ni, K.; Ma, L.; Chen, Z.; Zhao, Y.; Ruan, J.; Guo, S. Effect of different fertilizer regimes on the fungal community of acidic tea-garden soil. Acta Ecol. Sin. 2018, 38, 8158–8166. [Google Scholar]
- Liu, M.Y.; Burgos, A.; Ma, L.; Zhang, Q.; Tang, D.; Ruan, J. Lipidomics analysis unravels the effect of nitrogen fertilization on lipid metabolism in tea plant (Camellia sinensis L.). BMC Plant Biol. 2017, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chambers, D.H.; Chambers, E.; Adhikari, K.; Yoon, Y. Volatile aroma compounds in various brewed green teas. Molecules 2013, 18, 10024–10041. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.L.; Zhai, X.T.; Guo, D.Y.; Du, W.K.; Gao, T.; Zhou, J.; Schwab, W.G.; Song, C.K. Characterization of Key Odorants in Xinyang Maojian Green Tea and Their Changes During the Manufacturing Process. J. Agric. Food Chem. 2022, 70, 279–288. [Google Scholar] [CrossRef]
- Baba, R.; Kumazawa, K. Characterization of the Potent Odorants Contributing to the Characteristic Aroma of Chinese Green Tea Infusions by Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2014, 62, 8308–8313. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Huynh-Ba, T.; Blank, I.; Robert, F. Temporal Changes in Aroma Release of Longjing Tea Infusion: Interaction of Volatile and Nonvolatile Tea Components and Formation of 2-Butyl-2-octenal upon Aging. J. Agric. Food Chem. 2008, 56, 2160–2169. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, Y.; Liu, B.; Chen, Z.; Zheng, J.; Guan, M.; Shi, H.; Wang, Y.; Yang, W. Changes in the volatiles, chemical components, and antioxidant activities of Chinese jasmine tea during the scenting processes. Int. J. Food Prop. 2016, 20, 681–693. [Google Scholar] [CrossRef]
- He, C.J.; Guo, X.M.; Yang, Y.M.; Xie, Y.F.; Ju, F.Y.; Guo, W.B. Characterization of the aromatic profile in “zijuan” and “pu-erh” green teas by headspace solid-phase microextraction coupled with GC-O and GC-MS. Anal. Methods 2016, 8, 4727–4735. [Google Scholar] [CrossRef]
- Takei, Y.; Ishikawa, Y.; Hirao, N.; Fuchinoue, H.; Yamanishi, T. The Influence of the Amount of Supplied Fertilizer and Vinyl-House Cultivation on the Tea Aroma. Agric. Chem. 1978, 52, 505–512. [Google Scholar]
- Zheng, X.Q.; Li, Q.S.; Xiang, L.P.; Liang, Y.R. Recent Advances in Volatiles of Teas. Molecules 2016, 21, 338. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Zhang, N.; Zhao, M.Y.; Jing, T.T.; Jin, J.Y.; Wu, B.; Wan, X.C.; Schwab, W.G.; Song, C.K. Carotenoid Cleavage Dioxygenase 4 Catalyzes the Formation of Carotenoid-Derived Volatile β-Ionone during Tea (Camellia sinensis) Withering. J. Agric. Food Chem. 2020, 68, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Kanani, D.M.; Nikhade, B.P.; Balakrishnan, P.; Singh, G.; Pangarkar, V.G. Recovery of Valuable Tea Aroma Components by Pervaporation. Ind. Eng. Chem. Res. 2003, 42, 6924–6932. [Google Scholar] [CrossRef]
- Gulati, A.; Ravindranath, S.D. Seasonal Variations in Quality of Kangra Tea (Camellia sinensis (L.) O Kuntze) in Himachal Pradesh. J. Sci. Food Agric. 1996, 71, 231–236. [Google Scholar] [CrossRef]
- Yuan, Q.; Hernández, M.; Dumont, M.G.; Rui, J.; Fernández Scavino, A.; Conrad, R. Soil bacterial community mediates the effect of plant material on methanogenic decomposition of soil organic matter. Soil Biol. Biochem. 2018, 116, 99–109. [Google Scholar] [CrossRef]
- Sun, L. Exploration of Factors to Enhance Aromatic Quality in Green Tea; Murdoch University: Perth, Australia, 2022. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).