Yield and Fruit Quality Response of Pomegranate (Punica granatum) to Foliar Spray of Potassium, Calcium and Kaolin
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Set, Drop and Fruit Yield
2.2. Fruit Quality
2.2.1. Physical Fruit Characteristics
2.2.2. Chemical Fruit Characteristics
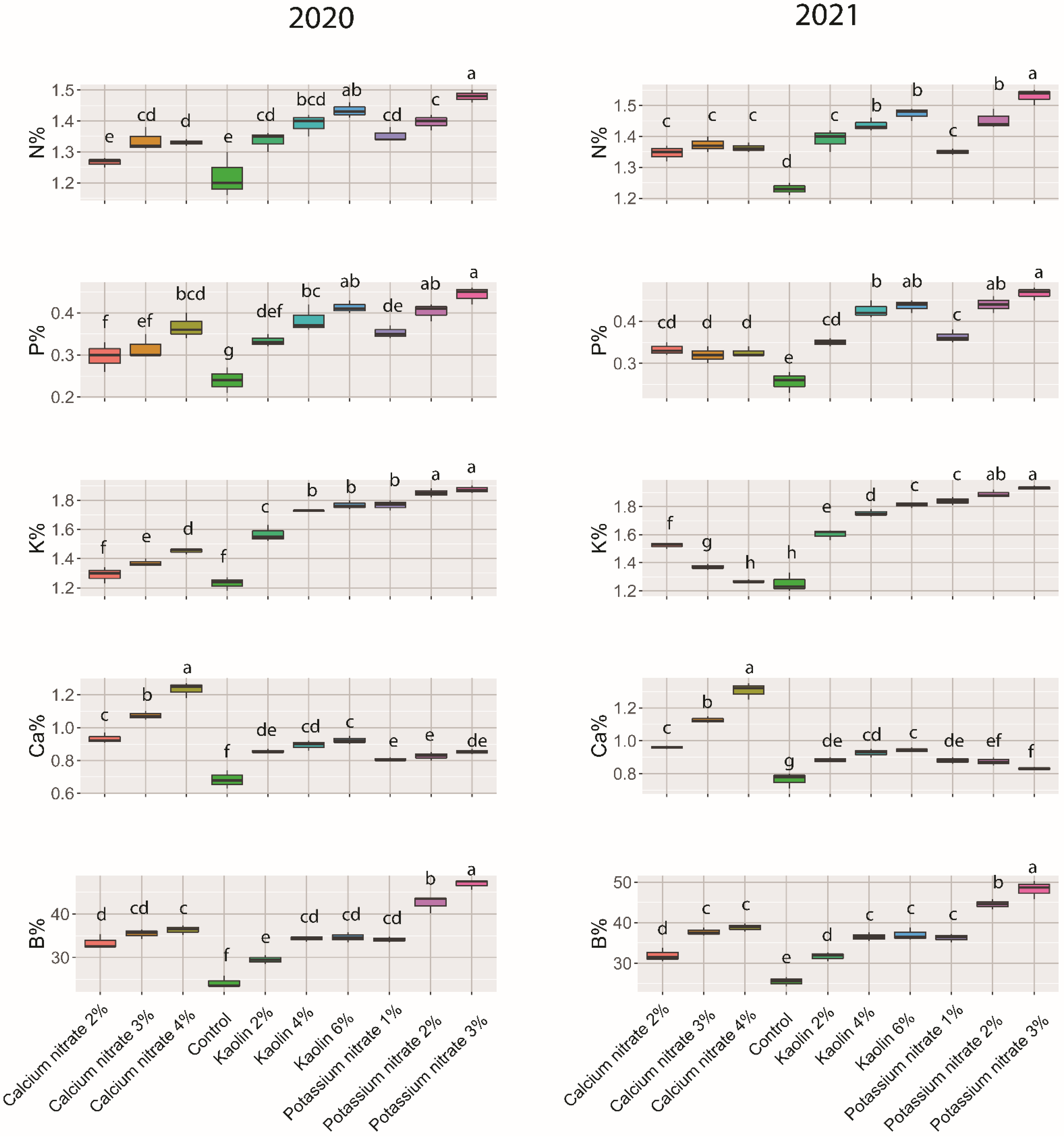
2.3. Leaf Nutritional Status
2.4. Statistical Analysis
3. Results
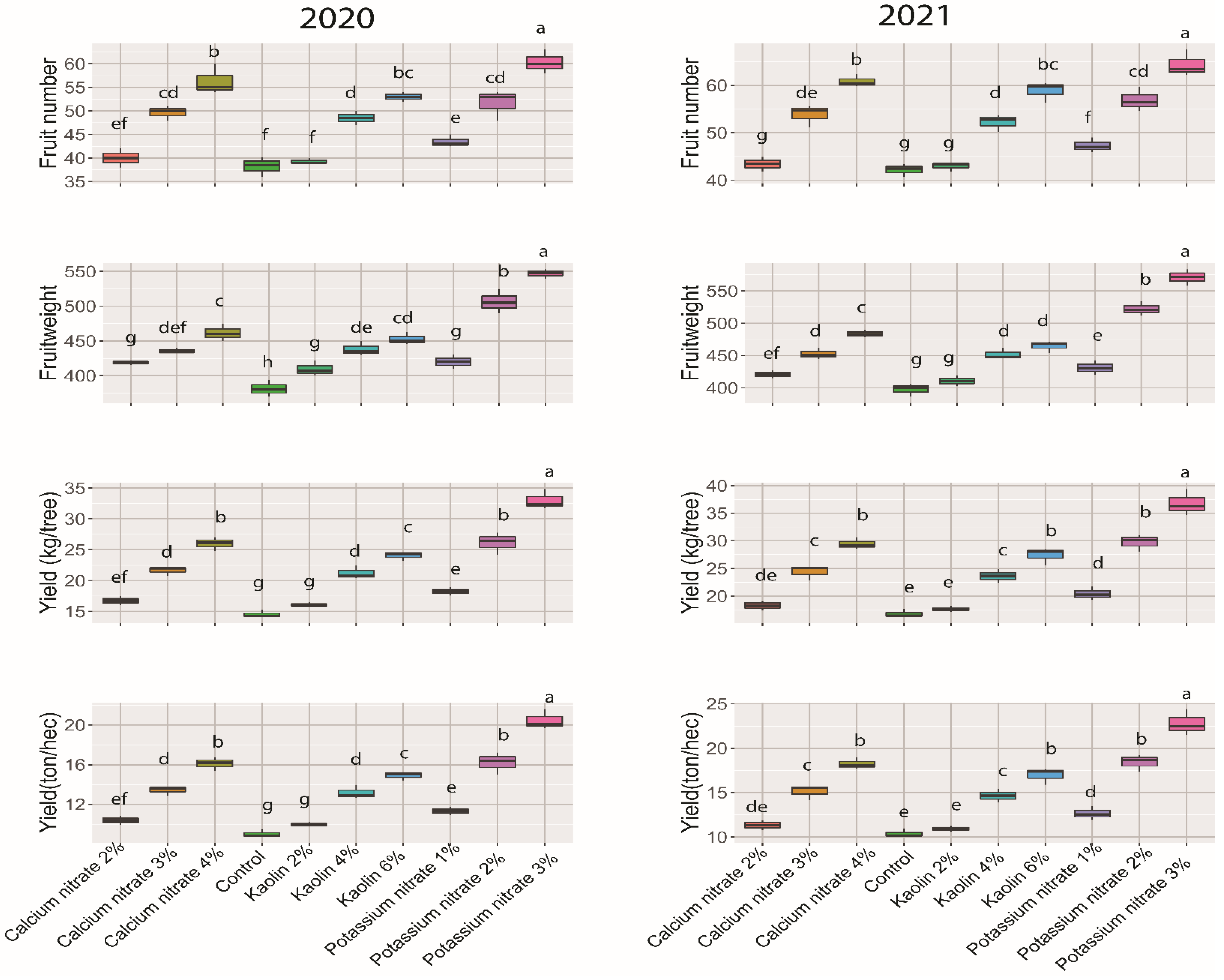
3.1. Fruit Set, Drop and Fruit Yield
3.2. Fruit Quality
3.3. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parvizi, H.; Sepaskhah, A.R. Effect of drip irrigation and fertilizer regimes on fruit quality of a pomegranate (Punica granatum (L.) cv. Rabab) orchard. Agric. Water Manag. 2015, 156, 70–78. [Google Scholar] [CrossRef]
- Al-Dosary, N.M.N.; Abdel-Sattar, M.; Aboukarima, A.M. Effect of Ammonium Sulphate Incorporated with Calcium Nitrate Fertilizers on Nutritional Status, Fruit Set and Yield of Pomegranate Trees cv. Wonderful. Agronomy 2022, 12, 971. [Google Scholar] [CrossRef]
- Hmid, I.; Hanine, H.; Elothmani, D.; Oukabli, A. The physico-chemical characteristics of Morrocan pomegranate and evaluation of the antioxidant activity for their juices. J. Saudi Soc. Agric. Sci. 2018, 17, 302–309. [Google Scholar] [CrossRef]
- Palou, L.; Crisosto, C.H.; Garner, D. Combination of postharvest antifungal chemical treatments and controlled atmosphere storage to control gray mold and improve storability of ‘Wonderful’pomegranates. Postharvest Biol. Technol. 2007, 43, 133–142. [Google Scholar] [CrossRef]
- Melgarejo, P.; Martınez, J.; Hernández, F.; Martınez-Font, R.; Barrows, P.; Erez, A. Kaolin treatment to reduce pomegranate sunburn. Sci. Hortic. 2004, 100, 349–353. [Google Scholar] [CrossRef]
- Yazici, K.; Kaynak, L. Effects of kaolin and shading treatments on sunburn on fruit of Hicaznar cultivar of pomegranate (Punica granatum L. cv. Hicaznar). In Proceedings of the I International Symposium on Pomegranate and Minor Mediterranean Fruits, Adana, Turkey, 16–19 October 2006; pp. 167–174. [Google Scholar]
- Glenn, D.M.; Puterka, G.J.; Drake, S.R.; Unruh, T.R.; Knight, A.L.; Baherle, P.; Prado, E.; Baugher, T.A. Particle film application influences apple leaf physiology, fruit yield, and fruit quality. J. Am. Soc. Hortic. Sci. 2001, 126, 175–181. [Google Scholar] [CrossRef]
- Pareek, S.; Valero, D.; Serrano, M. Postharvest biology and technology of pomegranate. J. Sci. Food Agric. 2015, 95, 2360–2379. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Kumar, R.; Bakshi, P.; Srivastava, J. Fruit Cracking: A Challenging Problem of Fruit Industry; Krishi Sandesh: San Vittore Olona, Italy, 2010. [Google Scholar]
- Abd El-Rhman, I. Physiological studies on cracking phenomena of pomegranates. J. Appl. Sci. Res. 2010, 6, 696–703. [Google Scholar]
- Samra, B.; Shalan, A. Studies on thinning, bagging and aluminum silicate spraying on yield and quality of Wanderfull pomegranate. J. Plant Prod. Sci. 2013, 4, 219–227. [Google Scholar] [CrossRef]
- Bonomelli, C.; Ruiz, R. Effects of foliar and soil calcium application on yield and quality of table grape cv.‘Thompson Seedless’. J. Plant Nutr. 2010, 33, 299–314. [Google Scholar] [CrossRef]
- Sharma, N.; Belsare, C. Effect of plant bio-regulators and nutrients on fruit cracking and quality in pomegranate (Punica granatum L.) ‘G-137′ in Himachal Pradesh. Acta Hortic. 2011, 890, 347–352. [Google Scholar] [CrossRef]
- Khalil, H.A.; Aly, H.S. Cracking and fruit quality of pomegranate (Punica granatum L.) as affected by pre-harvest sprays of some growth regulators and mineral nutrients. J. Hortic. Sci. Ornam. Plants 2013, 5, 71–76. [Google Scholar]
- Korkmaz, N.; Aşkın, M.A.; Ercişli, S.; Okatan, V. Foliar application of calcium nitrate, boric acid and gibberellic acid affects yield and quality of pomegranate (Punica granatum L.). Acta Sci. Pol. Hortorum Cultus 2016, 15, 105–112. [Google Scholar]
- Sutanu, M.; Aniruddha, Y.; Meena, K. Effect of calcium and boron on growth, yield and quality of pomegranate (Punica granatum L.). Int. J. Plant Sci. 2017, 12, 108–113. [Google Scholar]
- Bakeer, S. Effect of ammonium nitrate fertilizer and calcium chloride foliar spray on fruit cracking and sunburn of Manfalouty pomegranate trees. Sci. Hortic. 2016, 209, 300–308. [Google Scholar] [CrossRef]
- Merwad, M.; Eisa, R.; Merwad, A. Effect of GA3 and some nutrients on pomegranate under South Sinai Governorate conditions. Int. J. Chemtech Res. 2016, 9, 104–113. [Google Scholar]
- Ehteshami, S.; Sarikhani, H.; Ershadi, A. Effect of kaolin and gibberellic acid application on some qualitative characteristics and reducing the sunburn in pomegranate fruits (Punica granatum) CV.‘Rabab Neiriz’. Plant Prod. Technol. 2011, 11, 15–23. [Google Scholar]
- Mengel, K. Potassium. In Handbook of Plant Nutrition, 1st ed.; Barker, A.V., Pilbeam, D.J., Eds.; CRC Taylor and Francis: New York, NY, USA, 2007; pp. 91–120. [Google Scholar]
- Fageria, N.; Dos Santos, A.; De Moraes, M. Yield, potassium uptake, and use efficiency in upland rice genotypes. Commun. Soil Sci. Plant Anal. 2010, 41, 2676–2684. [Google Scholar] [CrossRef]
- Khayyat, M.; Tehranifar, A.; Zaree, M.; Karimian, Z.; Aminifard, M.; Vazifeshenas, M.; Amini, S.; Noori, Y.; Shakeri, M. Effects of potassium nitrate spraying on fruit characteristics of ‘Malas Yazdi’pomegranate. J. Plant Nutr. 2012, 35, 1387–1393. [Google Scholar] [CrossRef]
- Dhillon, W.; Gill, P.; Singh, N. Effect of nitrogen, phosphorus and potassium fertilization on growth, yield and quality of pomegranate‘Kandhari’. Acta Hortic. 2011, 890, 327–332. [Google Scholar] [CrossRef]
- Davarpanah, S.; Aakari, M.; Babalar, M.; Zarei, M.; Aghayeh, R. Effect of foliar application of phosphorus, potassium and iron on physical and chemical properties of pomegranate fruit. Jordan J. Biol. Sci. 2017, 13, 693–705. [Google Scholar]
- Schupp, J.R.; Fallahi, E.; Chun, I.-J. Effect of particle film on fruit sunburn, maturity and quality of ‘Fuji’ and ‘Honeycrisp’ apples. HortTechnology 2002, 12, 87–90. [Google Scholar] [CrossRef]
- Magness, J.R.; Taylor, G.F. An Improved Type of Pressure Tester for the Determination of Fruit Maturity; United States Department of Agriculture: Washington, DC, USA, 1925; p. 1982.
- AOAC, C.A. Ofcial methods of analysis of the Association of Analytical Chemists International. In Ofcial Methods Gaithersburg; Official Methods: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Nangle, E.J.; Gardner, D.S.; Metzger, J.D.; Rodriguez-Saona, L.; Guisti, M.M.; Danneberger, T.K.; Petrella, D.P. Pigment Changes in Cool-Season Turfgrasses in Response to Ultraviolet-B Light Irradiance. Agron. J. 2015, 107, 41–50. [Google Scholar] [CrossRef]
- Nielsen, S.S. Vitamin C determination by indophenol method. In Food Analysis Laboratory Manual; Springer: Berlin/Heidelberg, Germany, 2017; pp. 143–146. [Google Scholar]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histoenzymology; A Text Manual; Kalyani Publishers: New Delhi, India, 1980. [Google Scholar]
- Nielsen, S.S. Introduction to food analysis. In Food Analysis; Springer: Cham, Switzerland, 2017; pp. 3–16. [Google Scholar]
- Pal, S.K. Methods of Soil and Plant Analysis; New India Publishing Agency: New Delhi, India, 2019. [Google Scholar]
- Wilde, S.A.; Corey, R.B.; Lyer, I.G.; Voigt, G.K. Soil and Plant Analysis for Tree Culture, 3rd ed.; Oxford Publishing Co.: New Delhi, India, 1985; pp. 1–218. [Google Scholar]
- Wang, Y.; Yang, R.; Zheng, J.; Shen, Z.; Xu, X.J.E.; Safety, E. Exogenous foliar application of fulvic acid alleviate cadmium toxicity in lettuce (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 2019, 167, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bowden, M.; Diamond, D. The determination of phosphorus in a microfluidic manifold demonstrating long-term reagent lifetime and chemical stability utilising a colorimetric method. Sens. Actuators B Chem. 2003, 90, 170–174. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of soil, plant, and water analysis: A manual for the West Asia and North Africa region. Int. Cent. Agric. Res. Dry Areas 2013, 244, 134299328. [Google Scholar]
- Carter, M.R.E. Soil Sampling and Methods of Analysis; Canadian Society of Soil Science; Lewis Publishers: London, UK; Tokyo, Japan, 1993. [Google Scholar]
- Ali, M.M.; Rizwan, H.M.; Yousef, A.F.; Zhi, C.; Chen, F. Analysis of toxic elements in leaves and fruits of loquat by inductively coupled plasma-mass spectrometry (ICP-MS). Acta Sci. Pol. Hortorum Cultus 2021, 20, 33–42. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 6th ed.; Iowa State University Press: Ames, IA, USA, 1990; p. 507. [Google Scholar]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Elmer, P.; Spiers, T.; Wood, P. Effects of pre-harvest foliar calcium sprays on fruit calcium levels and brown rot of peaches. Crop Prot. 2007, 26, 11–18. [Google Scholar] [CrossRef]
- Chen, F.; Liu, H.; Yang, H.; Lai, S.; Cheng, X.; Xin, Y.; Yang, B.; Hou, H.; Yao, Y.; Zhang, S. Quality attributes and cell wall properties of strawberries (Fragaria annanassa Duch.) under calcium chloride treatment. Food Chem. 2011, 126, 450–459. [Google Scholar] [CrossRef]
- Nautiyal, P.; Papnai, G.; Arya, M.; Tiwari, R. Effect of different chemicals on fruit cracking of pomegranate in hilly region of Uttarakhand. J. Hill Agric. 2018, 9, 270–273. [Google Scholar] [CrossRef]
- Parthiban, S.; Indirani, R.; Subbiah, A.; Saraswathy, S.; Nireshkumar, N. Effect of Calcium, Boron and Micronutrient Formulations on Berry Cracking in Grapes var. Muscat Hamburg. Madras Agric. J. 2022, 108, 1. [Google Scholar]
- Attia, S. Manipulation of splitting, sunburn and enhancing coloration of “Wonderful” pomegranates by preharvest foliar applications. IJRDO J. Agric. Res. 2017, 3, 1–9. [Google Scholar]
- Huang, D.; Gong, X.; Liu, Y.; Zeng, G.; Lai, C.; Bashir, H.; Zhou, L.; Wang, D.; Xu, P.; Cheng, M. Effects of calcium at toxic concentrations of cadmium in plants. Planta 2017, 245, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, S.; Tehranifar, A.; Abadía, J.; Val, J.; Davarynejad, G.; Aran, M.; Khorassani, R. Foliar calcium fertilization reduces fruit cracking in pomegranate (Punica granatum cv. Ardestani). Sci. Hortic. 2018, 230, 86–91. [Google Scholar] [CrossRef]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Bharty, S.K.; Maji, S.; Prakash, S. Effect of GA3 and 2, 4-D on vegetative growth and yield of pomegranate (Punica granatum L.) cv. Bhagwa. J. Pharm. Innov. 2021, 10, 487–489. [Google Scholar]
- Jifon, J.L.; Syvertsen, J.P. Kaolin Particle Film Applications Can Increase Photosynthesis and Water Use Efficiency ofRuby Red’Grapefruit Leaves. J. Am. Soc. Hortic. Sci. 2003, 128, 107–112. [Google Scholar] [CrossRef]
- Del Saavedra, R.G.; Escaff, G.M.; Hernandéz, V.J. Kaolin effects in processing tomato production in Chile. In Proceedings of the IX International Symposium on the Processing Tomato, Melbourne, Australia, 30 November 2006; pp. 191–198. [Google Scholar]
- Rosati, A.; Metcalf, S.G.; Buchner, R.P.; Fulton, A.E.; Lampinen, B.D. Effects of kaolin application on light absorption and distribution, radiation use efficiency and photosynthesis of almond and walnut canopies. Ann. Bot. 2007, 99, 255–263. [Google Scholar] [CrossRef]
- Lombardini, L.; Harris, M.K.; Glenn, D.M. Effects of particle film application on leaf gas exchange, water relations, nut yield, and insect populations in mature pecan trees. HortScience 2005, 40, 1376–1380. [Google Scholar] [CrossRef]
- Weerakkody, P.; Jobling, J.; Infante, M.M.V.; Rogers, G. The effect of maturity, sunburn and the application of sunscreens on the internal and external qualities of pomegranate fruit grown in Australia. Sci. Hortic. 2010, 124, 57–61. [Google Scholar] [CrossRef]
- Gindaba, J.; Wand, S.J. Comparative effects of evaporative cooling, kaolin particle film, and shade net on sunburn and fruit quality in apples. HortScience 2005, 40, 592–596. [Google Scholar] [CrossRef]
- Wand, S.J.; Theron, K.I.; Ackerman, J.; Marais, S.J. Harvest and post-harvest apple fruit quality following applications of kaolin particle film in South African orchards. Sci. Hortic. 2006, 107, 271–276. [Google Scholar] [CrossRef]
- Glenn, D.M.; Cooley, N.; Walker, R.; Clingeleffer, P.; Shellie, K. Impact of kaolin particle film and water deficit on wine grape water use efficiency and plant water relations. HortScience 2010, 45, 1178–1187. [Google Scholar] [CrossRef]
- Pace, B.; Boari, F.; Cantore, V.; Leo, L.; Vanadia, S.; De Palma, E.; Phillips, N. Effect of particle film technology on temperature, yield and quality of processing tomato. In Proceedings of the X International Symposium on the Processing Tomato, Tunis, Tunisia, 11 November 2007; pp. 287–294. [Google Scholar]
- Ergun, M.J. Postharvest quality of ‘Galaxy’apple fruit in response to kaolin-based particle film application. J. Agric. Sci. Technol. 2012, 14, 599–607. [Google Scholar]
- Colavita, G.; Blackhall, V.; Valdez, S. Effect of kaolin particle films on the temperature and solar injury of pear fruits. In Proceedings of the XI International Pear Symposium, General Roca, Argentina, 23–26 November 2010; pp. 609–615. [Google Scholar]
- Glenn, D.M. The mechanisms of plant stress mitigation by kaolin-based particle films and applications in horticultural and agricultural crops. HortScience 2012, 47, 710–711. [Google Scholar] [CrossRef]
- Denaxa, N.-K.; Roussos, P.A.; Damvakaris, T.; Stournaras, V. Comparative effects of exogenous glycine betaine, kaolin clay particles and Ambiol on photosynthesis, leaf sclerophylly indexes and heat load of olive cv. Chondrolia Chalkidikis under drought. Sci. Hortic. 2012, 137, 87–94. [Google Scholar] [CrossRef]
- Hegazi, A.; Samra, N.; El-Baz, E.; Khalil, B.M.; Gawish, M. Improving fruit quality of manfaloty and wonderfull pomegranates by using bagging and some spray treatments with gibberellic acid, calcium chloride and kaolin. J. Plant Prod. 2014, 5, 779–792. [Google Scholar] [CrossRef]
- Gharaghani, A.; Eshghi, S.; Khajenouri, Y.; Rahemi, M. Effect of kaolin on tree physiology, superficial sunburn and fruit quantitative and qualitative characteristics of two commercial apple cultivars. Iran. J. Hort. Sci. 2015, 46, 475–486. [Google Scholar]
- Segura-Monroy, S.; Uribe-Vallejo, A.; Ramirez-Godoy, A.; Restrepo-Diaz, H. Effect of kaolin application on growth, water use efficiency, and leaf epidermis characteristics of Physallis peruviana seedlings under two irrigation regimes. J. Agric. Sci. Technol. 2015, 17, 1585–1596. [Google Scholar]
- Delgado, R.; González, M.R.; Martín, P. Interaction effects of nitrogen and potassium fertilization on anthocyanin composition and chromatic features of Tempranillo grapes. Int. J. Wine Res. 2006, 40, 141. [Google Scholar] [CrossRef]
- Egilla, J.; Davies, F.; Boutton, T. Drought stress influences leaf water content, photosynthesis, and water-use efficiency of Hibiscus rosa-sinensis at three potassium concentrations. Photosynthetica 2005, 43, 135–140. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; pp. 178–189. [Google Scholar]
- Hegazi, E.; Samira, S.; Mohamed, M.; El-Sonbaty, M.; El-Naby, S.; El-Sharony, T. Effect of potassium nitrate on vegetative growth, nutritional status, yield and fruit quality of Olive cv.“Picual”. J. Hortic. Sci. Ornam. Plants 2011, 3, 252–258. [Google Scholar]
- Barranco, D.; Ercan, H.; Munoz, D.; Belaj, A.; Arquero, O. Factors influencing the efficiency of foliar sprays of monopotassium phosphate in the olive. Int. J. Plant Prod. 2010, 4, 235–240. [Google Scholar]
- Karimi, R. Potassium-induced freezing tolerance is associated with endogenous abscisic acid, polyamines and soluble sugars changes in grapevine. Sci. Hortic. 2017, 215, 184–194. [Google Scholar] [CrossRef]
- Thirupathi, N.; Ghosh, S. Effect of foliar feeding of KNO3 and K2SO4 on yield and quality of some pomegranate cultivars grown in laterite soils of west Bengal. J. Trop. Agric. 2015, 33, 2835–2839. [Google Scholar]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Gen. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, S.K.; Rajasheker, G.; Kishor, P.; Kumar, S.A.; Kumari, P.H.; Saritha, K.; Rathnagiri, P.; Pandey, G.K. Role of protein phosphatases in signaling, potassium transport, and abiotic stress responses. In Protein Phosphatases and Stress Management in Plants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 203–232. [Google Scholar]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Assaha, D.V.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, T.; Singh, S.; Tuteja, N.; Prasad, R.; Singh, J. Potassium: A key modulator for cell homeostasis. J. Biotechnol. 2020, 324, 198–210. [Google Scholar] [PubMed]
- Aksu, G.; Altay, H. The effects of potassium applications on drought stress in sugar beet. Sugar Technol. 2020, 22, 1092–1102. [Google Scholar] [CrossRef]
- Norozi, M.; ValizadehKaji, B.; Karimi, R.; Nikoogoftar Sedghi, M. Effects of foliar application of potassium and zinc on pistachio (Pistacia vera L.) fruit yield. Int. J. Hortic. Sci. 2019, 6, 113–123. [Google Scholar]
- Souri, M.K.; Hatamian, M. Aminochelates in plant nutrition: A review. J. Plant Nutr. 2019, 42, 67–78. [Google Scholar] [CrossRef]
- Medan, R.A. Effect of foliar application of Potassium and calcium on vegetative growth, yield and fruit quality of “ROYAL” apricot trees. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 106–112. [Google Scholar]
- Moradinezhad, F.; Dorostkar, M. Pre-harvest foliar application of calcium chloride and potassium nitrate influences growth and quality of apricot (Prunus armeniaca L.) fruit cv.‘Shahroudi’. J. Soil Sci. Plant Nut. 2021, 21, 1642–1652. [Google Scholar] [CrossRef]

| pH | CaCO3 % | EC dS/m | O.M | Textural Class | Sand % | Silt % | Clay % | ||
|---|---|---|---|---|---|---|---|---|---|
| 8.2 | 40.5 | 1.74 | 1.32 | Sandy Loam | 74.5 | 12.2 | 13.3 | ||
| Nutrients (mg/kg) | Soluble Anions (meq/L) | Soluble Cations (meq/L) | |||||||
| P | K | N | HCO3- | Cl- | SO42− | Ca2+ | Mg2+ | Na+ | K+ |
| 64.30 | 75.5 | 30.72 | 3.8 | 9.4 | 4.20 | 6.75 | 2.66 | 5.64 | 2.35 |
| Treatment | Fruit Set% | Fruit Drop% | Sunburn% | Fruit Cracking% | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| Control | 0 | 7.83 f | 8.8 e | 12.42 a | 12.49 a | 12.35 a | 11.9 a | 12.30 a | 11.55 a |
| Calcium nitrate | 2% | 9.11 de | 9.92 de | 10.66 b | 10.67 b | 9.7 b | 9.32 b | 8.28 b | 7.89 bc |
| 3% | 10.86 c | 12.54 c | 7.85 f | 7.67 ef | 8.04 cd | 7.58 c | 6.19 e | 5.58 fg | |
| 4% | 12.62 b | 14.46 b | 6.96 g | 6.78 f | 7.14 e | 6.30 d | 5.57 f | 5.08 g | |
| Kaolin | 2% | 8.74 ef | 9.29 de | 12.03 a | 12.16 a | 9.56 b | 9.06 b | 8.55 b | 8.36 b |
| 4% | 12.39 b | 13.57 bc | 9.29 d | 9.68 bc | 7.49 de | 7.04 c | 7.16 cd | 6.58 de | |
| 6% | 12.67 b | 14.55 b | 8.49 e | 8.85 cd | 6.82 e | 6.12 d | 6.60 de | 5.90 ef | |
| Potassium nitrate | 1% | 9.99 cd | 10.55 d | 10.03 c | 9.84 bc | 9.69 b | 9.31 b | 8.33 b | 8.14 bc |
| 2% | 12.89 ab | 14.69 b | 7.99 ef | 8.15 de | 8.20 c | 7.52 c | 7.64 c | 7.33 cd | |
| 3% | 13.92 a | 15.95 a | 7.05 g | 6.83 f | 6.96 e | 6.26 d | 6.57 de | 6.51 e | |
| LSD0.05 | 1.16 | 1.20 | 0.57 | 1.03 | 0.65 | 0.59 | 0.62 | 0.78 | |
| Treatment | Sunburned Fruit Number | Fruit Cracking Number | Marketable Fruit Number | Marketable Yield (kg/Tree) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| Control | 0 | 4.72 a | 5.02 a | 4.69 a | 4.86 a | 28.82 g | 32.30 g | 10.98 g | 12.84 f |
| Calcium nitrate | 2% | 3.88 bc | 4.04 bcde | 3.32 cd | 3.43 cde | 32.80 ef | 3593 f | 13.73 ef | 15.13 de |
| 3% | 4.00 bc | 4.09 bcd | 3.08 d | 3.01 e | 42.59 d | 46.74 cd | 18.55 d | 21.16 c | |
| 4% | 4.02 bc | 3.83 cde | 3.14 d | 3.10 de | 49.17 b | 53.90 ab | 22.68 a | 26.08 b | |
| Kaolin | 2% | 3.76 bc | 3.89 cde | 3.37 cd | 3.59 cd | 32.21 f | 35.45 f | 13.19 f | 14.56 ef |
| 4% | 3.63 c | 3.68 de | 3.48 cd | 3.44 cde | 41.39 d | 45.11 d | 18.15 d | 20.40 c | |
| 6% | 3.61 c | 3.60 e | 3.5 cd | 3.47 cde | 45.89 c | 51.79 b | 20.79 c | 24.08 b | |
| Potassium nitrate | 1% | 4.21 b | 4.40 b | 3.62 bc | 3.86 bc | 35.67 e | 39.07 e | 14.98 e | 16.86 d |
| 2% | 4.23 b | 4.28 bc | 3.95 b | 4.17 b | 43.48 cd | 48.42 c | 22.01 bc | 25.29 b | |
| 3% | 4.19 b | 4.03 bcde | 3.96 b | 4.18 b | 52.18 a | 56.15 a | 28.55 a | 32.10 a | |
| LSD0.05 | 0.43 | 0.42 | 0.41 | 0.46 | 2.98 | 2.79 | 1.51 | 1.93 | |
| Treatment | Fruit Volume (cm3) | Fruit Length (cm) | Fruit Diameter (cm) | Fruit Firmness (Ib/inch2) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| Control | 0 | 407.67 f | 414.00 g | 7.69 f | 7.67 f | 7.6 f | 8.41 d | 20.00 e | 21.00 f |
| Calcium nitrate | 2% | 445.00 de | 443.67 ef | 8.70 de | 8.33 e | 9.22 d | 8.52 d | 25.00 cd | 27.00 d |
| 3% | 475.33 c | 473.00 d | 9.39 bc | 9.19 cd | 9.51 cd | 9.63 c | 32.33 a | 35.33 ab | |
| 4% | 499.33 b | 507.00 c | 9.53 ab | 9.51 bcd | 9.75 bc | 10.35 ab | 33.00 a | 37.00 a | |
| Kaolin | 2% | 426.00 ef | 436.67 f | 8.19 ef | 8.85 de | 8.25 e | 9.44 c | 24.33 cd | 26.67 de |
| 4% | 454.33 cd | 476.67 d | 8.80 cde | 9.26 cd | 9.41 cd | 9.77 c | 27.67 bc | 32.00 c | |
| 6% | 469.67 c | 488.33 d | 9.11 bcd | 9.71 abc | 10.05 b | 9.98 bc | 31.00 ab | 32.67 bc | |
| Potassium nitrate | 1% | 442.67 de | 455.67 e | 8.72 de | 8.91 de | 9.39 cd | 9.63 c | 2267 de | 23.67 ef |
| 2% | 537.00 a | 543.67 b | 9.54 ab | 10.00 ab | 10.11 b | 10.66 a | 23.67 cde | 24.33 de | |
| 3% | 558.00 a | 591.67 a | 10.10 a | 10.19 a | 10.58 a | 10.88 a | 24.00 cd | 26.33 de | |
| LSD0.05 | 22.36 | 15.97 | 0.58 | 0.62 | 0.45 | 0.55 | 3.54 | 2.80 | |
| Treatment | Grain Weight (g) | Peel Weight (g) | Juice % | ||||
|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| Control | 0 | 245.33 f | 253.67 f | 136.00 cd | 143.81 cd | 64.07 d | 71.45 c |
| Calcium nitrate | 2% | 273.67 d | 264.67 e | 145.00 bcd | 156.27 bc | 68.82 c | 73.49 bc |
| 3% | 292.67 c | 301.67 c | 143.00 bcd | 151.05 c | 71.65 bc | 75.07 b | |
| 4% | 333.00 b | 330.67 b | 128.67 d | 153.15 c | 73.54 ab | 76.42 ab | |
| Kaolin | 2% | 259.33 e | 279.67 d | 150.33 bcd | 131.09 d | 68.53 c | 73.95 bc |
| 4% | 275.00 d | 280.67 d | 163.33 ab | 171.45 b | 73.14 ab | 74.34 bc | |
| 6% | 295.00 c | 305.67 c | 158.00 bc | 159.02 bc | 73.47 ab | 76.51 ab | |
| Potassium nitrate | 1% | 293.67 c | 301.67 c | 126.33 d | 129.59 d | 72.34 bc | 75.34 b |
| 2% | 345.00 b | 350.33 a | 161.33 ab | 172.03 b | 73.83 ab | 76.67 ab | |
| 3% | 362.67 a | 357.00 a | 184.33 a | 214.31 a | 76.68 a | 78.68 a | |
| LSD0.05 | 13.16 | 9.86 | 22.39 | 15.51 | 3.69 | 2.86 | |
| Treatment | TSS % | Total Sugars % | Reduced Sugars % | Non-Reduced Sugars % | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| Control | 0 | 14.93 e | 16.3 d | 10.08 h | 10.75 g | 5.99 f | 6.50 e | 4.09 b | 4.25 c |
| Calcium nitrate | 2% | 15.8 d | 17.17 c | 11.08 fg | 11.45 ef | 6.35 ef | 6.54 e | 4.73 b | 4.91 bc |
| 3% | 15.93 d | 17.30 bc | 11.26 efg | 11.97 de | 6.39 e | 7.10 cd | 4.87 ab | 4.87 bc | |
| 4% | 15.98 cd | 17.50 bc | 11.80 cde | 12.5 bcd | 6.83 d | 7.17 c | 4.96 ab | 5.33 ab | |
| Kaolin | 2% | 15.62 de | 17.13 c | 10.73 g | 10.98 fg | 6.39 e | 6.82 de | 4.34 b | 4.16 c |
| 4% | 15.82 d | 17.27 bc | 11.56 def | 11.49 ef | 6.95 cd | 6.53 e | 4.61 b | 4.96 bc | |
| 6% | 16.73 bc | 17.63 b | 12.34 bc | 12.72 bc | 7.31 bc | 7.59 ab | 5.03 ab | 5.13 b | |
| Potassium nitrate | 1% | 16.31 bcd | 17.50 bc | 12.06 bcd | 12.04 cde | 7.13 cd | 7.49 b | 4.93 ab | 4.55 bc |
| 2% | 16.87 ab | 18.30 a | 12.72 b | 12.97 b | 7.67 ab | 7.73 ab | 5.04 ab | 5.24 ab | |
| 3% | 17.50 a | 18.50 a | 13.48 a | 13.85 a | 7.79 a | 7.86 a | 5.69 a | 5.99 a | |
| LSD0.05 | 0.72 | 0.34 | 0.63 | 0.65 | 0.37 | 0.31 | 0.84 | 0.75 | |
| Treatment | Anthocyanin (mg/100 mg) | Acidity % | TSS-Acid Ratio | VC (mg/100 mL) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| Control | 0 | 0.50 f | 0.50 h | 1.10 a | 1.11 a | 13.53 g | 14.70 g | 10.87 f | 11.16 e |
| Calcium nitrate | 2% | 0.56 e | 0.61 g | 1.06 a | 1.06 b | 14.93 f | 16.15 f | 13.83 e | 14.96 d |
| 3% | 0.59 de | 0.65 efg | 0.95 b | 0.89 cd | 16.84 e | 19.46 e | 15.43 d | 16.34 c | |
| 4% | 0.62 cd | 0.67 def | 0.85 cd | 0.85 de | 18.81 cd | 20.61 cd | 15.86 cd | 16.78 c | |
| Kaolin | 2% | 0.63 cd | 0.63 fg | 0.87 c | 0.90 c | 18.03 de | 18.97 e | 14.03 e | 14.25 d |
| 4% | 0.64 cd | 0.69 de | 0.85 cd | 0.87 cde | 18.61 cd | 19.92 de | 16.6 c | 16.97 c | |
| 6% | 0.67 c | 0.70 d | 0.85 cd | 0.83 e | 19.81 bc | 21.26 c | 17.71 b | 18.33 b | |
| Potassium nitrate | 1% | 0.77 b | 0.76 c | 0.84 cd | 0.85 de | 19.50 c | 20.68 cd | 15.08 d | 16.06 c |
| 2% | 0.81 b | 0.83 b | 0.80 d | 0.77 f | 21.10 b | 23.77 b | 17.85 b | 18.46 b | |
| 3% | 0.87 a | 0.88 a | 0.75 e | 0.74 f | 23.45 a | 25.01 a | 19.58 a | 20.07 a | |
| LSD0.05 | 0.05 | 0.04 | 0.05 | 0.04 | 1.32 | 1.00 | 0.87 | 0.85 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Saif, A.M.; Mosa, W.F.A.; Saleh, A.A.; Ali, M.M.; Sas-Paszt, L.; Abada, H.S.; Abdel-Sattar, M. Yield and Fruit Quality Response of Pomegranate (Punica granatum) to Foliar Spray of Potassium, Calcium and Kaolin. Horticulturae 2022, 8, 946. https://doi.org/10.3390/horticulturae8100946
Al-Saif AM, Mosa WFA, Saleh AA, Ali MM, Sas-Paszt L, Abada HS, Abdel-Sattar M. Yield and Fruit Quality Response of Pomegranate (Punica granatum) to Foliar Spray of Potassium, Calcium and Kaolin. Horticulturae. 2022; 8(10):946. https://doi.org/10.3390/horticulturae8100946
Chicago/Turabian StyleAl-Saif, Adel M., Walid F. A. Mosa, Abaidalah A. Saleh, Muhammad Moaaz Ali, Lidia Sas-Paszt, Hesham S. Abada, and Mahmoud Abdel-Sattar. 2022. "Yield and Fruit Quality Response of Pomegranate (Punica granatum) to Foliar Spray of Potassium, Calcium and Kaolin" Horticulturae 8, no. 10: 946. https://doi.org/10.3390/horticulturae8100946
APA StyleAl-Saif, A. M., Mosa, W. F. A., Saleh, A. A., Ali, M. M., Sas-Paszt, L., Abada, H. S., & Abdel-Sattar, M. (2022). Yield and Fruit Quality Response of Pomegranate (Punica granatum) to Foliar Spray of Potassium, Calcium and Kaolin. Horticulturae, 8(10), 946. https://doi.org/10.3390/horticulturae8100946







