Climate Variables of the Sites of Origin and Genotype Influence on Phenolic Compounds Accumulation in Cultivars of Myrtus communis L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Agrometeorological Data
2.2. Preparation of Extracts
2.3. Determination of Total Phenols, Tannins, and Anthocyanins
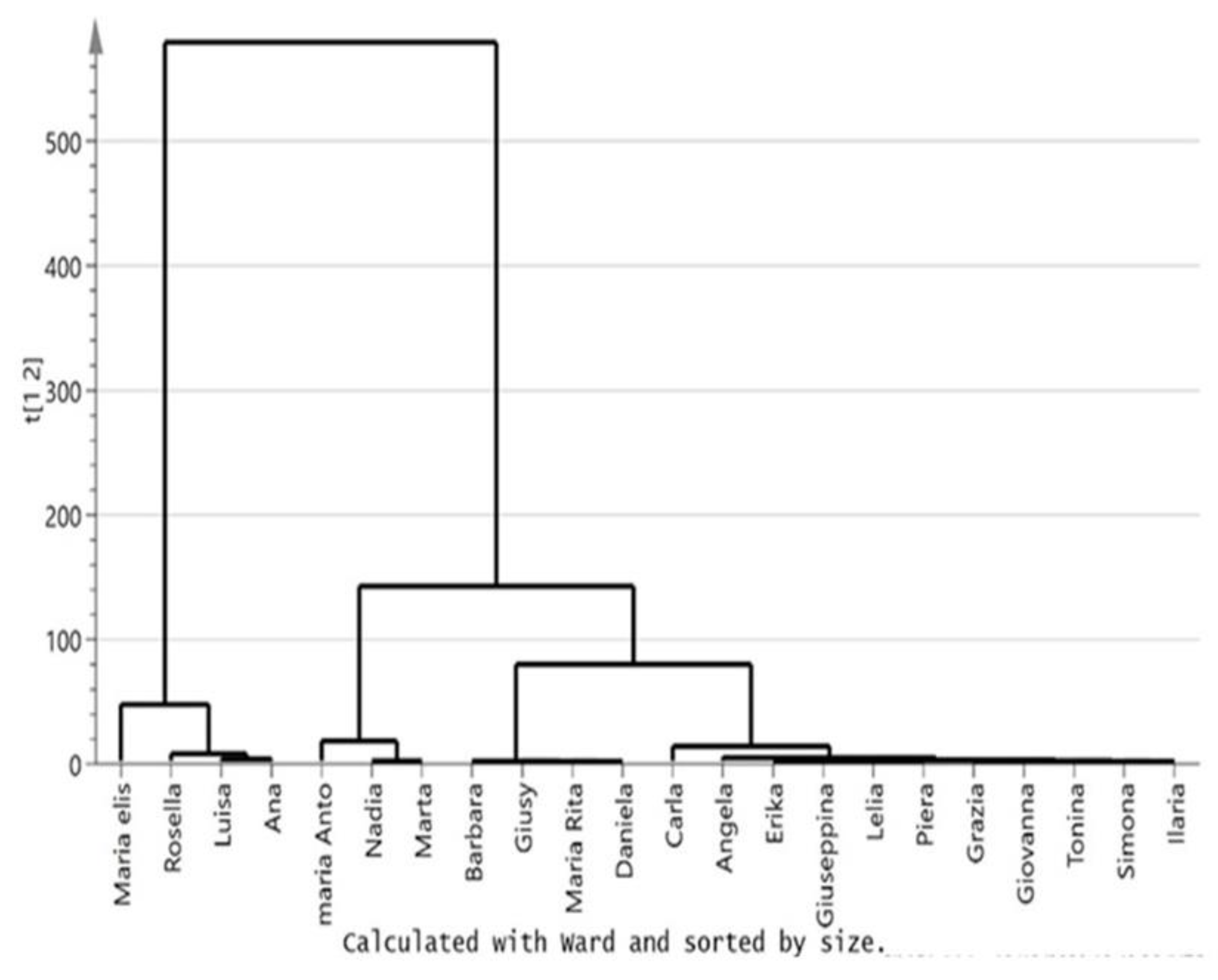
2.4. Statistical Analysis
3. Results
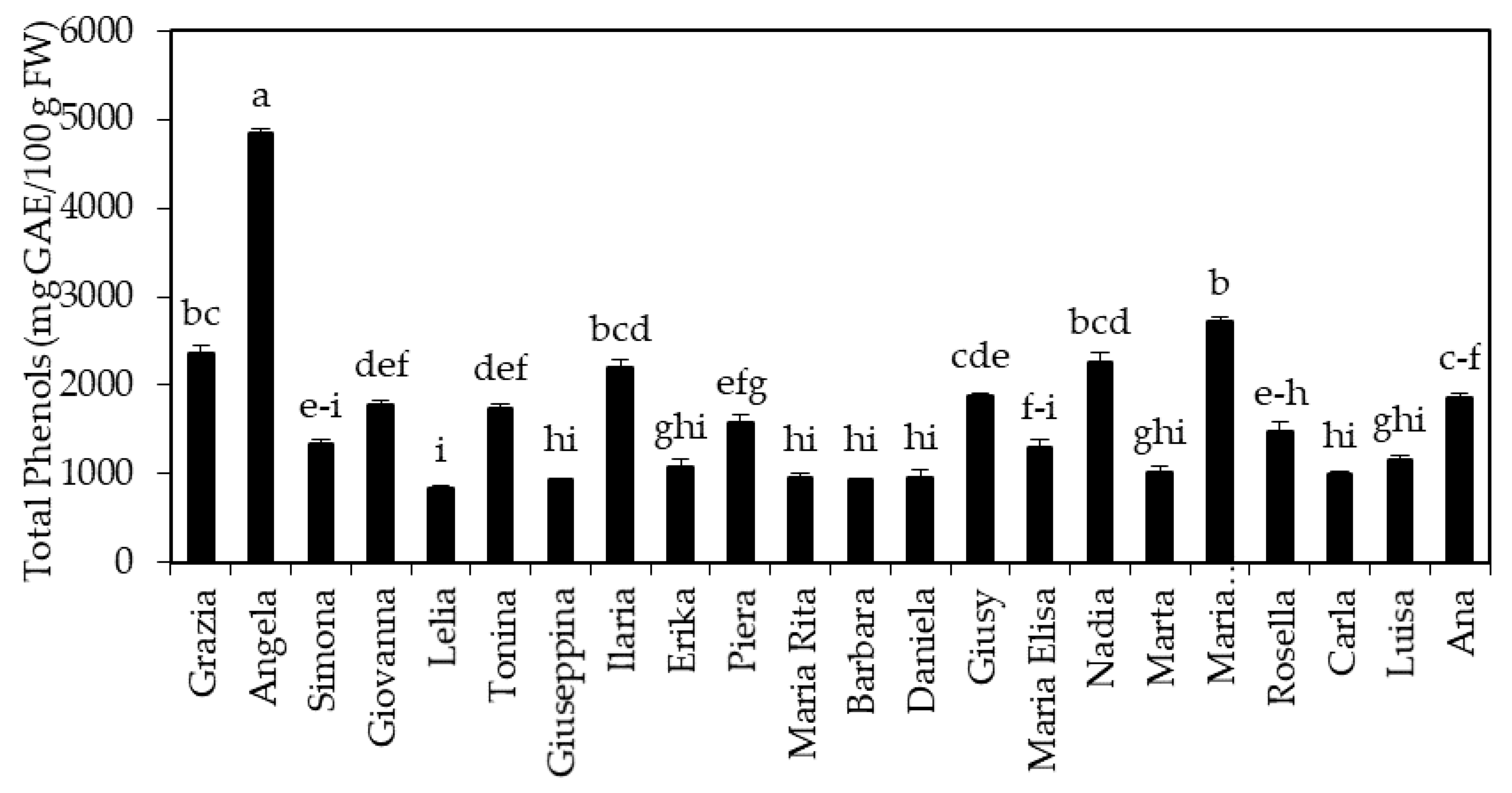
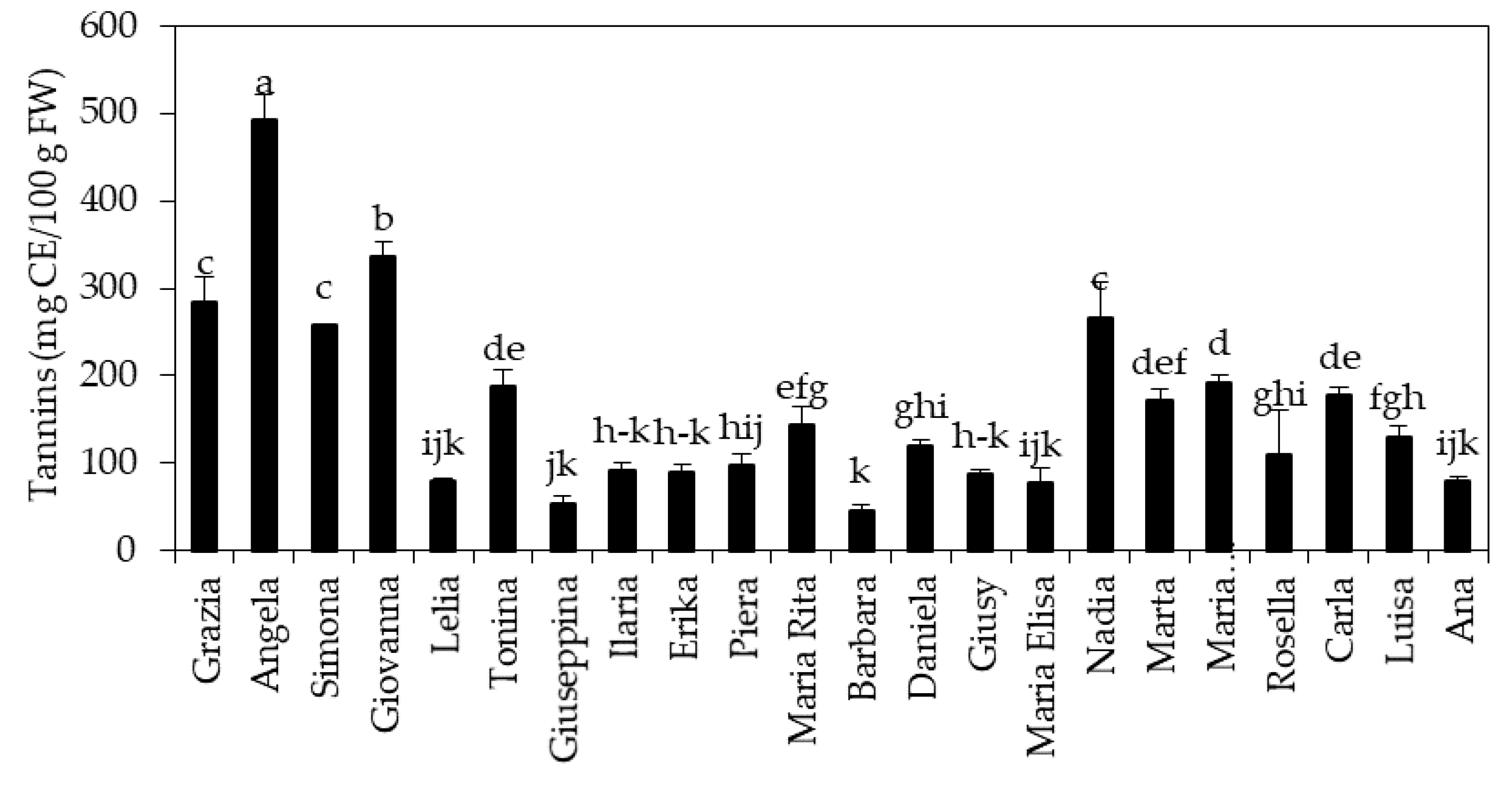
3.1. Concentration of Phenolic Compounds
3.2. Agrometeorological Parameters of the Sites of Origin of the Cultivars
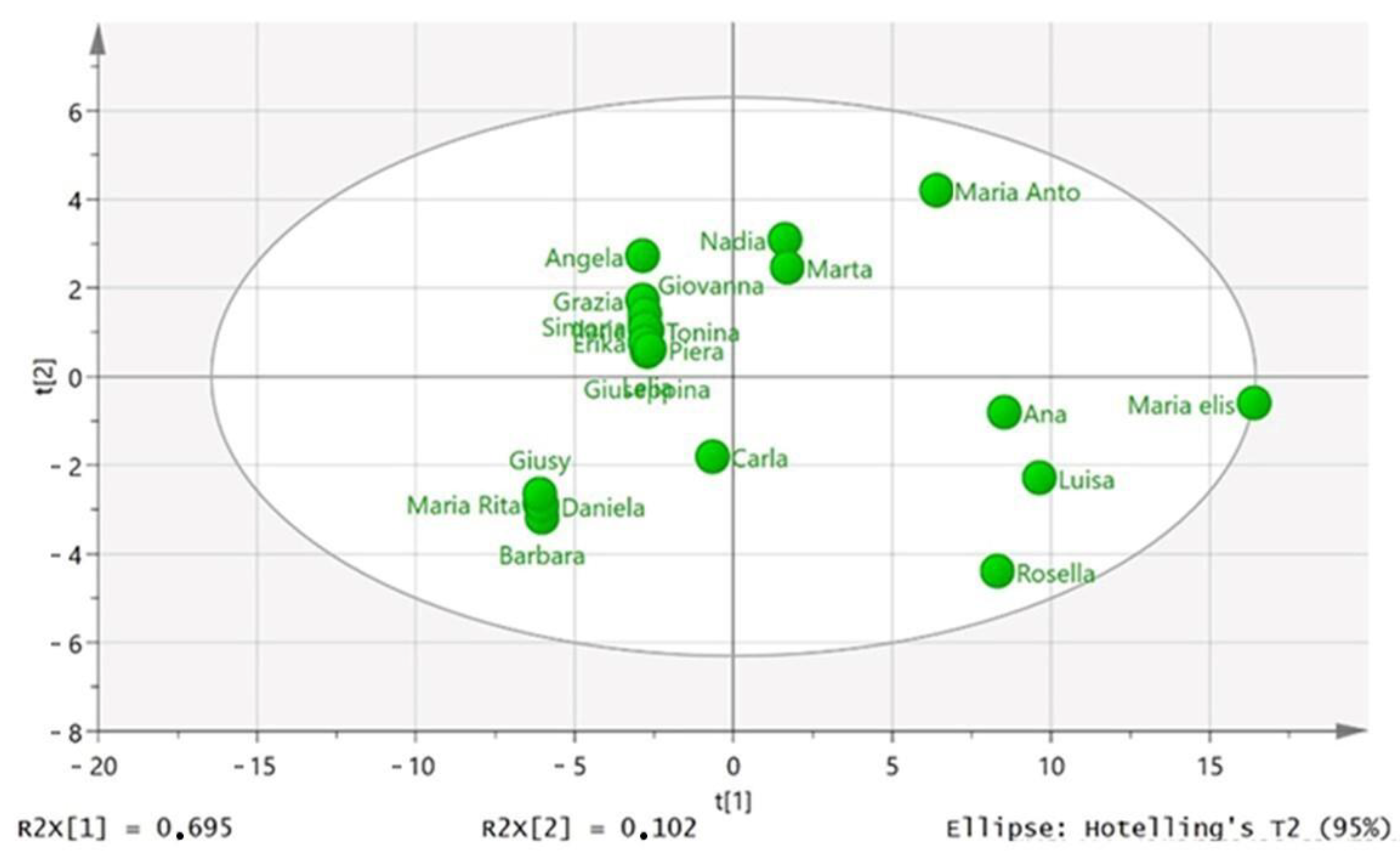
3.3. Correlations between Phenolic Compounds and Agrometeorological Data and Chemometric Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Anttonen, M.J.; Hoppula, K.I.; Nestby, R.; Verheul, M.J.; Karjalainen, R.O. Influence of fertilization, mulch color, early forcing, fruit order, planting date, shading, growing environment, and genotype on the contents of selected phenolics in strawberry (Fragaria× ananassa Duch.) fruit. J. Agric. Food Chem. 2006, 54, 2614–2620. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Han, W.; Wang, M. Ultraviolet and environmental stresses involved in the induction and regulation of anthocyanin biosynthesis: A review. Afr. J. Biotechnol. 2008, 7, 25. [Google Scholar]
- Lo Piero, A.R.; Puglisi, I.; Rapisarda, P.; Petrone, G. Anthocyanins accumulation and related gene expression in red orange fruit induced by low temperature storage. J. Agric. Food Chem. 2005, 53, 9083–9088. [Google Scholar] [CrossRef]
- Ubi, B.E.; Honda, C.; Bessho, H.; Kondo, S.; Wada, M.; Kobayashi, S.; Moriguchi, T. Expression analysis of anthocyanin biosynthetic genes in apple skin: Effect of UV-B and temperature. Plant Sci. 2006, 170, 571–578. [Google Scholar] [CrossRef]
- Lin-Wang, K.U.I.; Micheletti, D.; Palmer, J.; Volz, R.; Lozano, L.; Espley, R.; Iglesias, I. High temperature reduces apple fruit colour via modulation of the anthocyanin regulatory complex. Plant Cell Environ. 2011, 34, 1176–1190. [Google Scholar] [CrossRef]
- Borsani, O.; Gonzalez-Neves, G.; Ferrer, M.; Monza, J. Anthocyanins accumulation and genes-related expression in berries of cv. Tannat (Vitis vinifera L.). J. Appl. Hortic. 2010, 12, 3–9. [Google Scholar] [CrossRef][Green Version]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- Costa, C.; Graça, A.; Fontes, N.; Teixeira, M.; Gerós, H.; Santos, J.A. The interplay between atmospheric conditions and grape berry quality parameters in Portugal. Appl. Sci. 2020, 10, 4943. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Zhu, L.; Huang, Y.; Lu, J. Influence of growing season on phenolic compounds and antioxidant properties of grape berries from vines grown in subtropical climate. J. Agric. Food Chem. 2011, 59, 1078–1086. [Google Scholar] [CrossRef]
- Marin, A.; Ferreres, F.; Barbera, G.G.; Gil, M.I. Weather variability influences color and phenolic content of pigmented baby leaf lettuces throughout the season. J. Agric. Food Chem. 2015, 63, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; He, Y.N.; Yue, T.X.; Wang, J.; Zhang, Z.W. Effects of climatic conditions and soil properties on Cabernet Sauvignon berry growth and anthocyanin profiles. Molecules 2014, 19, 13683–13703. [Google Scholar] [CrossRef] [PubMed]
- Sárosi, S.; Bernáth, J.; Burchi, G.; Antonetti, M.; Bertoli, A.; Pistelli, L.; Benvenuti, S. Effect of different plant origins and climatic conditions on the total phenolic content and total antioxidant capacity of self-heal (Prunella vulgaris L.). In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): A New Look at Medicinal and 925, Lisbon, Portugal, 22–27 August 2010; pp. 49–55. [Google Scholar]
- Jasicka-Misiak, I.; Poliwoda, A.; Dereń, M.; Kafarski, P. Phenolic compounds and abscisic acid as potential markers for the floral origin of two Polish unifloral honeys. Food Chem. 2012, 131, 1149–1156. [Google Scholar] [CrossRef]
- Guo, J.; Yuan, Y.; Dou, P.; Yue, T. Multivariate statistical analysis of the polyphenolic constituents in kiwifruit juices to trace fruit varieties and geographical origins. Food Chem. 2017, 232, 552–559. [Google Scholar] [CrossRef]
- Giampieri, F.; Cianciosi, D.; Forbes-Hernández, T.Y. Myrtle (Myrtus communis L.) berries, seeds, leaves, and essential oils: New undiscovered sources of natural compounds with promising health benefits. Food Front. 2020, 1, 276–295. [Google Scholar] [CrossRef]
- Sanna, D.; Mulas, M.; Molinu, M.G.; Fadda, A. Oxidative stability of plant hydroalcoholic extracts assessed by EPR spin trapping under forced ageing conditions: A myrtle case study. Food Chem. 2019, 271, 753–761. [Google Scholar] [CrossRef]
- Aidi-Wannes, W.; Marzouk, B. Differences between Myrtle Fruit Parts (Myrtus communis var. italica) in Phenolics and Antioxidant Contents. J. Food Biochem. 2013, 37, 585–594. [Google Scholar] [CrossRef]
- Barboni, T.; Cannac, M.; Massi, L.; Perez-Ramirez, Y.; Chiaramonti, N. Variability of polyphenol compounds in Myrtus communis L. (Myrtaceae) berries from Corsica. Molecules 2010, 15, 7849–7860. [Google Scholar] [CrossRef]
- Mulas, M. The myrtle (Myrtus communis L.) case, from a wild shrub to a new fruit crop. Acta Hortic. 2012, 948, 235–242. [Google Scholar] [CrossRef]
- Mulas, M.; Cani, M.R. Germplasm evaluation of spontaneous myrtle (Myrtus communis L.) for cultivar selection and crop development. J. Herbs Spices Med. Plants 1999, 6, 31–49. [Google Scholar] [CrossRef]
- Mele, C.; Corona, L.; Melito, S.; Raggi, L.; Mulas, M. The genetic diversity of selections and wild populations of myrtle revealed by molecular geographic contexts. Ind. Crops Prod. 2019, 132, 168–176. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Mirzakhani, M.; Pirbalouti, A.G. Essential oil variation among 21 wild myrtle (Myrtus communis L.) populations collected from different geographical regions in Iran. Ind. Crops Prod. 2013, 51, 328–333. [Google Scholar] [CrossRef]
- Piras, F.M.; Dettori, M.F.; Magnani, A. ToF-SIMS PCA analysis of Myrtus communis L. Appl. Surf. Sci. 2009, 255, 7805–7811. [Google Scholar] [CrossRef]
- González de Peredo, A.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Jiménez-Cantizano, A.; Ferreiro-González, M.; Amores-Arrocha, A.; Barbero, G. Development of new analytical microwave-assisted extraction methods for bioactive compounds from myrtle (Myrtus communis L.). Molecules 2018, 23, 2992. [Google Scholar] [CrossRef] [PubMed]
- Sarais, G.; D’Urso, G.; Lai, C.; Pirisi, F.M.; Pizza, C.; Montoro, P. Targeted and untargeted mass spectrometric approaches in discrimination between Myrtus communis cultivars from Sardinia region. J. Mass Spectrom. 2016, 51, 704–715. [Google Scholar] [CrossRef]
- Canu, S.; Rosati, L.; Fiori, M.; Motroni, A.; Filigheddu, R.; Farris, E. Bioclimate map of Sardinia (Italy). J. Maps 2015, 11, 711–718. [Google Scholar] [CrossRef]
- Caloiero, T.; Coscarelli, R.; Gaudio, R.; Leonardo, G.P. Precipitation trend and concentration in the Sardinia region. Theor. Appl. Climatol. 2019, 137, 297–307. [Google Scholar] [CrossRef]
- Montaldo, N.; Sarigu, A. Potential links between the North Atlantic Oscillation and decreasing precipitation and runoff on a Mediterranean area. J. Hydrol. 2017, 553, 419–437. [Google Scholar] [CrossRef]
- Medda, S.; Mulas, M. Fruit Quality Characters of Myrtle (Myrtus communis L.) Selections: Review of a Domestication Process. Sustainability 2021, 13, 8785. [Google Scholar] [CrossRef]
- Fadda, A.; Mulas, M. Chemical changes during myrtle (Myrtus communis L.) fruit development and ripening. Sci. Hort. 2010, 125, 477–485. [Google Scholar] [CrossRef]
- RAS. 2019. Available online: https://www.regione.sardegna.it/autoritadibacino/monitoraggio/settoreidrografico/ (accessed on 27 August 2020).
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Fredes, C.; Yousef, G.G.; Robert, P.; Grace, M.H.; Lila, M.A.; Gómez, M.; Montenegro, G. Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [Mol.] Stuntz) from different geographical regions in Chile. J. Sci. Food Agric. 2014, 94, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Vega-Gálvez, A.; Ah-Hen, K.; Chacana, M.; Vergara, J.; Martínez-Monzó, J.; García-Segovia, P.; Di Scala, K. Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny Smith) slices. Food Chem. 2012, 132, 51–59. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. A comparison of fruit quality parameters of wild bilberry (Vaccinium myrtillus L.) growing at different locations. J. Sci. Food Agric. 2015, 95, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Echeverría, G.; Carbonneau, A. Effect of berry weight and its components on the contents of sugars and anthocyanins of three varieties of Vitis vinifera L. under different water supply conditions. S. Afr. J. Enol. Viticult. 2014, 35, 103–113. [Google Scholar] [CrossRef]
- Barnuud, N.N.; Zerihun, A.; Gibberd, M.; Bates, B. Berry composition and climate: Responses and empirical models. Int. J. Biometeorol. 2014, 58, 1207–1223. [Google Scholar] [CrossRef]
- Borochov-Neori, H.; Judeinstein, S.; Harari, M.; Bar-Yaakov, I.; Patil, B.S.; Lurie, S.; Holland, D. Climate effects on anthocyanin accumulation and composition in the pomegranate (Punica granatum L.) fruit arils. J. Agric. Food Chem. 2011, 59, 5325–5334. [Google Scholar] [CrossRef]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Barril, C. Impact of short temperature exposure of Vitis vinifera L. cv. Shiraz grapevine bunches on berry development, primary metabolism and tannin accumulation. Environ. Exp. Bot. 2019, 168, 103866. [Google Scholar] [CrossRef]
- Bucchetti, B.; Matthews, M.A.; Falginella, L.; Peterlunger, E.; Castellarin, S.D. Effect of water deficit on Merlot grape tannins and anthocyanins across four seasons. Sci. Hort. 2011, 128, 297–305. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Stander, M.A.; Fawole, O.A.; Opara, U.L. Effect of fruit maturity and growing location on the postharvest contents of flavonoids, phenolic acids, vitamin C and antioxidant activity of pomegranate juice (cv. Wonderful). Sci. Hort. 2014, 179, 36–45. [Google Scholar] [CrossRef]
- Vaneková, Z.; Vanek, M.; Škvarenina, J.; Nagy, M. The Influence of Local Habitat and Microclimate on the Levels of Secondary Metabolites in Slovak Bilberry (Vaccinium myrtillus L.) Fruit. Plants 2020, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Rieger, G.; Muller, M.; Guttenberger, H.; Bucar, F. Influence of altitudinal variation on the content of phenolic compounds in wild populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus. J. Agr. Food Chem. 2008, 56, 9080–9086. [Google Scholar] [CrossRef] [PubMed]
- Rosłon, W.; Osińska, E.; Ewelina Pióro-Jabrucka, E.; Grabowska, A. Morphological and chemical variability of wild populations of bilberry (Vaccinium myrtillus L.). Pol. J. Environ. Stud. 2011, 20, 237–243. [Google Scholar]
- Wang, L.J.; Su, S.; Wu, J.; Du, H.; Li, S.S.; Huo, J.W.; Wang, L.S. Variation of anthocyanins and flavonols in Vaccinium uliginosum berry in Lesser Khingan Mountains and its antioxidant activity. Food Chem. 2014, 160, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, E.; Michailidis, M.; Tanou, G.; Scossa, F.; Sarrou, E.; Stamatakis, G.; Molassiotis, A. Decoding altitude-activated regulatory mechanisms occurring during apple peel ripening. Hortic. Res. 2020, 7, 1–12. [Google Scholar] [CrossRef]
- Medda, S.; Sanchez-Ballesta, M.T.; Romero, I.; Dessena, L.; Mulas, M. Expression of structural flavonoid biosynthesis genes in dark-Blue and White Myrtle Berries (Myrtus communis L.). Plants 2021, 10, 316. [Google Scholar] [CrossRef]
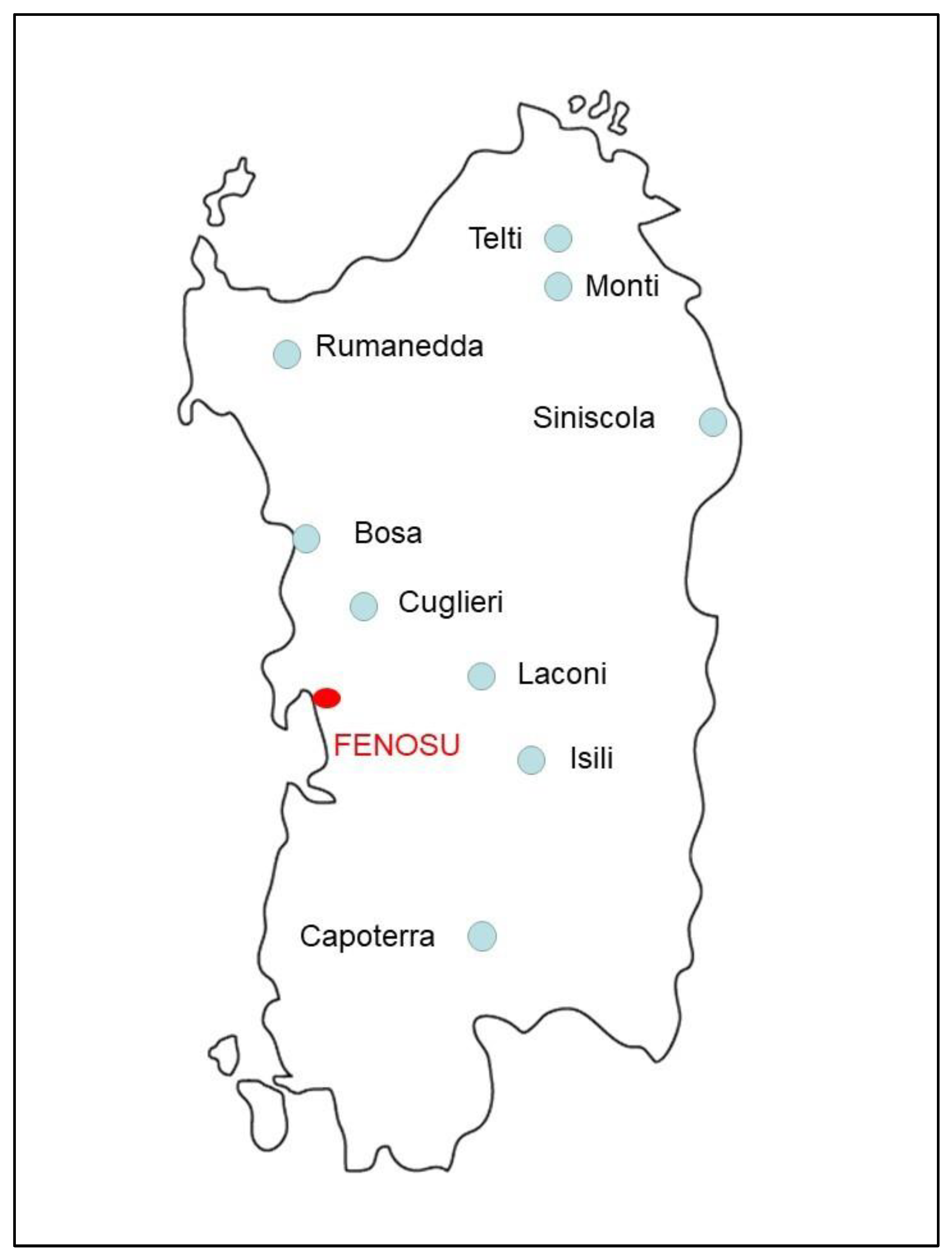

| Site of Origin | Altitude (m a.s.l.) | WGS84 | Cultivar |
|---|---|---|---|
| Rumanedda | 45 | 8.35484044, 40.68616703 | “Grazia”, “Angela”, “Simona”, “Giovanna”, “Lelia”, “Tonina”, “Giuseppina”, “Ilaria”, “Erika”, “Piera” |
| Monti | 506 | 9.193589, 40.482492 | “Luisa” |
| Telti | 103 | 9.35427989, 40.87739254 | “Ana” |
| Siniscola | 40 | 9.69324407, 40.57296208 | “Carla” |
| Bosa | 83 | 8.49761184, 40.29915939 | “Nadia”, “Marta” |
| Cuglieri | 235 | 8.56761404, 40.18907507 | “Maria Antonietta” |
| Laconi | 707 | 9.05133847, 39.85371886 | “Sofia” |
| Isili | 523 | 9.10765215, 39.74006983 | “Rosella” |
| Capoterra | 151 | 8.96688623, 39.17913536 | “Maria Rita”, “Barbara”, “Daniela”, “Giusy” |
| T Mean | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| J | F | M | A | M | J | J | A | S | O | N | D | |
| Total phenols | 0.009 | −0.019 | −0.045 | −0.044 | −0.086 | −0.04 | −0.216 | −0.192 | −0.104 | −0.077 | 0.015 | 0.013 |
| Anthocyanins | −0.287 * | −0.245 * | −0.218 | −0.208 | −0.194 | 0.199 | −0.097 | −0.104 | −0.161 | −0.189 | −0.261 * | −0.270 * |
| Tannins | 0.155 | 0.157 | 0.120 | 0.116 | 0.058 | 0.157 | −0.145 | −0.121 | 0.062 | 0.108 | 0.175 | 0.185 |
| T max | ||||||||||||
| J | F | M | A | M | J | J | A | S | O | N | D | |
| Total phenols | −0.062 | −0.063 | −0.071 | −0.062 | −0.083 | −0.276 * | −0.353 ** | −0.254 * | −0.108 | −0.055 | −0.038 | −0.040 |
| Anthocyanins | −0.236 | −0.216 | −0.191 | −0.173 | −0.159 | −0.034 | −0.013 | −0.140 | −0.194 | −0.254 * | −0.264 * | −0.260 * |
| Tannins | 0.185 | 0.201 | 0.203 | 0.215 | 0.200 | −0.019 | −0.184 | −0.178 | 0.139 | 0.161 | 0.176 | 0.185 |
| T min | ||||||||||||
| J | F | M | A | M | J | J | A | S | O | N | D | |
| Total phenols | 0.113 | 0.114 | 0.1 | 0.099 | 0.046 | −0.012 | −0.017 | −0.023 | 0.011 | 0.063 | 0.106 | 0.112 |
| Anthocyanins | −0.300 * | −0.272 * | −0.28 4* | −0.274 * | −0.250 * | −0.260 | −0.153 | −0.158 | −0.191 | −0.222 | −0.263 * | −0.261 * |
| Tannins | 0.128 | 0.155 | 0.104 | 0.139 | 0.092 | 0.048 | 0.059 | 0.036 | 0.123 | 0.153 | 0.172 | 0.191 |
| Rainfall | ||||||||||||
| J | F | M | A | M | J | J | A | S | O | N | D | |
| Total phenols | −0.065 | −0.089 | −0.115 | −0.156 | 0.194 | 0.227 | −0.097 | −0.073 | −0.109 | 0.124 | 0.214 | −0.037 |
| Anthocyanins | 0.1333 | 0.164 | 0.069 | 0.130 | −0.034 | −0.016 | 0.256 * | 0.213 | 0.218 | 0.035 | 0.017 | 0.174 |
| Tannins | −0.186 | −0.206 | −0.253 * | −0.320 ** | 0.096 | 0.156 | −0.188 | −0.127 | −0.287 * | 0.208 | 0.244 * | −0.140 |
| Total Phenols | Anthocyanins | Tannins | Altitude | |
|---|---|---|---|---|
| Total phenols | −0.53 ** | 0.72 ** | −0.14 | |
| Anthocyanins | −0.49 ** | 0.27 * | ||
| Tannins | −0.27 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medda, S.; Fadda, A.; Mulas, M. Climate Variables of the Sites of Origin and Genotype Influence on Phenolic Compounds Accumulation in Cultivars of Myrtus communis L. Horticulturae 2022, 8, 928. https://doi.org/10.3390/horticulturae8100928
Medda S, Fadda A, Mulas M. Climate Variables of the Sites of Origin and Genotype Influence on Phenolic Compounds Accumulation in Cultivars of Myrtus communis L. Horticulturae. 2022; 8(10):928. https://doi.org/10.3390/horticulturae8100928
Chicago/Turabian StyleMedda, Silvia, Angela Fadda, and Maurizio Mulas. 2022. "Climate Variables of the Sites of Origin and Genotype Influence on Phenolic Compounds Accumulation in Cultivars of Myrtus communis L." Horticulturae 8, no. 10: 928. https://doi.org/10.3390/horticulturae8100928
APA StyleMedda, S., Fadda, A., & Mulas, M. (2022). Climate Variables of the Sites of Origin and Genotype Influence on Phenolic Compounds Accumulation in Cultivars of Myrtus communis L. Horticulturae, 8(10), 928. https://doi.org/10.3390/horticulturae8100928







