Novel Approaches for Sustainable Horticultural Crop Production: Advances and Prospects
Abstract
1. Introduction
2. Importance of Microbial Endophytes and Potential Use as Bioinoculants in Horticultural Crop Production
2.1. Microbial Endophytes Diversity and Function in Plants
2.2. Microbial Endophytes-Mediated Biofertilization and Biostimulation
2.3. Microbial Endophytes-Induced Stress Tolerance/Resistance
2.4. Endophytes for Management of Postharvest Decays
2.5. Limitations of Using Microbial Endophytes and Future Prospects
3. Potential Uses of Nanomaterials in Horticultural Production
3.1. Nanocapsules
3.2. Nanotubes
3.3. Metal-Based Nanoparticles
3.4. Limitations and Future Prospects of NMs’ Use in Horticulure
4. Novel Class of Phytohormones Strigolactones: Perspectives of Their Application in Horticulture
4.1. Multidirectional SL Regulation of Shoot and Root Architecture
4.2. SL-Regulated Plant Interaction to Biotic Stimuli
4.3. Regulation by SLs of the Plant Tolerance to Abiotic Stresses
5. Controlled Environment Horticulture Using Artificial Light
5.1. Fast Development in Controlled Environment Horticulture
5.2. Light as the Most Challenging Environmental Issue in CEH
5.3. Importance of Lighting Strategy in CEH
6. CRISPR Technology in Horticulture
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. 2017. Available online: https://www.fao.org/publications/card/en/c/37388c4e-22fc-48bc-b8f2-453f7b954b5d (accessed on 29 June 2022).
- Food and Agriculture Organization of the United Nations. 2022. Available online: https://www.fao.org/publications/card/en/c/CB9910EN (accessed on 14 August 2022).
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd ed.; Elsevier Academic Press: San Diego, CA, USA, 2019; p. 516. [Google Scholar]
- Lobell, D.B.; Gourdji, S.M. The influence of climate change on global crop productivity. Plant Physiol. 2012, 160, 1686–1697. [Google Scholar] [CrossRef] [PubMed]
- Aliniaeifard, S.; Van Meeteren, U. Natural genetic variation in stomatal response can help to increase acclimation of plants to dried environments. Acta Hortic. 2016, 1190, 71–76. [Google Scholar] [CrossRef]
- Kalhor, M.S.; Aliniaeifard, S.; Seif, M.; Asayesh, E.J.; Bernard, F.; Hassani, B.; Li, T. Enhanced salt tolerance and photosynthetic performance: Implication of ɤ-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol. Biochem. 2018, 130, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Shomali, A.; Aliniaeifard, S. Overview of signal transduction in plants under salt and drought stresses. Salt and drought stress tolerance in plants. In Signaling Networks and Adaptive Mechanisms; Hasanuzzaman, M., Tanveer, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 231–258. [Google Scholar]
- Sousaraei, N.; Mashayekhi, K.; Mousavizadeh, S.J.; Akbarpour, V.; Medina, J.; Aliniaeifard, S. Screening of tomato landraces for drought tolerance based on growth and chlorophyll fluorescence analyses. Hortic. Environ. Biotechnol. 2021, 62, 521–535. [Google Scholar] [CrossRef]
- Sarraf, M.; Vishwakarma, K.; Kumar, V.; Arif, N.; Das, S.; Johnson, R.; Janeeshma, E.; Puthur, J.T.; Aliniaeifard, S.; Chauhan, D.K. Metal/metalloid-based nanomaterials for plant abiotic stress tolerance: An overview of the mechanisms. Plants 2022, 11, 316. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Niknam, V.; Aliniaeifard, S.; Didaran, F.; Tsaniklidis, G.; Fanourakis, D.; Teymoorzadeh, M.; Mousavi, S.H.; Bosacchi, M.; Li, T. The regulatory role of γ-Aminobutyric acid in chickpea plants depends on drought tolerance and water scarcity level. Sci. Rep. 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Tian, H.; Lu, C.; Pan, S.; Yang, J.; Miao, R.; Ren, W.; Yu, Q.; Fu, B.; Jin, F.-F.; Lu, Y. Optimizing resource use efficiencies in the food–energy–water nexus for sustainable agriculture: From conceptual model to decision support system. Curr. Opin. Environ. Sustain. 2018, 33, 104–113. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Olmo, R.; Wetzels, S.U.; Armanhi, J.S.L.; Arruda, P.; Berg, G.; Cernava, T.; Cotter, P.D.; Araujo, S.C.; de Souza, R.S.C.; Ferrocino, I.; et al. Microbiome research as an effective driver of success stories in agrifood systems—A selection of case studies. Front. Microbiol. 2022, 13, 834622. [Google Scholar] [CrossRef]
- Glick, B.R.; Gamalero, E. Recent developments in the study of plant microbiomes. Microorganisms 2021, 9, 1533. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Compant, S.; Cambon, M.C.; Vacher, C.; Mitter, B.; Samad, A.; Sessitsch, A. The plant endosphere world–bacterial life within plants. Environ. Microbiol. 2021, 23, 1812–1829. [Google Scholar] [CrossRef]
- Carro-Huerga, G.; Compant, S.; Gorfer, M.; Cardoza, R.E.; Schmoll, M.; Gutiérrez, S.; Casquero, P.A. Colonization of Vitis vinifera L. by the endophyte Trichoderma sp. strain T154: Biocontrol activity against Phaeoacremonium minimum. Front. Plant Sci. 2020, 11, 1170. [Google Scholar] [CrossRef]
- Mei, C.; Amaradasa, B.S.; Chretien, R.L.; Liu, D.; Snead, G.; Samtani, J.B.; Lowman, S. A Potential application of endophytic bacteria in strawberry production. Horticulturae 2021, 7, 504. [Google Scholar] [CrossRef]
- Chu, L.L.; Bae, H. Bacterial endophytes from ginseng and their biotechnological application. J. Ginseng Res. 2022, 46, 1–10. [Google Scholar] [CrossRef]
- Farouk, S.; AL-Huqail, A.A.; El-Gamal, S.M.A. Improvement of phytopharmaceutical and alkaloid production in periwinkle plants by endophyte and abiotic elicitors. Horticulturae 2022, 8, 237. [Google Scholar] [CrossRef]
- Szymańska, S.; Tyburski, J.; Piernik, A.; Sikora, M.; Mazur, J.; Katarzyna, H. Raising beet tolerance to salinity through bioaugmentation with halotolerant endophytes. Agronomy 2020, 10, 1571. [Google Scholar] [CrossRef]
- Neelipally, R.T.K.R.; Anoruo, A.O.; Nelson, S. Effect of Co-inoculation of Bradyrhizobium and Trichoderma on growth, development, and yield of Arachis hypogaea L. (peanut). Agronomy 2020, 10, 1415. [Google Scholar] [CrossRef]
- Kaushal, M. Microbes in cahoots with plants: MIST to hit the jackpot of agricultural productivity during drought. Int. J. Mol. Sci. 2019, 20, 1769. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Malfanova, N.; Kamilova, F.; Validov, S.; Shcherbakov, A.; Chebotar, V.; Tikhonovich, I.; Lugtenberg, B. Characterization of Bacillus subtilis HC8, a novel plant-beneficial endophytic strain from giant hogweed. Microb. Biotechnol. 2011, 4, 523–532. [Google Scholar] [CrossRef]
- Malfanova, N.; Lugtenberg, B.J.J.; Berg, G. Bacterial endophytes: Who and where, and what are they doing there? In Molecular Microbial Ecology of the Rhizosphere; De Bruijned, F.J., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 393–403. [Google Scholar]
- Khan, A.A.; Wang, T.; Hussain, T.; Ali, F.; Shi, F.; Latef, A.A.H.A.; Ali, O.M.; Hayat, K.; Mehmood, S. Halotolerant-Koccuria rhizophila (14asp)-induced amendment of salt stress in pea plants by limiting Na+ uptake and elevating production of antioxidants. Agronomy 2021, 11, 1907. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Wang, Y.; Zhang, J.; Wan, S.; Huang, Y.; Yun, T.; Xie, J.; Wang, W. Biocontrol potential of endophytic Streptomyces malaysiensis 8ZJF-21 from medicinal plant against banana Fusarium wilt caused by Fusarium oxysporum f. sp. cubense tropical race 4. Front. Plant Sci. 2022, 11, 874819. [Google Scholar] [CrossRef]
- Nifakos, K.; Tsalgatidou, P.C.; Thomloudi, E.E.; Skagia, A.; Kotopoulis, D.; Baira, E.; Delis, C.; Papadimitriou, K.; Markellou, E.; Venieraki, A.; et al. Genomic analysis and secondary metabolites production of the endophytic Bacillus velezensis Bvel1: A biocontrol agent against Botrytis cinerea causing Bunch Rot in post-harvest table grapes. Plants 2021, 10, 1716. [Google Scholar] [CrossRef]
- Feng, B.; Chen, D.; Jin, R.; Li, E.; Li, P. Bioactivities evaluation of an endophytic bacterial strain Bacillus velezensis JRX-YG39 inhabiting wild grape. BMC Microbiol. 2022, 22, 170. [Google Scholar] [CrossRef]
- Lastochkina, O.; Seifi Kalhor, M.; Aliniaeifard, S.; Baymiev, A.; Pusenkova, L.; Garipova, S.; Kulabuhova, D.; Maksimov, I. Bacillus spp.: Efficient biotic strategy to control postharvest diseases of fruits and vegetables. Plants 2019, 8, 97. [Google Scholar] [CrossRef]
- Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Rumyantsev, S.D.; Cherepanova, E.A.; Alekseev, V.Y.; Sarvarova, E.R.; Kasimova, A.R.; Maksimov, I.V. By modulating the hormonal balance and ribonuclease activity of tomato plants Bacillus subtilis induces defense response against potato Virus X and potato Virus Y. BioMolecules 2022, 12, 288. [Google Scholar] [CrossRef]
- Rashad, Y.M.; Abdalla, S.A.; Sleem, M.M. Endophytic Bacillus subtilis SR22 triggers defense responses in tomato against rhizoctonia root rot. Plants 2022, 11, 2051. [Google Scholar] [CrossRef]
- Lastochkina, O. Bacillus subtilis-mediated abiotic stress tolerance in plants. In Bacilli and AgroBiotechnology: Phytostimulation and Biocontrol; Islam, M., Rahman, M., Pandey, P., Boehme, M., Haesaert, G., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 97–133. [Google Scholar]
- Lastochkina, O.V. Adaptation and tolerance of wheat plants to drought mediated by natural growth regulators Bacillus spp.: Mechanisms and practical importance (review). Sel’skokhozyaistvennaya Biol. [Agric. Biol.] 2021, 56, 843–867. [Google Scholar] [CrossRef]
- Steinwand, M.A.; Pamela, C.R. Crop biotechnology and the future of food. Nat. Food 2020, 1, 273–283. [Google Scholar] [CrossRef]
- Traxler, G. The GMO experience in North and South America. Int. J. Tech. Glob. 2006, 2, 46–64. [Google Scholar] [CrossRef]
- Bisht, D.S.; Bhatia, V.; Bhattacharya, R. Improving plant-resistance to insect-pests and pathogens: The new opportunities through targeted genome editing. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2019; Volume 96, pp. 65–76. [Google Scholar]
- Rana, R.A.; Siddiqui, M.N.; Skalicky, M.; Brestic, M.; Hossain, A.; Kayesh, E.; Popov, M.; Hejnak, V.; Gupta, D.R.; Mahmud, N.U.; et al. Prospects of nanotechnology in improving the productivity and quality of horticultural crops. Horticulturae 2021, 7, 332. [Google Scholar] [CrossRef]
- Nakandala, N.D.U.S.; Ranaweera, L.T. Strigolactone, a novel hormone with essential functions in planta possesses a significant value as a cancer therapeutic agent: A review. JTVA 2020, 1, 106–153. [Google Scholar]
- Raza, A.; Javed, R.; Zahid, Z.; Sharif, R.; Hafeez, M.B.; Ghouri, M.Z.; Siddiqui, M. Strigolactones for sustainable plant growth and production under adverse environmental conditions. In Plant Performance Under Environmental Stress; Husen, A., Ed.; Springer: Cham, Switzerland, 2021; pp. 129–166. [Google Scholar]
- Avgoustaki, D.D.; Xydis, G. Indoor vertical farming in the urban nexus context: Business growth and resource savings. Sustainability 2020, 12, 1965. [Google Scholar] [CrossRef]
- Jin, W.; Formiga Lopez, D.; Heuvelink, E.; Marcelis, L.F. Light use efficiency of lettuce cultivation in vertical farms compared with greenhouse and field. Food Energy Secur. 2022, e391. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, M.; Bhandari, B. Nanotechnology—A shelf life extension strategy for fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2020, 60, 1706–1721. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: Influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Sci. Total Environ. 2020, 738, 140240. [Google Scholar] [CrossRef]
- Pandey, A.; Sharma, M.; Pandey, G.K. Emerging roles of strigolactones in plant responses to stress and development. Front. Plant Sci. 2016, 7, 434. [Google Scholar] [CrossRef]
- Dor, E.; Plakhine, D.; Joel, D.M.; Larose, H.; Westwood, J.H.; Smirnov, E.; Ziadna, H.; Hershenhorn, J. A new race of sunflower broomrape (Orobanche cumana) with a wider host range due to changes in seed response to strigolactones. Weed Sci. 2020, 68, 134–142. [Google Scholar] [CrossRef]
- Omoarelojie, L.O.; Kulkarn, M.G. Strigolactone analog (rac-GR24) enhances chilling tolerance in mung bean seedlings. South Afr. J. Bot. 2021, 140, 173–181. [Google Scholar] [CrossRef]
- Zhang, X.H.; Ma, C. GR24-mediated enhancement of salt tolerance and roles of H2O2 and Ca2+ in regulating this enhancement in cucumber. J. Plant Physiol. 2022, 270, 153640. [Google Scholar] [CrossRef]
- Graamans, L.; Baeza, E.; Van Den Dobbelsteen, A.; Tsafaras, I.; Stanghellini, C. Plant factories versus greenhouses: Comparison of resource use efficiency. Agric. Syst. 2018, 160, 31–43. [Google Scholar] [CrossRef]
- Moosavi-Nezhad, M.; Salehi, R.; Aliniaeifard, S.; Winans, K.S.; Nabavi-Pelesaraei, A. An analysis of energy use and economic and environmental impacts in conventional tunnel and LED-equipped vertical systems in healing and acclimatization of grafted watermelon seedlings. J. Clean. Prod. 2022, 361, 132069. [Google Scholar] [CrossRef]
- Marcelis, L.F.; Costa, J.M.; Heuvelink, E. Achieving sustainable greenhouse production: Present status, recent advances and future developments. In Achieving Sustainable Greenhouse Cultivation; Marcelis, L., Heuvelink, E., Eds.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2019; pp. 1–14. [Google Scholar]
- Esmaili, M.; Aliniaeifard, S.; Mashal, M.; Ghorbanzadeh, P.; Seif, M.; Gavilan, M.U.; Carrillo, F.F.; Lastochkina, O.; Tao, L. CO2 enrichment and increasing light intensity till a threshold level, enhance growth and water use efficiency of lettuce plants in controlled environment. Not. Bot. Horti. Agrobot. Cluj Napoca 2020, 48, 2244–2262. [Google Scholar] [CrossRef]
- Esmaili, M.; Aliniaeifard, S.; Mashal, M.; Vakilian, K.A.; Ghorbanzadeh, P.; Azadegan, B.; Seif, M.; Didaran, F. Assessment of adaptive neuro-fuzzy inference system (ANFIS) to predict production and water productivity of lettuce in response to different light intensities and CO2 concentrations. Agric. Water Manag. 2021, 258, 107201. [Google Scholar] [CrossRef]
- Vasileva, E.N.; Akhtemova, G.A.; Zhukov, V.A.; Tikhonovich, I.A. Endophytic microorganisms in fundamental research and agriculture. Ecol. Genet. 2019, 17, 19–32. [Google Scholar] [CrossRef]
- Glick, B.R. Beneficial Plant-Bacterial Interactions, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–383. [Google Scholar]
- Gamalero, E.; Bona, E.; Glick, B.R. Current techniques to study beneficial plant-microbe interactions. Microorganisms 2022, 10, 1380. [Google Scholar] [CrossRef]
- Baez-Rogelio, A.; Morales-García, Y.E.; Quintero-Hernández, V.; Muñoz-Rojas, J. Next generation of microbial inoculants for agriculture and bioremediation. Microb. Biotechnol. 2017, 10, 19–21. [Google Scholar] [CrossRef]
- Ma, Y. Beneficial bacteria for disease suppression and plant growth promotion. In Plant-Microbe Interactions in Agroecological Perspectives; Singh, D., Singh, H., Prabha, R., Eds.; Springer: Singapore, 2017; pp. 513–529. [Google Scholar]
- Lastochkina, O.; Baymiev, A.; Shayahmetova, A.; Garshina, D.; Koryakov, I.; Shpirnaya, I.; Pusenkova, L.; Mardanshin, I.; Kasnak, C.; Palamutoglu, R. Effects of endophytic Bacillus subtilis and salicylic acid on postharvest diseases (Phytophthora infestans, Fusarium oxysporum) development in stored potato tubers. Plants 2020, 9, 76. [Google Scholar] [CrossRef]
- Tran, T.P.H.; Wang, S.-L.; Nguyen, V.B.; Tran, D.M.; Nguyen, D.S.; Nguyen, A.D. Study of novel endophytic bacteria for biocontrol of black pepper root-knot nematodes in the central highlands of Vietnam. Agronomy 2019, 9, 714. [Google Scholar] [CrossRef]
- Maksimov, I.V.; Blagova, D.K.; Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Cherepanova, E.A.; Sarvarova, S.D.; Rumyantsev, V.; Alekseev, Y.; Khayrullin, R.M. Recombinant Bacillus subtilis 26DCryChS line with gene Btcry1Ia encoding Cry1Ia toxin from Bacillus thuringiensis promotes integrated wheat defense against pathogen Stagonospora nodorum Berk. and greenbug Schizaphis graminum Rond. Biol. Control 2020, 144, 104242. [Google Scholar] [CrossRef]
- Miljakovic, D.; Marinkovic, J.; Balesevic-Tubic, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Cantoro, R.; Palazzini, J.M.; Yerkovich, N.; Miralles, D.J.; Chulze, S.N. Bacillus velezensis RC 218 as a biocontrol agent against Fusarium graminearum: Effect on penetration, growth and TRI5 expression in wheat spikes. Biol. Control 2021, 66, 259–270. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; AL-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.J. What is there in seeds? Vertically transmitted endophytic resources for sustainable improvement in plant growth. Front. Plant Sci. 2018, 9, 24. [Google Scholar] [CrossRef]
- Garipova, S.R. Prospects of using endophytic bacteria for bioremediation of arable soils polluted by residual amounts of pesticides and xenobiotics. Uspekhi. Sovremennoi. Biologii. 2014, 134, 35–47. (In Russia) [Google Scholar]
- Chebotar, V.K.; Malfanova, N.V.; Shcherbakov, A.V.; Ahtemova, G.A.; Borisov, A.Y.; Lugtenberg, B.; Tikhonovich, I.A. Endophytic bacteria in microbial preparations that improve plant development (review). Appl. Biochem. Microbiol. 2015, 51, 271–277. [Google Scholar] [CrossRef]
- Iqbal, A.; Arshad, M.; Hashmi, I.; Karthikeyan, R.; Gentry, T.J.; Schwab, A.P. Biodegradation of phenol and benzene by endophytic bacterial strains isolated from refinery wastewater-fed Cannabis sativa. Environ. Technol. 2018, 39, 1705–1714. [Google Scholar] [CrossRef]
- Hussain, A.; Kamran, M.A.; Javed, M.T.; Hayat, K.; Farooq, M.A.; Ali, N.; Ali, M.; Manghwar, H.; Jan, F. Individual and combinatorial application of Kocuria rhizophila and citric acid on phytoextraction of multi-metal contaminated soils by Glycine max L. Environ. Exp. Bot. 2019, 159, 23–33. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, Y.; Wang, P.; Zhu, L.; Zheng, J.; Li, R.; Ruan, L.; Peng, D.; Sun, M. Complete genome sequence of Bacillus subtilis BSn5, an endophytic bacterium of Amorphophallus konjac with antimicrobial activity for the plant pathogen Erwinia carotovora carotovora. J. Bacteriol. 2011, 193, 2070–2071. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, F.O.; Monteiro, R.A.; Wassem, R.; Cruz, L.M.; Ayub, R.A.; Colauto, N.B.; Fernandez, M.A.; Fungaro, M.H.P.; Grisard, E.C.; Hungria, M.; et al. Genome of Herbaspirillum seropedicae strain SmR1, a specialized diazotrophic endophyte of tropical grasses. PLoS Genet. 2011, 7, e1002064. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.M.; Marina, M.; Pieckenstain, F.L. The communities of tomato (Solanum lycopersicum L.) leaf endophytic bacteria, analyzed by 16S-ribosomal RNA gene pyrosequencing. FEMS Microbiol. Lett. 2014, 351, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, H.; Zhang, T.; Lou, K. Illumina-based analysis of endophytic bacterial diversity and space-time dynamics in sugar beet on the north slope of Tianshan mountain. Appl. Microbiol. Biotechnol. 2014, 98, 6375–6385. [Google Scholar] [CrossRef]
- Žiarovská, J.; Medo, J.; Kysel, M.; Zamiešková, L.; Kačániová, M. Endophytic bacterial microbiome diversity in early developmental stage plant tissues of wheat varieties. Plants 2020, 9, 266. [Google Scholar] [CrossRef]
- Hallmann, J.; Quadt-Hallmann, A.; Mahaffee, W.F.; Kloepper, J.W. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997, 43, 895–914. [Google Scholar] [CrossRef]
- Compant, S.; Mitter, B.; Colli-Mull, J.G.; Gangl, H.; Sessitsch, A. Endophytes of grapevine flowers, berries, and seeds: Identification of cultivable bacteria, comparison with other plant parts, and visualization of niches of colonization. Microb. Ecol. 2011, 62, 188–197. [Google Scholar] [CrossRef]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- Frank, A.C.; Saldierna Guzmán, J.P.; Shay, J.E. Transmission of bacterial endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.N.; Yadav, N.; Dhaliwal, H.S.; Saxena, A.K. Endophytic microbes: Biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie Van Leeuwenhoek 2020, 113, 1075–1107. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Ali, M.S.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K.H. Bacillus velezensis: A valuable member of bioactive molecules within plant microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef]
- Lastochkina, O.; Pusenkova, L.; Garshina, D.; Kasnak, C.; Palamutoglu, R.; Shpirnaya, I.; Mardanshin, I.; Maksimov, I. Improving the biocontrol potential of endophytic bacteria Bacillus subtilis with salicylic acid against Phytophthora infestans-caused postharvest potato tuber late blight and impact on stored tubers quality. Horticulturae 2022, 8, 117. [Google Scholar] [CrossRef]
- González, M.F.; Ali, M.S.; Baek, K.-H. Endophyte Bacillus velezensis isolated from Citrus spp. controls streptomycin-resistant Xanthomonas citri subsp. citri that causes citrus bacterial canker. Agronomy 2019, 9, 470. [Google Scholar]
- Chatterjee, P.; Samaddar, S.; Anandham, R.; Kang, Y.; Kim, K.; Selvakumar, G.; Sa, T. Beneficial soil bacterium Pseudomonas frederiksbergensis OS261 augments salt tolerance and promotes red pepper plant growth. Front. Plant Sci. 2017, 8, 705. [Google Scholar] [CrossRef]
- Del Carmen Orozco-Mosqueda, M.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant growth-promoting bacteria as bioinoculants: Attributes and challenges for sustainable crop improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Lastochkina, O.; Garshina, D.; Allagulova, C.; Pusenkova, L.; Garipova, S.; Maslennikova, D.; Fedorova, K.; Shpirnaya, I.; Ibragimov, A.; Koryakov, I.; et al. Potential aspects of plant growth promoting bacteria to improve horticultural crop production. Int. J. Hort. Sci. Technol. 2021, 8, 103–122. [Google Scholar]
- Mingot-Ureta, C.; Lopez-Moya, F.; Lopez-Llorca, L.V. Isolates of the nematophagous fungus Pochonia chlamydosporia are endophytic in banana roots and promote plant growth. Agronomy 2020, 10, 1299. [Google Scholar] [CrossRef]
- Vukelić, I.D.; Prokić, L.T.; Racić, G.M.; Pešić, M.B.; Bojović, M.M.; Sierka, E.M.; Kalaji, H.M.; Panković, D.M. Effects of Trichoderma harzianum on photosynthetic characteristics and fruit quality of tomato plants. Int. J. Mol. Sci. 2021, 22, 6961. [Google Scholar] [CrossRef]
- Acuña-Rodríguez, I.S.; Ballesteros, G.I.; Atala, C.; Gundel, P.E.; Molina-Montenegro, M.A. Hardening blueberry plants to face drought and cold events by the application of fungal endophytes. Agronomy 2022, 12, 1000. [Google Scholar] [CrossRef]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006, 23, 753–771. [Google Scholar] [CrossRef]
- Martínez-Hidalgo, P.; Flores-Félix, J.D.; Sánchez-Juanes, F.; Rivas, R.; Mateos, P.F.; Santa Regina, I.; Peix, Á.; Martínez-Molina, E.; Igual, J.M.; Velázquez, E. Identification of canola roots endophytic bacteria and analysis of their potential as biofertilizers for canola crops with special emphasis on sporulating bacteria. Agronomy 2021, 11, 1796. [Google Scholar] [CrossRef]
- Hwang, H.H.; Chien, P.R.; Huang, F.C.; Hung, S.H.; Kuo, C.H.; Deng, W.L.; Chiang, E.I.; Huang, C.C. A plant endophytic bacterium, Burkholderia seminalis strain 869t2, promotes plant growth in arabidopsis, pak choi, chinese amaranth, lettuces, and other vegetables. Microorganisms 2021, 9, 1703. [Google Scholar] [CrossRef]
- Vannucchi, F.; Imperato, V.; Saran, A.; Staykov, S.; D’Haen, J.; Sebastiani, L.; Vangronsveld, J.; Thijs, S. Inoculated seed endophytes modify the poplar responses to trace elements in polluted soil. Agronomy 2021, 11, 1987. [Google Scholar] [CrossRef]
- González, V.; Armijos, E.; Garcés-Claver, A. Fungal endophytes as biocontrol agents against the main soil-borne diseases of melon and watermelon in Spain. Agronomy 2020, 10, 820. [Google Scholar] [CrossRef]
- Defez, R.; Andreozzi, A.; Bianco, C. The overproduction of indole-3-acetic acid (IAA) in endophytes upregulates nitrogen fixation in both bacterial cultures and inoculated rice plants. Microb. Ecol. 2017, 74, 441–452. [Google Scholar] [CrossRef]
- Pandey, P.K.; Singh, M.C.; Singh, S.S.A.K.; Kumar, M.; Pathak, M.; Shakywar, R.C.; Pandey, A.K. Inside the plants: Endophytic bacteria and their functional attributes for plant growth promotion. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 11–21. [Google Scholar] [CrossRef]
- Masood, S.; Zhao, X.Q.; Shen, R.F. Bacillus pumilus promotes the growth and nitrogen uptake of tomato plants under nitrogen fertilization. Sci. Hortic. 2020, 272, 109581. [Google Scholar]
- Asad, S.A.; Farooq, M.; Afzal, A.; West, H. Integrated phytobial heavy metal remediation strategies for a sustainable clean environment-a review. Chemosphere 2019, 217, 925–941. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Babalola, O.O.; Emmanuel, O.C.; Adeleke, B.S.; Odelade, K.A.; Nwachukwu, B.C.; Ayiti, O.E.; Adegboyega, T.T.; Igiehon, N.O. Rhizosphere microbiome cooperations: Strategies for sustainable crop production. Curr. Microbiol. 2021, 78, 1069–1085. [Google Scholar] [CrossRef]
- Sessitsch, A.; Hardoim, P.; Döring, J.; Weilharter, A.; Krause, A.; Woyke, T.; Mitter, B.; Hauberg-Lotte, L.; Friedrich, F.; Rahalkar, M.; et al. Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. MPMI 2012, 25, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Raji, M.; Thangavelu, M. Isolation and screening of potassium solubilizing bacteria from saxicolous habitat and their impact on tomato growth in different soil types. Arch. Microbiol. 2021, 203, 3147–3161. [Google Scholar] [CrossRef]
- Shore, S.; Sathisha, G. Screening of endophytic colonizing bacteria for cytokinin-like compounds: Crude cell-free broth of endophytic colonizing bacteria is unsuitable in cucumber cotyledon bioassay. World J. Agric. Sci. 2010, 6, 345–352. [Google Scholar]
- Khan, M.; Asaf, S.; Khan, A.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.M.; Lee, I.J. Plant growth-promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant Biol. 2020, 22, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.-Y.; Dodd, I.C.; Chen, J.E.; Phang, S.-M.; Chin, C.F.; Yow, Y.-Y.; Ratnayeke, S. Regulation of algal and cyanobacterial auxin production, physiology, and application in agriculture: An overview. J. Appl. Phycol. 2021, 33, 2995–3023. [Google Scholar] [CrossRef]
- Moreno-Gavíra, A.; Diánez, F.; Sánchez-Montesinos, B.; Santos, M. Paecilomyces variotii as a plant-growth promoter in horticulture. Agronomy 2020, 10, 597. [Google Scholar] [CrossRef]
- Kuklinski-Sorbal, J.; Araujo, W.L.; Mendes, R.; Geraldi, I.O.; Pizzirani-Kleiner, A.A.; Azevedo, J.L. Isolation and characterisation of soybean-associated bacteria and their potential for plant growth promotion. Environ. Microbiol. 2004, 6, 1244–1251. [Google Scholar] [CrossRef]
- Numponsak, T.; Kumla, J.; Suwannarach, N.; Matsui, K.; Lumyong, S. Biosynthetic pathway and optimal conditions for the production of indole-3-acetic acid by an endophytic fungus, Colletotrichum fructicola CMU-A109. PLoS ONE 2018, 13, 17. [Google Scholar] [CrossRef]
- Baron, C.B.; Rigobelo, E.C. Endophytic fungi: A tool for plant growth promotion and sustainable agriculture. Mycology 2022, 13, 39–55. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Bhore, S.J.; Ravichantar, N.; Loh, C.Y. Screening of endophytic bacteria isolated from leaves of Sambung Nyawa [Gynura procumbens (Lour.) Merr.] for cytokinin-like compounds. Bioinformation 2010, 5, 191. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; Martínez-Montiel, N.; García-Sánchez, J.; Pérez-y-Terrón, R.; Martínez-Contreras, R.D. Gibberellin biosynthesis and metabolism: A convergent route for plants, fungi and bacteria. Microbiol. Res. 2018, 208, 85–98. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Fadiji, A.E.; Ayilara, M.S.; Igiehon, O.N.; Nwachukwu, B.C.; Babalola, O.O. Strategies to enhance the use of endophytes as bioinoculants in agriculture. Horticulturae 2022, 8, 498. [Google Scholar] [CrossRef]
- Bömke, C.; Rojas, M.C.; Gong, F.; Hedden, P.; Tudzynski, B. Isolation and characterization of the gibberellin biosynthetic gene cluster in Sphaceloma manihoticola. Appl. Environ. Microbiol. 2008, 74, 5325–5339. [Google Scholar] [CrossRef]
- Khan, S.A.; Hamayun, M.; Yoon, H.; Kim, H.Y.; Suh, S.J.; Hwang, S.K.; Kim, J.M.; Lee, I.J.; Choo, Y.S.; Yoon, U.H.; et al. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 2008, 8, 231. [Google Scholar] [CrossRef]
- Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 2015, 35, 62–74. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, S.A.; Ahmad, N.; Tang, D.S.; Kang, S.M.; Na, C.I.; Sohn, E.Y.; Hwang, Y.H.; Shin, D.H.; Lee, B.H.; et al. Cladosporium sphaerospermum as a new plant growth-promoting endophyte from the roots of Glycine max (L.). Merr. World J. Microbiol. Biotechnol. 2009, 25, 627–632. [Google Scholar]
- Hamayun, M.; Khan, S.A.; Iqbal, I.; Na, C.I.; Khan, A.L.; Hwang, Y.H.; Lee, B.H.; Lee, I.J. Chrysosporium pseudomerdarium produces gibberellins and promotes plant growth. J. Microbiol. 2009, 47, 425–430. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, S.A.; Khan, A.L.; Rehman, G.; Kim, Y.H.; Iqbal, I.; Hussain, J.; Sohn, E.Y.; Lee, I.J. Gibberellin production and plant growth promotion from pure cultures of Cladosporium sp. MH-6 isolated from cucumber (Cucumis sativus L.). Mycologia 2010, 102, 989–995. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Jung, H.Y.; Lee, J.H.; Lee, I.J. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: An example of Paecilomyces formosus LHL10. BMC Microbiol. 2012, 12, 3. [Google Scholar] [CrossRef]
- Khan, A.L.; Shinwari, Z.K.; Kim, Y.; Waqas, M.; Hamayun, M.; Kamran, M.; Lee, I.J. Role of endophyte Chaetomium globosum LK4 in growth of Capisum annuum by production of gibberellins and indole acetic acid. Pak. J. Bot. 2012, 44, 1601–1607. [Google Scholar]
- Wang, H.; Zhang, R.; Duan, Y.; Jiang, W.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. The endophytic strain Trichoderma asperellum 6S-2: An efficient biocontrol agent against apple replant disease in China and a potential plant-growth-promoting fungus. J. Fungi 2021, 7, 1050. [Google Scholar] [CrossRef]
- Baron, N.C.; de Souza Pollo, A.; Rigobelo, E.C. Purpureocillium lilacinum and Metarhizium marquandii as plant growth-promoting fungi. PeerJ 2020, 8, e9005. [Google Scholar] [CrossRef]
- Cheng, X.F.; Xie, M.M.; Li, Y.; Liu, B.Y.; Liu, C.Y.; Wu, Q.S.; Kuča, K. Effects of field inoculation with arbuscular mycorrhizal fungi and endophytic fungi on fruit quality and soil properties of Newhall navel orange. Appl. Soil Ecol. 2022, 170, 104308. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant growth promoting and biocontrol activity of Streptomyces as endophytes. Int. J. Mol. Sci. 2018, 19, E952. [Google Scholar] [CrossRef]
- Berg, G.; Krechel, A.; Ditz, M.; Sikora, R.A.; Ulrich, A.; Hallmann, J. Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi. FEMS Microbiol. Ecol. 2005, 51, 215–229. [Google Scholar] [CrossRef]
- Weilharter, A.; Mitter, B.; Shin, M.V.; Chain, P.S.; Nowak, J.; Sessitsch, A. Complete genome sequence of the plant growth-promoting endophyte Burkholderia phytofirmans strain PsJN. J. Bacteriol. 2011, 193, 3383–3384. [Google Scholar] [CrossRef]
- Chowdappa, P.; Mohan Kumar, S.P.; Jyothi Lakshmi, M.; Upreti, K.K. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum. Biol. Control 2013, 65, 109–117. [Google Scholar] [CrossRef]
- Khan, A.L.; Waqas, M.; Kang, S.; Al-Harrasi, A.; Hussain, J.; Al-Rawahi, A.; Al-Khiziri, S.; Ullah, I.; Ali, L.; Jung, H.Y.; et al. Bacterial endophyte Sphingomonas LK11 produces gibberellins and IAA and promotes tomato plant growth. J. Microbiol. 2014, 52, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zhang, C.; Ye, Y.; Wen, J.; Wu, Y.; Wang, H.; Li, H.M.; Cai, S.X.; Cai, W.T.; Cheng, Z.Q.; et al. Beneficial traits of bacterial endophytes belonging to the core communities of the tomato root microbiome. Agric. Ecosyst. Environ. 2017, 247, 149–156. [Google Scholar] [CrossRef]
- Sefloo, N.G.; Wieczorek, K.; Steinkellner, S.; Hage-Ahmed, K. Serendipita species trigger cultivar-specific responses to Fusarium wilt in tomato. Agronomy 2019, 9, 595. [Google Scholar] [CrossRef] [PubMed]
- Giassi, V.; Kiritani, C.; Kupper, K.C. Bacteria as growth-promoting agents for citrus rootstocks. Microbiol. Res. 2016, 190, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.S.A.; Tozzi, J.P.L.; Terrasan, C.R.F.; Bettiol, W. Endophytic microorganisms from coffee tissues as plant growth promoters and biocontrol agents of coffee leaf rust. Biol. Control. 2012, 63, 62–67. [Google Scholar] [CrossRef]
- Haidar, R.; Roudet, J.; Bonnard, O.; Dufour, M.C.; Corio-Costet, M.F.; Fert, M.; Gautier, T.; Deschamps, A.; Fermaud, M. Screening and modes of action of antagonistic bacteria to control the fungal pathogen Phaeomoniella chlamydospora involved in grapevine. Microbiol. Res. 2016, 192, 172–184. [Google Scholar] [CrossRef]
- Ferrigo, D.; Causin, R.; Raiola, A. Effect of potential biocontrol agents selected among grapevine endophytes and commercial products on crown gall disease. Bio. Control 2017, 62, 821–833. [Google Scholar] [CrossRef]
- Baldan, E.; Nigris, S.; Populin, F.; Zottini, M.; Squartini, A.; Baldan, B. Identification of culturable bacterial endophyte community isolated from tissues of Vitis vinifera “Glera”. Plant Biosyst. 2014, 148, 508–516. [Google Scholar] [CrossRef]
- Thomas, P.; Sekhar, A.C. Live cell imaging reveals extensive intracellular cytoplasmic colonization of banana by normally non-cultivable endophytic bacteria. AoB Plants 2014, 6, plu002. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Shurigin, V.V.; Hashem, A.; Abd_Allah, E.F. Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front. Microbiol. 2017, 8, 1887. [Google Scholar] [CrossRef]
- Moura, G.G.D.; Barros, A.V.; Machado, F.; Martins, A.D.; Silva, C.M.D.; Durango, L.G.C.; Forim, M.; Alves, E.; Pasqual, M.; Doria, J. Endophytic bacteria from strawberry plants control gray mold in fruits via production of antifungal compounds against Botrytis cinerea L. Microbiol. Res. 2021, 251, 126793. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, R.; Yuan, Z.; Chen, P. Biological prevention and control of pitaya fruit canker disease using endophytic fungi isolated from papaya. Arch. Microbiol. 2021, 203, 4033–4040. [Google Scholar] [CrossRef]
- Sdiri, Y.; Lopes, T.; Rodrigues, N.; Silva, K.; Rodrigues, I.; Pereira, J.A.; Baptista, P. Biocontrol ability and production of volatile organic compounds as a potential mechanism of action of olive endophytes against Colletotrichum acutatum. Microorganisms 2022, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Huang, L.; Liu, Q.; Xu, S.; Wen, Z.; Qin, S.; Li, T.; Feng, Y. Positive effects of applying endophytic bacteria in eggplant-Sedum intercropping system on Cd phytoremediation and vegetable production in cadmium polluted greenhouse. J. Environ. Sci. 2022, 115, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Díaz-González, S.; Marín, P.; Sánchez, R.; Arribas, C.; Kruse, J.; González-Melendi, P.; Brunner, F.; Sacristán, S. Mutualistic fungal endophyte Colletotrichum tofieldiae Ct0861 colonizes and increases growth and yield of maize and tomato plants. Agronomy 2020, 10, 1493. [Google Scholar] [CrossRef]
- Ngo, V.A.; Wang, S.-L.; Nguyen, V.B.; Doan, C.T.; Tran, T.N.; Tran, D.M.; Tran, T.D.; Nguyen, A.D. Phytophthora antagonism of endophytic bacteria isolated from roots of black pepper (Piper nigrum L.). Agronomy 2020, 10, 286. [Google Scholar] [CrossRef]
- Akbaba, M.; Ozaktan, H. Biocontrol of angular leaf spot disease and colonization of cucumber (Cucumis sativus L.) by endophytic bacteria. Egypt. J. Biol. Pest Control. 2018, 28, 14. [Google Scholar] [CrossRef]
- Grabka, R.; d’Entremont, T.W.; Adams, S.J.; Walker, A.K.; Tanney, J.B.; Abbasi, P.A.; Ali, S. Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Plants 2022, 11, 384. [Google Scholar] [CrossRef]
- Cherif, H.; Marasco, R.; Rolli, E.; Ferjani, R.; Fusi, M.; Soussi, A.; Mapelli, F.; Blilou, I.; Borin, S.; Boudabous, A.; et al. Oasis desert farming selects environment-specific date palm root endophytic communities and cultivable bacteria that promote resistance to drought. Environ. Microbiol. Rep. 2015, 7, 668–678. [Google Scholar] [CrossRef]
- Sorokan, A.; Benkovskaya, G.; Burkhanova, G.; Blagova, D.; Maksimov, I. Endophytic strain Bacillus subtilis 26DCryChS producing cry1ia toxin from Bacillus thuringiensis promotes multifaceted potato defense against Phytophthora infestans (Mont.) de Bary and pest Leptinotarsa decemlineata Say. Plants 2020, 9, 1115. [Google Scholar] [CrossRef]
- Rocha, F.Y.O.; Negrisoli, A.S.; Júnior, G.F.; de Matos, P.M.; Rossi, C.N.; Vidal, M.S.; Baldani, J.I. Endophytic Bacillus bacteria living in sugarcane plant tissues and Telchin licus licus Larvae (Drury) (Lepidoptera: Castniidae): The symbiosis that may open new paths in the biological control. Front. Microbiol. 2021, 12, 659965. [Google Scholar] [CrossRef]
- Azevedo, J.L.; Maccheroni, W.; Pereira, J.O.; De Araújo, W.L. Endophytic microorganisms: A review on insect control and recent advances on tropical plants. Electron. J. Biotechnol. 2000, 3, 40–65. [Google Scholar] [CrossRef]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef]
- Lastochkina, O.; Garshina, D.; Allagulova, C.; Fedorova, K.; Koryakov, I.; Vladimirova, A. Application of endophytic Bacillus subtilis and salicylic acid to improve wheat growth and tolerance under combined drought and Fusarium root rot stresses. Agronomy 2020, 10, 1343. [Google Scholar] [CrossRef]
- Wu, W.; Chen, W.; Liu, S.; Wu, J.; Zhu, Y.; Qin, L.; Zhu, B. Beneficial relationships between endophytic bacteria and medicinal plants. Front. Plant Sci. 2021, 12, 646146. [Google Scholar] [CrossRef]
- Wei, G.; Kloepper, J.W.; Tuzun, S. Induction of systemic resistance of cucumber to Colletotrichum orbiculare by select strains of plant growth-promoting rhizobacteria. Phytopathology 1991, 81, 1508–1512. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.M. Bacterial endophytes as elicitors of induced systemic resistance. In Microbial Root Endophytes Soil Biology; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 33–52. [Google Scholar]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Manohar Jebakumar, R.; Selvarajan, R. Biopriming of micropropagated banana plants at pre-or post-BBTV inoculation stage with rhizosphere and endophytic bacteria determines their ability to induce systemic resistance against BBTV in cultivar Grand Naine. Biocontrol. Sci. Tech 2018, 28, 1074–1090. [Google Scholar] [CrossRef]
- Nowak, J.; Shulaev, V. Priming for transplant stress resistance in in vitro propagation. In Vitro Cell. Dev. Biol. Plant 2003, 39, 107–124. [Google Scholar] [CrossRef]
- Garipova, S.R.; Irgalina, R.S.; Dmitrieva, D.F.; Kutueva, A.G. Evaluation of new strains of endophytic Bacilli and Rhizobia when inoculated of common bean Ufimskaya variety under South Ural. Dokl. Bashkirskogo Univ. 2016, 1, 705–710. (In Russia) [Google Scholar]
- Garipova, S.R.; Garifullina, D.V.; Markova, O.V. Bacterial endophyte associations of nodules increasing the productivity of legumes. Agrokhimiya 2010, 11, 50–58. (In Russia) [Google Scholar]
- Mercado-Blanco, J.; Lugtenberg, B. Biotechnological applications of bacterial endophytes. Curr. Biotechnol. 2014, 3, 60–75. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Karlidag, H.; Esitken, A.; Yildirim, E.; Donmez, M.F.; Turan, M. Effects of plant growth promoting bacteria on yield, growth, leaf water content, membrane permeability, and ionic composition of strawberry under saline conditions. J. Plant Nutr. 2010, 34, 34–45. [Google Scholar] [CrossRef]
- Karlidag, H.; Yildirim, E.; Turan, M.; Pehluvan, M.; Donmez, F. Plant growth-promoting rhizobacteria mitigate deleterious effects of salt stress on strawberry plants (Fragaria×ananassa). Hortscience 2013, 48, 563–567. [Google Scholar] [CrossRef]
- Martins, S.J.; Rocha, G.A.; de Melo, H.C.; de Castro Georg, R.; Ulhôa, C.J.; de Campos Dianese, É.; Dunlap, C.A. Plant-associated bacteria mitigate drought stress in soybean. Environ. Sci. Pollut. Res. 2018, 25, 13676–13686. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.K.; Dey, R.; Sherathia, D.N.; Devidayal Mangalassery, S.; Kumar, A.; Rupapara, R.B.; Mandaliya, M.; Rawal, P.; Bhadania, R.A.; Thomas, M.; et al. Alleviation of salinity stress in peanut by application of endophytic bacteria. Front. Microbiol. 2021, 12, 650771. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Aliniaeifard, S.; Garshina, D.; Garipova, S.; Pusenkova, L.; Allagulova, C.; Fedorova, K.; Baymiev, A.; Koryakov, I.; Sobhani, M. Seed priming with endophytic Bacillus subtilis strain-specifically improves growth of Phaseolus vulgaris plants under normal and salinity conditions and exerts anti-stress effect through induced lignin deposition in roots and decreased oxidative and osmotic damages. J. Plant Physiol. 2021, 263, 153462. [Google Scholar] [PubMed]
- Bacilio, M.; Moreno, M.; Bashan, Y. Mitigation of negative effects of progressive soil salinity gradients by application of humic acids and inoculation with Pseudomonas stutzeri in a salt-tolerant and a salt-susceptible pepper. Appl. Soil Ecol. 2016, 107, 394–404. [Google Scholar] [CrossRef]
- Kidd, P.S.; Álvarez, A.; Álvarez-López, V.; Cerdeira-Pérez, A.; Rodríguez-Garrido, B.; Prieto-Fernández, A.; Chalot, M. Beneficial traits of root endophytes and rhizobacteria associated with plants growing in phytomanaged soils with mixed trace metal-polycyclic aromatic hydrocarbon contamination. Chemosphere 2021, 277, 130272. [Google Scholar] [CrossRef]
- Ait, B.E.; Nowak, J.; Clement, C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, I strain PsJN. Appl. Environ. Microbiol. 2006, 72, 7246–7252. [Google Scholar]
- Fernandez, O.; Theocharis, A.; Bordiec, S.; Feil, R.; Jacquens, L.; Clement, C.; Fontaine, F.; Barka, E.A. Burkholderia phytofirmans PsJN acclimates grapevine to cold by modulating carbohydrate metabolism. MPMI 2012, 25, 496–504. [Google Scholar] [CrossRef]
- Theocharis, A.; Bordiec, S.; Fernandez, O.; Paquis, S.; DhondtCordelier, S.; Baillieul, F.; Clement, C.; Barka, E.A. Burkholderia phytofirmans PsJN primes Vitis vinifera L. and confers a better tolerance to low nonfreezing temperatures. MPMI 2012, 25, 241–249. [Google Scholar] [CrossRef]
- De Almeida Lopes, K.B.; Carpentieri-Pipolo, V.; Fira, D.; Balatti, P.A.; López, S.M.Y.; Oro, T.H.; Pagliosa, E.S.; Degrassi, G. Screening of bacterial endophytes as potential biocontrol agents against soybean diseases. J. Appl. Microbiol. 2018, 125, 1466–1481. [Google Scholar] [CrossRef]
- Cherepanova, E.A.; Galyautdinov, I.V.; Burkhanova, G.F.; Maksimov, I.V. isolation and identification of lipopeptides of Bacillus subtilis 26D. Appl. Biochem. Microbiol. 2021, 57, 636–642. [Google Scholar] [CrossRef]
- Li, Z.; Wen, W.; Qin, M.; He, Y.; Xu, D.; Li, L. Biosynthetic mechanisms of secondary metabolites promoted by the interaction between endophytes and plant hosts. Front. Microbiol. 2022, 13, 928967. [Google Scholar] [CrossRef]
- Sorokan, A.; Burkhanova, G.; Alekseev, V.; Maksimov, I. The influence of co-treatment with Bacillus thuringiensis B-5351 and salicylic acid on the resistance of potato plants to Phytophthora infestans (Mont.) de Bary. Tomsk. State Univ. J. Biol. 2021, 53, 109–130. [Google Scholar] [CrossRef]
- Pusenkova, L.I.; Il’yasova, E.Y.; Lastochkina, O.V.; Maksimov, I.V.; Leonova, S.A. Changes in the species composition of the rhizosphere and phyllosphere of sugar beet under the impact of biological preparations based on endophytic bacteria and their metabolites. Eurasian Soil Sci. 2016, 49, 1136–1144. [Google Scholar] [CrossRef]
- Lastochkina, O.V.; Pusenkova, L.I.; Il’yasova, E.Y.; Aliniaeifard, S. Effect of Bacillus subtilis based biologicals on physiological and biochemical parameters of sugar beet (Beta vulgaris L.) plants infected with Alternaria alternata. Agrobiology 2018, 53, 958–968. [Google Scholar]
- Taghavi, S.; van der Lelie, D.; Hoffman, A.; Zhang, Y.B.; Walla, M.D.; Vangronsveld, J.; Newman, L.; Monchyet, S. Genome sequence of the plant growth promoting endophytic bacterium Enterobacter 638. PLoS Genet. 2010, 6, e1000943. [Google Scholar] [CrossRef]
- Castillo, U.; Harper, J.K.; Strobel, G.A.; Sears, J.; Alesi, K.; Ford, E.; Lin, J.; Hunter, M.; Maranta, M.; Ge, H.; et al. Kakadumycins, novel antibiotics from Streptomyces sp. NRRL 30566, an endophyte of Grevillea pteridifolia. FEMS Microbiol. Lett. 2003, 224, 183–190. [Google Scholar] [CrossRef]
- Ezra, D.; Castillo, U.F.; Strobel, G.A.; Hess, W.M.; Porter, H.; Jensen, J.B.; Condron, M.A.M.; Teplow, D.B.; Sears, J.; Maranta, M.; et al. Coronamycins, peptide antibiotics produced by a verticillate Streptomyces (MSU-2110) endophytic on Monstera sp. Microbiology 2004, 150, 785–793. [Google Scholar] [CrossRef]
- Ding, L.; Maier, A.; Fiebig, H.H.; Linc, W.H.; Hertweck, C. A family of multicyclic indolosesquiterpenes from a bacterial endophyte. Org. Biomol. Chem. 2011, 9, 4029–4031. [Google Scholar] [CrossRef]
- Maksimov, I.V.; Abizgil’dina, R.R.; Pusenkova, L.I. Plant growth promoting rhizobacteria as alternative to chemical crop protectors from pathogens (review). Appl. Biochem. Microbiol. 2011, 47, 333–345. [Google Scholar] [CrossRef]
- Riley, M.A.; Wertz, J.E. Bacteriocins: Evolution, ecology, and application. Annu. Rev. Microbiol. 2002, 56, 117–137. [Google Scholar] [CrossRef]
- Ryan, R.P.; Ryan, D.J.; Sun, Y.C.; Li, F.M.; Wang, Y.; Dowling, D.N. An acquired efflux system is responsible for copper resistance in Xanthomonas strain IG-8 isolated from China. FEMS Microbiol. Lett. 2007, 268, 40–46. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef]
- Song, Q.Y.; Li, F.; Nan, Z.B.; Coulter, J.A.; Wei, W.J. Do Epichloë endophytes and their grass symbiosis only produce toxic alkaloids to insects and livestock? J. Agric. Food Chem. 2020, 68, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Tooker, J.F.; Giron, D. The evolution of endophagy in herbivorous insects. Front. Plant Sci. 2020, 11, 581816. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; Loper, J.E. Characterization of siderophore production by the biological control agent Enterobacter cloacae. MPMI 1994, 7, 440–448. [Google Scholar] [CrossRef]
- Verma, S. Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J. Biotechnol. 2001, 91, 127–141. [Google Scholar] [CrossRef]
- Pirttila, A.M.; Joensuu, P.; Pospiech, H.; Jalonen, J.; Hohtolaet, A. Bud endophytes of Scots pine produce adenine derivatives and other compounds that affect morphology and mitigate browning of callus cultures. Physiol. Plant 2004, 121, 305–312. [Google Scholar] [CrossRef]
- Eslahi, N.; Kowsari, M.; Motallebi, M.; Zamani, M.R.; Moghadasi, Z. Influence of recombinant Trichoderma strains on growth of bean (Phaseolus vulgaris L.) by increased root colonization and induction of root growth related genes. Sci. Hortic. 2020, 261, 108932. [Google Scholar] [CrossRef]
- Card, S.; Johnson, L.; Teasdale, S.; Caradus, J. Deciphering endophyte behaviour: The link between endophyte biology and efficacious biological control agents. FEMS Microbiol. Ecol. 2016, 92, 19. [Google Scholar] [CrossRef]
- Forchetti, G.; Masciarelli, O.; Alemano, S.; Alvarez, D.; Abdala, G. Endophytic bacteria in sunflower (Helianthus annuus L.): Isolation, characterization, and production of jasmonates and abscisic acid in culture medium. Appl. Microbiol. Biotechnol. 2007, 76, 1145–1152. [Google Scholar] [CrossRef]
- Dar, N.A.; Amin, I.; Wani, W.; Wani, S.A.; Shikari, A.B.; Wani, S.H.; Masoodi, K.Z. Abscisic acid: A key regulator of abiotic stress tolerance in plants. Plant Gene 2017, 11, 106–111. [Google Scholar] [CrossRef]
- Rostami, S.; Azhdarpoor, A. The application of plant growth regulators to improve phytoremediation of contaminated soils: A review. Chemosphere 2019, 220, 818–827. [Google Scholar] [CrossRef]
- Cohen, A.C.; Travaglia, C.N.; Bottini, R.; Piccoli, P.N. Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany 2009, 87, 455–462. [Google Scholar] [CrossRef]
- Sorokan, A.; Veselova, S.; Benkovskaya, G.; Maksimov, I. Endophytic strain Bacillus subtilis 26D increases levels of phytohormones and repairs growth of potato plants after Colorado potato beetle damage. Plants 2021, 10, 923. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Poor, P.; Janda, T. Salicylic Acid: A versatile signaling molecule in plants. J. Plant Growth Regul. 2022, 41, 1887–1890. [Google Scholar] [CrossRef]
- Tao, X.; Wu, Q.; Li, J.; Wang, D.; Nassarawa, S.S.; Ying, T. Ethylene biosynthesis and signal transduction are enhanced during accelerated ripening of postharvest tomato treated with exogenous methyl jasmonate. Sci. Hortic. 2021, 281, 109965. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Joe, M.M.; Islam, R.; Sa, T. ACC deaminase containing diazotrophic endophytic bacteria ameliorate salt stress in Catharanthus roseus through reduced ethylene levels and induction of antioxidative defense systems. Symbiosis 2012, 56, 77–86. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Freitas, H. Inoculation of plant growth promoting bacterium Achromobacter xylosoxidans strain Ax10 for the improvement of copper phytoextraction by Brassica Juncea. J. Environ. Manag. 2009, 90, 831–837. [Google Scholar] [CrossRef]
- Zhang, Y.F.; He, L.Y.; Chen, Z.J.; Wang, Q.Y.; Qian, M.; Sheng, X.F. Characterization of ACC deaminase producing endophytic bacteria isolated from copper tolerant plants and their potential in promoting the growth and copper accumulation of Brassica napus. Chemosphere 2011, 83, 57–62. [Google Scholar] [CrossRef]
- Zhang, Y.F.; He, L.Y.; Chen, Z.J.; Zhang, W.-H.; Wang, Q.Y.; Qian, M.; Sheng, X.F. Characterization of lead resistant and ACC deaminase-producing endophytic bacteria and their potential in promoting lead accumulation of rape. J. Hazard. Mat. 2011, 186, 1720–1725. [Google Scholar] [CrossRef]
- Siciliano, S.D.; Fortin, N.; Mihoc, A.; Wisse, G.; Labelle, S.; Beaumier, D.; Ouellette, D.; Roy, R.; Whyte, L.G.; Banks, M.K.; et al. Selection of specific endophytic bacterial genotypes by plants in response to soil contamination. Appl. Environ. Microbiol. 2001, 67, 2469–2475. [Google Scholar] [CrossRef]
- Moore, F.P.; Barac, T.; Borremans, B.; Oeyen, L.; Vangronsveld, J.; van der Lelie, D.; Campbell, C.D.; Moore, E.R.B. Endophytic bacterial diversity in poplar trees growing on a BTEX-contaminated site: The characterisation of isolates with potential to enhance phytoremediation. Syst. Appl. Microbiol. 2006, 29, 539–556. [Google Scholar] [CrossRef]
- Mufti, R.; Amna Rafique, M.; Haq, F.; Chaudhury, H.J. Genetic diversity and metal resistance assessment of endophytes isolated from Oxalis corniculata. Soil Environ. 2015, 34, 89–99. [Google Scholar]
- Haq, F.; Butt, M.; Ali, H.; Chaudhary, H.J. Biosorption of cadmium and chromium from water by endophytic Kocuria rhizophila: Equilibrium and kinetic studies. Desalination Water Treat 2016, 57, 19946–19958. [Google Scholar] [CrossRef]
- Shanmugam, V.; Pothiraj, G.; Dauda, W.P. Endophytes for postharvest disease management in vegetables and fruits. In Postharvest Handling and Diseases of Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2021; pp. 93–110. [Google Scholar]
- Trias, R.; Badosa, E.; Montesinos, E.; Bañeras, L. Bioprotective Leuconostoc strains against Listeria monocytogenes in fresh fruits and vegetables. Int. J. Food Microbiol. 2008, 127, 91–98. [Google Scholar] [CrossRef]
- Chaouachi, M.; Marzouk, T.; Jallouli, S.; Elkahoui, S.; Gentzbittel, L.; Ben, C.; Djébali, N. Activity assessment of tomato endophytic bacteria bioactive compounds for the postharvest biocontrol of Botrytis cinerea. Postharvest Biol. Tech. 2021, 172, 111389. [Google Scholar] [CrossRef]
- Mari, M.; Guizzardi, M.; Pratella, G.C. Biological control of gray mold in pears by antagonistic bacteria. Biol. Control. 1996, 7, 30–37. [Google Scholar] [CrossRef]
- Hassan, E.A.; Mostafa, Y.S.; Alamri, S.; Hashem, M.; Nafady, N.A. Biosafe management of Botrytis grey mold of strawberry fruit by novel bioagents. Plants 2021, 10, 2737. [Google Scholar] [CrossRef] [PubMed]
- Saminathan, T.; García, M.; Ghimire, B.; Lopez, C.; Bodunrin, A.; Nimmakayala, P.; Abburi, V.L.; Levi, A.; Balagurusamy, N.; Reddy, U.K. Metagenomic and metatranscriptomic analyses of diverse watermelon cultivars reveal the role of fruit associated microbiome in carbohydrate metabolism and ripening of mature fruits. Front. Plant Sci. 2018, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Pusenkova, L.I.; Garipova, S.R.; Lastochkina, O.V.; Fedorova, K.A.; Mardanshin, I.S. Influence of endophytic bacteria Bacillus subtilis on harvest, quality of tubes and post-harvest diseases of potato. Agrochem. Her. J. 2021, 5, 73–79. [Google Scholar]
- Sun, M.; Liu, J.; Li, J.; Huang, Y. Endophytic bacterium Serratia plymuthica From Chinese leek suppressed apple ring rot on postharvest apple fruit. Front. Microbiol. 2022, 12, 802887. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Shi, B.; Wang, L.; Huang, T.; Zhou, Z.; Hou, H.; Tu, H. Isolation and characterization of Bacillus velezensis strain P2-1 for biocontrol of apple postharvest decay caused by Botryosphaeria dothidea. Front. Microbiol. 2022, 12, 808938. [Google Scholar] [CrossRef] [PubMed]
- Khruengsai, S.; Pripdeevech, P.; D’Souza, P.E.; Panuwet, P. Biofumigation activities of volatile compounds from two Trichoderma afroharzianum strains against Fusarium infections in fresh chilies. J. Sci. Food Agric. 2021, 101, 5861–5871. [Google Scholar] [CrossRef]
- Damasceno, C.L.; Duarte, E.A.A.; dos Santos, L.B.P.R.; de Oliveira, T.A.S.; de Jesus, F.N.; de Oliveira, L.M.; Soares, A.C.F. Postharvest biocontrol of anthracnose in bananas by endophytic and soil rhizosphere bacteria associated with sisal (Agave sisalana) in Brazil. Biol. Control 2019, 137, 104016. [Google Scholar] [CrossRef]
- Aiello, D.; Restuccia, C.; Stefani, E.; Vitale, A.; Cirvilleri, G. Postharvest biocontrol ability of Pseudomonas synxantha against Monilinia fructicola and Monilinia fructigena on stone fruit. Postharvest Biol. Tech. 2019, 149, 83–89. [Google Scholar] [CrossRef]
- Pang, L.; Xia, B.; Liu, X.; Yi, Y.; Jiang, L.; Chen, C.; Li, P.; Zhang, M.; Deng, X.; Wang, R. Improvement of antifungal activity of a culture filtrate of endophytic Bacillus amyloliquefaciens isolated from kiwifruit and its effect on postharvest quality of kiwifruit. J. Food Biochem. 2021, 45, e13551. [Google Scholar] [CrossRef]
- Archana, T.J.; Gogoi, R.; Kaur, C.; Varghese, E.; Sharma, R.R.; Srivastav, M.; Tomar, M.; Kumar, M.; Kumar, A. Bacterial volatile mediated suppression of postharvest anthracnose and quality enhancement in mango. Postharvest Biol. Technol. 2021, 177, 111525. [Google Scholar] [CrossRef]
- Khruengsai, S.; Pripdeevech, P.; Tanapichatsakul, C.; Srisuwannapa, C.; D’Souza, P.E.; Panuwet, P. Antifungal properties of volatile organic compounds produced by Daldinia eschscholtzii MFLUCC 19-0493 isolated from Barleria prionitis leaves against Colletotrichum acutatum and its post-harvest infections on strawberry fruits. PeerJ 2021, 9, e11242. [Google Scholar] [CrossRef]
- Li, Y.; Xia, M.; He, P.; Yang, Q.; Wu, Y.; He, P.; Ahmed, A.; Li, X.; Wang, Y.; Munir, S.; et al. Developing Penicillium digitatum management strategies on post-harvest citrus fruits with metabolic components and colonization of Bacillus subtilis L1-21. Fungi 2022, 8, 80. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, H.; Zhou, J. The applications of nanotechnology in crop production. Molecules 2021, 26, 7070. [Google Scholar] [CrossRef]
- Mali, S.C.; Raj, S.; Trivedi, R. Nanotechnology a novel approach to enhance crop productivity. BB Rep. 2020, 24, 100821. [Google Scholar]
- Chen, H.; Zhi, H.; Liang, J.; Yu, M.; Cui, B.; Zhao, X.; Sun, C.; Wang, Y.; Cui, H.; Zeng, Z. Development of leaf-adhesive pesticide nanocapsules with pH-responsive release to enhance retention time on crop leaves and improve utilization efficiency. J. Mater. Chem. 2021, 9, 783–792. [Google Scholar] [CrossRef]
- Chen, H.; Zhi, H.; Feng, B.; Cui, B.; Zhao, X.; Sun, C.; Zeng, Z. Thermo-responsive quaternary ammonium chitosan nanocapsules with on-demand controlled pesticide release and maximally synergistic biological activity. J. Agric. Food Chem. 2022, 70, 7653–7661. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, nano-guard for pesticides: A new window for safe application. J. Agric. Food Chem. 2017, 64, 1447–1483. [Google Scholar] [CrossRef]
- Maroofpour, N.; Hejazi, M.J.; Hamishehkar, H.; Iranipour, S. Relative Toxicity and residual activity of nanocapsules and commercial formulations of pirimicarb and pymetrozine against Myzus persicae (Hemiptera: Aphididae). J. Econ. Entomol. 2019, 112, 2670–2675. [Google Scholar] [CrossRef]
- Rudakiya, D.; Patel, Y.; Chhaya, U.; Gupte, A. Carbon Nanotubes in Agriculture: Production, Potential, and Prospects. In Nanotechnology for Agriculture; Panpatte, D.G., Jhala, Y.K., Eds.; Springer Nature: Singapore, 2019; Chapter 8; pp. 121–130. [Google Scholar]
- Zhu, L.; Chen, L.; Gu, J.; Ma, H.; Wu, H. Carbon-based nanomaterials for sustainable agriculture: Their application as light converters, nanosensors, and delivery tools. Plants 2022, 11, 511. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Wong, M.H.; Lew, T.T.S.; Bisker, G.; Lee, M.A.; Kaplan, A.; Dong, J.; Liu, A.T.; Koman, V.B.; Sinclair, R.; et al. Nanosensor technology applied to living plant systems. Annu. Rev. Anal. Chem. 2017, 10, 113–140. [Google Scholar] [CrossRef]
- Patel, A.; Tiwari, S.; Parihar, P.; Singh, R.; Prasad, S.M. Carbon nanotubes as plant growth regulators: Impacts on growth, reproductive system, and soil microbial community. In Nanomaterials in Plants, Algae and Microorganism; Academic Press: Cambridge, MA, USA, 2019; pp. 23–42. [Google Scholar]
- Haghighi, M.; da Silva, J.A.T. The effect of carbon nanotubes on the seed germination and seedling growth of four vegetable species. J. Crop Sci. Biotechnol. 2014, 17, 201–208. [Google Scholar] [CrossRef]
- Canas, J.E.; Long, M.; Nations, S.; Vadan, R.; Dai, L.; Luo, M.; Olszyk, D. Nanomaterials in the environment effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species. Environ. Toxicol. Chem. 2008, 27, 1922–1931. [Google Scholar] [CrossRef]
- Singh, N.B.; Jain, P.; De, A.; Tomar, R. Green synthesis and applications of nanomaterials. Curr. Pharm. Biotechnol. 2021, 22, 1705–1747. [Google Scholar] [CrossRef]
- Akhtar, N.; Ilyas, N.; Meraj, T.A.; Pour-Aboughadareh, A.; Sayyed, R.Z.; Mashwani, Z.-U.-R.; Poczai, P. improvement of plant responses by nanobiofertilizer: A step towards sustainable agriculture. Nanomaterials 2022, 12, 965. [Google Scholar] [CrossRef]
- Chiralt, A.; Menzel, C.; Hernandez-García, E.; Collazo, S.; Gonzalez-Martinez, C. Use of by-products in edible coatings and biodegradable packaging materials for food preservation. In Sustainability of the Food System: Sovereignty, Waste, and Nutrients Bioavailability; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 101–127. [Google Scholar]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Edible coatings and antimicrobial nanoemulsions for enhancing shelf life and reducing foodborne pathogens of fruits and vegetables. Sustain. Mater. Technol. 2020, 26, e00215. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Q.; Picha, D.H.; Ferguson, M.H.; Ndukwe, I.E.; Azadi, P. Comparative performance of bio-based coatings formulated with cellulose, chitin, and chitosan nanomaterials suitable for fruit preservation. Carbohydr. Polym. 2021, 259, 117764. [Google Scholar] [CrossRef]
- Rovera, C.; Ghaani, M.; Farris, S. Nano-inspired oxygen barrier coatings for food packaging applications: An overview. In Trends in Food Science and Technology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 210–220. [Google Scholar]
- Yousuf, B.; Wu, S.; Siddiqui, M.W. Incorporating essential oils or compounds derived thereof into edible coatings: Effect on quality and shelf life of fresh/fresh cut produce. In Trends in Food Science and Technology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 245–257. [Google Scholar]
- Ali, M.H.; Sobze, J.M.; Pham, T.H.; Nadeem, M.; Liu, C.; Galagedara, L.; Cheema, M.; Thomas, R. Carbon nanotubes improved the germination and vigor of plant species from peatland ecosystem via remodeling the membrane lipidome. Nanomaterials 2020, 10, 1852. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, M.; Kim, H.Y.; Ding, B.; Park, S.J. A facile ultrasonic-assisted fabrication of nitrogen-doped carbon dots/BiOBr up-conversion nanocomposites for visible light photocatalytic enhancements. Sci. Rep. 2017, 7, 45086. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; de Silva, K.; Biris, A.S.; Dervishi, E.; Villagarcia, H. Carbon nanotubes induce growth enhancement of tobacco cells. Am. Chem. Soc. Nano 2012, 6, 2128–2135. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Kim, B.S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon nanotubes as plant growth regulators: Effects on tomato growth, reproductive system, and soil microbial community. Small 2013, 9, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Mao, X.; Zhuang, J.; Lei, B.; Li, Y.; Li, W.; Liu, Y. PVA-coated fluorescent carbon dot nanocapsules as an optical amplifier for enhanced photosynthesis of lettuce. ACS Sustain. Chem. Eng. 2020, 8, 3938–3949. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Yang, X.; Zhang, Y.; Hui, H.; Zhang, D.; Shu, J. Multi-walled carbon nanotubes enhanced the antioxidative system and alleviated salt stress in grape seedlings. Sci. Hortic. 2022, 293, 110698. [Google Scholar] [CrossRef]
- McGehee, D.L.; Lahiani, M.H.; Irin, F.; Green, M.J.; Khodakovskaya, M.V. Multiwalled carbon nanotubes dramatically affect the fruit metabolome of exposed tomato plants. ACS Appl. Mater. Interfaces 2017, 9, 32430–32435. [Google Scholar] [CrossRef]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Ali, S. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, A.K. Potential of engineered nanostructured biopolymer based coatings for perishable fruits with Coronavirus safety perspectives. Prog. Org. Coat. 2022, 163, 106632. [Google Scholar] [CrossRef]
- Ijaz, M.; Zafar, M.; Afsheen, S.; Iqbal, T. A Review on ag-nanostructures for enhancement in shelf time of fruits. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1475–1482. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- Abdulhameed, M.F.; Taha, A.A.; Ismail, R.A. Improvement of cabbage growth and yield by nanofertilizers and nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100437. [Google Scholar] [CrossRef]
- Jiang, H.; Lv, L.; Ahmed, T.; Jin, S.; Shahid, M.; Noman, M.; Osman, H.-E.H.; Wang, Y.; Sun, G.; Li, X. Effect of the nanoparticle exposures on the tomato bacterial wilt disease control by modulating the rhizosphere bacterial community. Int. J. Mol. Sci. 2022, 23, 414. [Google Scholar] [CrossRef]
- Mokarram-Kashtiban, S.; Hosseinim, S.M.; Kouchaksaraei, M.T.; Younesi, H. The impact of nanoparticles zero-valent iron (nZVI) and rhizosphere microorganisms on the phytoremediation ability of white willow and its response. Environ. Sci. Pollut. Res. 2019, 26, 10776–10789. [Google Scholar] [CrossRef]
- Faizan, F.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef]
- Sohail, S.L.; Ferrari, E.; Stierhof, Y.D.; Kemmerling, B.; Mashwani, Z.R. Molecular effects of biogenic zinc nanoparticles on the growth and development of Brassica napus L. revealed by proteomics and transcriptomics. Front. Plant Sci. 2022, 13, 798751. [Google Scholar] [CrossRef]
- Singh, M.D.; Kumar, A.B.N.; Chirag, G. Effects of time of application and concentrations of nano Zns on chlorophyll content (SPAD) of sunflower (Helianthus annuus L.). Int. J. Microbiol. Res. 2017, 9, 895–896. [Google Scholar]
- Gacem, M.A.; Chaibi, R. Cu-based nanoparticles as pesticides: Applications and mechanism of management of insect pests. In Copper Nanostructures: Next-Generation of Agrochemicals for Sustainable Agroecosystems; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 9; pp. 203–218. [Google Scholar]
- Valério, A.; Maass, D.; de Andrade, L.M.; Hotza, D.; de Oliveira, D.; de Andrade, C.J. Copper nanomaterials for pesticide detection. In Copper Nanostructures: Next-Generation of Agrochemicals for Sustainable Agroecosystems; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 10; pp. 219–241. [Google Scholar]
- Predoi, D.; Ghita, R.V.; Iconaru, S.L.; Cimpeanu, C.L.; Raita, S.M. Application of nanotechnology solutions in plants fertilization. In Urban Horticulture—Necessity of the Future; Solankey, S.S., Akhtar, S., Maldonado, A.I.L., Rodriguez-Fuentes, H., Vidales Contreras, J.A., Márquez Reyes, J.M., Eds.; Intechopen Limited: London, UK, 2020; Chapter 4; pp. 12–40. [Google Scholar]
- Servin, A.D.; Morales, M.I.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Munoz, B.; Zhao, L.; Nunez, J.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron verification of TiO2 accumulation in cucumber fruit: A possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environ. Sci. Technol. 2013, 47, 11592–11598. [Google Scholar] [CrossRef]
- Helal, M.; Sami, R.; Khojah, E.; Elhakem, A.; Benajiba, N.; Al Mushhin, A.A.M.; Fouda, N. Evaluating the coating process of titanium dioxide nanoparticles and sodium tripolyphosphate on cucumbers under chilling condition to extend the shelf life. Sci. Rep. 2021, 11, 20312. [Google Scholar] [CrossRef]
- Marchiol, L.; Mattiello, A.; Pošćić, F.; Fellet, G.; Zavalloni, C.; Carlino, E.; Musetti, R. Changes in physiological and agronomical parameters of barley (Hordeum vulgare) exposed to cerium and titanium dioxide nanoparticles. Int. J. Environ. Res. Public Health 2016, 13, 332. [Google Scholar] [CrossRef]
- Marchiol, L.; Mattiello, A. Application of nanotechnology in agriculture: Assessment of TiO2 nanoparticle effects on barley. In Application of Titanium Dioxide; Janus, M., Ed.; In Tech: London, UK, 2017; pp. 23–39. [Google Scholar]
- Zahra, Z.; Waseem, N.; Zahra, R.; Lee, H.; Badshah, M.A.; Mehmood, A.; Choi, H.-K.; Arshad, M. Growth and metabolic responses of rice (Oryza sativa L.) cultivated in phosphorus-deficient soil amended with TiO2 nanoparticles. J. Agric. Food Chem. 2017, 65, 5598–5606. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.-P.; Joshi, A.; Tian, D.-D.; Rajput, V.D.; Singh, M.; Arora, J.; Minkina, T.; Li, Y.-R. Recent trends in nano-fertilizers for sustainable agriculture under climate change for global food security. J. Nanomater. 2022, 12, 173. [Google Scholar] [CrossRef]
- Ahmed, B.; Shahid, M.; Khan, M.S.; Musarrat, J. Chromosomal aberrations, cell suppression and oxidative stress generation induced by metal oxide nanoparticles in onion (Allium cepa) bulb. Metallomics 2018, 10, 1315–1327. [Google Scholar] [CrossRef]
- Anjali, C.H.; Sharma, Y.; Mukherjee, A.; Chandrasekaran, N. Neem oil (Azadirachta indica) nanoemulsion: A potent larvicidal agent against Culex quinquefasciatus. Pest Manag. Sci. 2012, 68, 158–163. [Google Scholar] [CrossRef]
- Ostadi, A.; Javanmard, A.; Amani Machiani, M.; Sadeghpour, A.; Maggi, F.; Nouraein, M.; Morshedloo, M.R.; Hano, C.; Lorenzo, J.M. Co-application of TiO2 nanoparticles and arbuscular mycorrhizal fungi improves essential oil quantity and quality of sage (Salvia officinalis L.) in drought stress conditions. Plants 2022, 11, 1659. [Google Scholar] [CrossRef]
- Tahmasbi, D.; Zarghami, R.; Azghandi, A.V.; Chaichi, M. Effects of nanosilver and nitroxin biofertilizer on yield and yield components of potato minitubers. Int. J. Agric. Biol. 2011, 13, 986–990. [Google Scholar]
- Almutairi, Z.M. Influence of silver nano-particles on the salt resistance of tomato (Solanum lycopersicum) during germination. Int. J. Agric. Biol. 2016, 18, 449–457. [Google Scholar] [CrossRef]
- Kumari, M.; Pandey, S.; Bhattacharya, A.; Mishra, A.; Nautiyal, C.S. Protective role of biosynthesized silver nanoparticles against early blight disease in Solanum lycopersicum. Plant Physiol. Biochem. 2017, 121, 216–225. [Google Scholar] [CrossRef]
- Danish, M.; Altaf, M.; Robab, M.I.; Shahid, M.; Manoharadas, S.; Hussain, S.A.; Shaikh, H. green synthesized silver nanoparticles mitigate biotic stress induced by meloidogyne incognita in Trachyspermum ammi (L.) by improving growth, biochemical, and antioxidant enzyme activities. ACS Omega 2021, 6, 11389–11403. [Google Scholar] [CrossRef]
- Elatafi, E.; Fang, J. Effect of silver nitrate (AgNO3) and nano-silver (Ag-NPs) on Physiological characteristics of grapes and quality during storage period. Horticulturae 2022, 8, 419. [Google Scholar] [CrossRef]
- Gao, L.; Li, Q.; Zhao, Y.; Wang, H.; Liu, Y.; Sun, Y.; Wang, F.; Jia, W.; Hou, X. Silver nanoparticles biologically synthesised using tea leaf extracts and their use for extension of fruit shelf life. IET Nanobiotechnol. 2017, 11, 637–643. [Google Scholar] [CrossRef]
- Liu, J.; Ratnayake, K.; Joyce, D.C.; He, S.; Zhang, Z. Effects of three different nano-silver formulations on cut Acacia holosericea vase life. Postharvest Biol. Technol. 2012, 66, 8–15. [Google Scholar] [CrossRef]
- Amin, O.A.; Barsoom, M.A.; Bastawy, Z. The role of nano silver with sucrose on longevity of cut flowers of zinnia in vase. J. Middle East Appl. Sci. 2020, 10, 835–846. [Google Scholar]
- Decker, E.L.; Alder, A.; Hunn, S.; Ferguson, J.; Lehtonen, M.T.; Scheler, B.; Kerres, K.L.; Wiedemann, G.; Safavi-Rizi, V.; Nordzieke, S.; et al. Strigolactone biosynthesis is evolutionarily conserved, regulated by phosphate starvation and contributes to resistance against phytopathogenic fungi in a moss, Physcomitrella patens. New Phytol. 2017, 216, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Screpanti, C.; Fonné-Pfister, R. Strigolactone derivatives for potential crop enhancement applications. Bioorganic Med. Chem. Lett. 2016, 26, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Umehara, M. Possible roles of strigolactones during leaf senescence. Plants 2015, 4, 664–677. [Google Scholar] [CrossRef]
- Kohlen, W.; Charnikhova, T. The tomato CAROTENOID CLEAVAGE DIOXYGENASE 8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol. 2012, 196, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Ito, K. Effects of strigolactone signaling on Arabidopsis thaliana growth under nitrogen deficient stress condition. Plant Signal. Behav. 2016, 11, e1126031. [Google Scholar] [CrossRef]
- Van Ha, C.; Leyva-González, M.A. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar]
- Xu, M.; Xue, Z. Enhancement of the photosynthetic and removal performance for microalgae-based technologies by co-culture strategy and strigolactone induction. Bioresour. Technol. 2021, 339, 125579. [Google Scholar] [CrossRef]
- Kopta, T.; Antal, M. The influence of synthetic strigolactones and plant extracts on the morphological parameters of onion (Allium cepa). Adv. Hort. Sci. 2017, 31, 235–240. [Google Scholar]
- Min, Z.; Li, Z. Transcriptome analysis revealed hormone signaling response of grapevine buds to strigolactones. Sci. Hortic. 2021, 283, 109936. [Google Scholar] [CrossRef]
- Rasmussen, A.; Mason, M.G. Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiol. 2012, 158, 1976–1987. [Google Scholar] [CrossRef]
- Jia, K.P.; Luo, Q. Strigolactone-regulated hypocotyl elongation is dependent on cryptochrome and phytochrome signaling pathways in Arabidopsis. Mol. Plant 2014, 7, 528–540. [Google Scholar] [CrossRef]
- Kang, S.W. Developmental control of horticultural plants using strigolactone to improve marketability. Tsukuba J. Agric. For. 2017, 5, 1–8. [Google Scholar]
- Yu, C.; Chen, W. Comparative proteomic analysis of tomato (Solanum lycopersicum L.) shoots reveals crosstalk between strigolactone and auxin. Genomics 2021, 113, 3163–3173. [Google Scholar] [CrossRef]
- Agusti, J.; Herold, S. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc. Natl. Acad. Sci. USA 2011, 108, 20242–20247. [Google Scholar] [CrossRef]
- Guan, J.C.; Koch, K.E. Diverse roles of strigolactone signaling in maize architecture and the uncoupling of a branching-specific subnetwork. Plant Physiol. 2012, 160, 1303–1317. [Google Scholar] [CrossRef]
- Roumeliotis, E.; Kloosterman, B. The effects of auxin and strigolactones on tuber initiation and stolon architecture in potato. J. Exp. Bot. 2012, 63, 4539–4548. [Google Scholar] [CrossRef]
- Pasare, S.A.; Ducreux, L.J.M. The role of the potato (Solanum tuberosum) CCD8 gene in stolon and tuber development. New Phytol. 2013, 198, 1108–1120. [Google Scholar] [CrossRef]
- Cardoso, C.; Zhang, Y. Natural variation of rice strigolactone biosynthesis is associated with the deletion of two MAX1 orthologs. Proc. Natl. Acad. Sci. USA 2014, 111, 2379–2384. [Google Scholar] [CrossRef]
- Kyozuka, J.; Nomura, T. Origins and evolution of the dual functions of strigolactones as rhizosphere signaling molecules and plant hormones. Curr. Opin. Plant Biol. 2022, 65, 102154. [Google Scholar] [CrossRef]
- Foo, E.; Yoneyama, K. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol. Plant 2013, 6, 76–87. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N. Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta Physiol. Plant 2010, 32, 297–304. [Google Scholar] [CrossRef]
- Soto, M.J.; Fernandez-Aparicio, M. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biol. Biochem. 2010, 42, 383–385. [Google Scholar] [CrossRef]
- De Cuyper, C.; Fromentin, J. From lateral root density to nodule number, the strigolactone analogue GR24 shapes the root architecture of Medicago truncatula. J. Exp. Bot. 2015, 66, 137–146. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y. Strigolactone-triggered stomatal closure requires hydrogen peroxide synthesis and nitric oxide production in an abscisic acid-independent manner. New Phytol. 2018, 217, 290–304. [Google Scholar] [CrossRef]
- Torres-Vera, R.; García, J.M. Do strigolactones contribute to plant defence? Mol. Plant Pathol. 2014, 15, 211–216. [Google Scholar] [CrossRef]
- Piisilä, M.; Keceli, M.A. The F-box protein MAX2 contributes to resistance to bacterial phytopathogens in Arabidopsis thaliana. BMC Plant Biol. 2015, 15, 1–17. [Google Scholar] [CrossRef]
- Stes, E.; Depuydt, S. Strigolactones as anauxiliary hormonal defence mechanism against leafy gall syndrome in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 5123–5134. [Google Scholar] [CrossRef]
- Fu, X.; Wang, J. SMXLs regulate seed germination under salinity and drought stress in soybean. Plant Growth Regul. 2022, 96, 397–408. [Google Scholar] [CrossRef]
- Bu, Q.; Lv, T. Regulation of drought tolerance by the F-box protein MAX2 in Arabidopsis. Plant Physiol. 2014, 164, 424–439. [Google Scholar] [CrossRef]
- Min, Z.; Li, R. Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol. Biochem. 2019, 135, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L. Exogenous strigolactones alleviate the photosynthetic inhibition and oxidative damage of cucumber seedlings under salt stress. Sci. Hortic. 2022, 297, 110962. [Google Scholar] [CrossRef]
- Wang, W.N.; Min, Z. Physiological and transcriptomic analysis of Cabernet Sauvginon (Vitis vinifera L.) reveals the alleviating effect of exogenous strigolactones on the response of grapevine to drought stress. Plant Physiol. Biochem. 2021, 167, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tan, Z. Physiological mechanism of strigolactone enhancing tolerance to low light stress in cucumber seedlings. BMC Plant Biol. 2022, 22, 30. [Google Scholar] [CrossRef]
- Lu, T.; Yu, H. Improving plant growth and alleviating photosynthetic inhibition and oxidative stress from low-light stress with exogenous GR24 in tomato (Solanum lycopersicum L.) seedlings. Front. Plant Sci. 2019, 10, 490. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Y. Strigolactone maintains strawberry quality by regulating phenylpropanoid, NO, and H2S metabolism during storage. Postharvest Biol. Technol. 2021, 178, 111546. [Google Scholar] [CrossRef]
- Kozai, T. (Ed.) Smart Plant Factory: The Next Generation Indoor Vertical Farms; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustainability 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Khoramtabrizi, M.; Aliniaeifard, S.; Chegini, G. Effects of different artificial light spectra on growth of Lettuce in a continuous light plant factory system. Acta Hortic. 2020, 1271, 101–106. [Google Scholar] [CrossRef]
- Roberts, J.M.; Bruce, T.J.; Monaghan, J.M.; Pope, T.W.; Leather, S.R.; Beacham, A.M. Vertical farming systems bring new considerations for pest and disease management. Ann. Appl. Biol. 2020, 176, 226–232. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red: Blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef]
- Sapounas, A.; Katsoulas, N.; Slager, B.; Bezemer, R.; Lelieveld, C. Design, control, and performance aspects of semi-closed greenhouses. Agronomy 2020, 10, 1739. [Google Scholar] [CrossRef]
- Li, T.; Heuvelink, E.; Dueck, T.A.; Janse, J.; Gort, G.; Marcelis, L.F.M. Enhancement of crop photosynthesis by diffuse light: Quantifying the contributing factors. Ann. Bot. 2014, 114, 145–156. [Google Scholar] [CrossRef]
- Bayat, L.; Arab, M.; Aliniaeifard, S. Effects of different light spectra on high light stress tolerance in rose plants (Rosa hybrida cv.‘Samurai’). J. Plant Proc. Funct. 2020, 9, 93–103. [Google Scholar]
- Ghorbanzadeh, P.; Aliniaeifard, S.; Esmaeili, M.; Mashal, M.; Azadegan, B.; Seif, M. Dependency of growth, water use efficiency, chlorophyll fluorescence, and stomatal characteristics of lettuce plants to light intensity. J. Plant Growth Regul. 2021, 40, 2191–2207. [Google Scholar] [CrossRef]
- Esmaeili, S.; Aliniaeifard, S.; Dianati Daylami, S.; Karimi, S.; Shomali, A.; Didaran, F.; Telesiński, A.; Sierka, E.; Kalaji, H.M. Elevated light intensity compensates for nitrogen deficiency during chrysanthemum growth by improving water and nitrogen use efficiency. Sci. Rep. 2022, 12, 10002. [Google Scholar] [CrossRef]
- Ashrostaghi, T.; Aliniaeifard, S.; Shomali, A.; Azizinia, S.; Abbasi Koohpalekani, J.; Moosavi-Nezhad, M.; Gruda, N.S. Light intensity: The role player in cucumber response to cold stress. Agronomy 2022, 12, 201. [Google Scholar] [CrossRef]
- Javadi Asayesh, E.; Aliniaeifard, S.; Askari, N.; Roozban, M.R.; Sobhani, M.; Tsaniklidis, G.; Woltering, E.J.; Fanourakis, D. Supplementary light with increased blue fraction accelerates emergence and improves development of the inflorescence in Aechmea, Guzmania and Vriesea. Horticulturae 2021, 7, 485. [Google Scholar] [CrossRef]
- Heldt, H.-W.; Piechulla, B.; Heldt, F. Plant biochemistry; Translation of the 4th German edition; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Moradi, S.; Kafi, M.; Aliniaeifard, S.; Salami, S.A.; Shokrpour, M.; Pedersen, C.; Moosavi-Nezhad, M.; Wróbel, J.; Kalaji, H.M. Blue light improves photosynthetic performance and biomass partitioning toward harvestable organs in Saffron (Crocus sativus L.). Cells 2021, 10, 1994. [Google Scholar] [CrossRef]
- Seif, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Shomali, A.; Fanourakis, D.; Li, T.; Woltering, E. Monochromatic red light during plant growth decreases the size and improves the functionality of stomata in chrysanthemum. Funct. Plant Biol. 2021, 48, 515–528. [Google Scholar] [CrossRef]
- Hosseini, A.; Mehrjerdi, M.Z.; Aliniaeifard, S.; Seif, M. Photosynthetic and growth responses of green and purple basil plants under different spectral compositions. Physiol. Mol. Biol. Plants 2019, 25, 741–752. [Google Scholar] [CrossRef]
- Aalifar, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Serek, M. Blue light postpones senescence of carnation flowers through regulation of ethylene and abscisic acid pathway-related genes. Plant Physiol. Biochem. 2020, 151, 103–112. [Google Scholar] [CrossRef]
- Aalifar, M.; Aliniaeifard, S.; Arab, M.; Zare Mehrjerdi, M.; Dianati Daylami, S.; Serek, M.; Woltering, E.; Li, T. Blue light improves vase life of carnation cut flowers through its effect on the antioxidant defense system. Front. Plant Sci. 2020, 11, 511. [Google Scholar] [CrossRef]
- Bayat, L.; Arab, M.; Aliniaeifard, S.; Seif, M.; Lastochkina, O.; Li, T. Effects of growth under different light spectra on the subsequent high light tolerance in rose plants. AoB Plants 2018, 10, ply052. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Seif, M.; Arab, M.; Zare Mehrjerdi, M.; Li, T.; Lastochkina, O. Growth and photosynthetic performance of Calendula officinalis under monochromatic red light. Int. J. Hortic. Sci. Technol. 2018, 5, 123–132. [Google Scholar]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Moosavi-Nezhad, M.; Salehi, R.; Aliniaeifard, S.; Tsaniklidis, G.; Woltering, E.J.; Fanourakis, D.; Żuk-Gołaszewska, K.; Kalaji, H.M. Blue light improves photosynthetic performance during healing and acclimatization of grafted watermelon seedlings. Int. J. Mol. Sci. 2021, 22, 8043. [Google Scholar] [CrossRef]
- Hosseini, A.; Zare Mehrjerdi, M.; Aliniaeifard, S. Alteration of bioactive compounds in two varieties of Basil (Ocimum basilicum) grown under different light spectra. J. Essent. Oil-Bear. Plants 2018, 21, 913–923. [Google Scholar] [CrossRef]
- Kim, H.-H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red-and blue-light-emitting diodes. Hortscience 2004, 39, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Kubota, C.; Chia, P.; Yang, Z.; Li, Q. Applications of far-red light emitting diodes in plant production under controlled environments. In International Symposium on Advanced Technologies and Management Towards Sustainable Greenhouse Ecosystems: Greensys 2011; International Society for Horticultural Science: Leuven, Belgium, 2012; Volume 952. [Google Scholar]
- Orlando, M.; Trivellini, A.; Incrocci, L.; Ferrante, A.; Mensuali, A. The inclusion of green light in a red and blue light background impact the growth and functional quality of vegetable and flower microgreen species. Horticulturae 2022, 8, 217. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Aliniaeifard, S.; Shomali, A.; Didaran, F. Interaction of light intensity and CO concentration alters biomass partitioning in chrysanthemum. J. Hortic. Res. 2021, 29, 45–56. [Google Scholar] [CrossRef]
- Yamori, W. Photosynthetic response to fluctuating environments and photoprotective strategies under abiotic stress. J Plant Res 2016, 129, 379–395. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Chen, Y.; Fanourakis, D.; Tsaniklidis, G.; Aliniaeifard, S.; Yang, Q.; Li, T. Low UVA intensity during cultivation improves the lettuce shelf-life, an effect that is not sustained at higher intensity. Postharvest Biol. Technol. 2021, 172, 111376. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; van Meeteren, U. Greenhouse vapour pressure deficit and lighting conditions during growth can influence postharvest quality through the functioning of stomata. Acta Hortic. 2018, 1227, 677–684. [Google Scholar] [CrossRef]
- Popp, M.; Janett, H.P.; Lüttge, U.; Medina, E. Metabolite gradients and carbohydrate translocation in rosette leaves of CAM and C3 bromeliads. New Phytol. 2003, 157, 649–656. [Google Scholar] [CrossRef]
- Appolloni, E.; Orsini, F.; Pennisi, G.; Gabarrell Durany, X.; Paucek, I.; Gianquinto, G. Supplemental LED lighting effectively enhances the yield and quality of greenhouse truss tomato production: Results of a meta-analysis. Front. Plant Sci. 2021, 12, 596927. [Google Scholar] [CrossRef]
- Horibe, T. Use of Light Stimuli as a Postharvest Technology for Cut Flowers. Front. Plant Sci. 2020, 11, 573490. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Falahi, Z.; Dianati Daylami, S.; Li, T.; Woltering, E. Postharvest spectral light composition affects chilling injury in anthurium cut flowers. Front. Plant Sci. 2020, 11, 846. [Google Scholar] [CrossRef]
- Rabka, M.; Mariyanayagam, D.; Shukla, P. IoT-Based Horticulture Monitoring System. In Intelligent Sustainable Systems. Lecture Notes in Networks and Systems; Springer: Singapore, 2022; pp. 765–774. [Google Scholar]
- Geilfus, S.M. (Ed.) Controlled environment horticulture; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Esmaili, M.; Mashal, M.; Aliniaeifard, S.; Urrestarazu, M.; Carrillo, F.F. Impact of silicon on chemical properties of drainage water from lettuce following determination of proper cultivar and light spectrum. Commun. Soil Sci. Plant Anal. 2021, 52, 756–768. [Google Scholar] [CrossRef]
- Shomali, A.; Aliniaeifard, S.; Didaran, F.; Lotfi, M.; Mohammadian, M.; Seif, M.; Strobel, W.R.; Sierka, E.; Kalaji, H.M. Synergistic effects of melatonin and gamma-aminobutyric acid on protection of photosynthesis system in response to multiple abiotic stressors. Cells 2021, 10, 1631. [Google Scholar] [CrossRef]
- Job, D. Plant biotechnology in agriculture. Biochimie 2002, 84, 1105–1110. [Google Scholar] [CrossRef]
- Newell, C.A. Plant transformation technology. Mol. Biotech. 2000, 16, 53–65. [Google Scholar] [CrossRef]
- Mohanty, D.; Chandra, A.; Tandon, R. Germline transformation for crop improvement. In Molecular Breeding for Sustainable Crop Improvement; Springer: Cham, Switzerland, 2016; pp. 343–395. [Google Scholar]
- Lowe, K.; Wu, E.; Wang, N.; Hoerster, G.; Hastings, C.; Cho, M.J.; Scelonge, C.; Lenderts, B.; Chamberlin, M.; Cushatt, J.; et al. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell 2016, 28, 1998–2015. [Google Scholar] [CrossRef]
- Baltes, N. Plant Genome engineering with sequence-specific nucleases: Methods for editing DNA in whole plants. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2014. [Google Scholar]
- Bak, R.O.; Gomez-Ospina, N.; Porteus, M.H. Gene editing on center stage. Trends Genet. 2018, 34, 600–611. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shukla, P. Gene editing for cell engineering: Trends and applications. Crit. Rev. Biotech. 2017, 37, 672–684. [Google Scholar] [CrossRef]
- Urnov, F.D.; Rebar, E.J.; Holmes, M.C.; Zhang, H.S.; Gregory, P.D. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 2010, 11, 636–646. [Google Scholar] [CrossRef]
- Joung, J.K.; Sander, J.D. TALENs: A widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 2013, 14, 49–55. [Google Scholar] [CrossRef]
- Hafez, M.; Hausner, G. Homing endonucleases: DNA scissors on a mission. Genome 2012, 55, 553–569. [Google Scholar] [CrossRef]
- Mao, Y.; Botella, J.R.; Liu, Y.; Zhu, J.K. Gene editing in plants: Progress and challenges. Natl. Sci. Rev. 2019, 6, 421–437. [Google Scholar] [CrossRef]
- Knott, G.J.; Doudna, J.A. CRISPR-Cas guides the future of genetic engineering. Science 2018, 361, 866–869. [Google Scholar] [CrossRef]
- Leenay, R.T.; Beisel, C.L. Deciphering, communicating, and engineering the CRISPR PAM. J. Mol. Biol. 2017, 429, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Scherer, S. Guide to the Human Genome; Cold Spring Harbor Laboratory Press: Harbor, NY, USA, 2010. [Google Scholar]
- Ahmad, M.; Ali, Q.; Hafeez, M.M.; Malik, A. Improvement for biotic and abiotic stress tolerance in crop plants. Biol. Clin. Sci. Res. J. 2021, 50, 1–9. [Google Scholar] [CrossRef]
- Dwivedi, S.; Goldman, I.; Ortiz, R. Pursuing the potential of heirloom cultivars to improve adaptation, nutritional, and culinary features of food crops. Agronomy 2019, 9, 441. [Google Scholar] [CrossRef]
- Bullock, D.W.; Wilson, W.W.; Neadeau, J. Gene editing versus genetic modification in the research and development of new crop traits: An economic comparison. Am. J. Agric. Econ. 2021, 103, 1700–1719. [Google Scholar] [CrossRef]
- Sun, Z.; Li, N.; Huang, G.; Xu, J.; Pan, Y.; Wang, Z.; Tang, Q.; Song, M.; Wang, X. Site-specific gene targeting using Transcription Activator-Like Effector (TALE)-based nuclease in Brassica oleracea. J. Integr. Plant Biol. 2013, 55, 1092–1103. [Google Scholar] [CrossRef]
- Xu, J.; Hua, K.; Lang, Z. Genome editing for horticultural crop improvement. Hortic. Res. 2019, 6, 113. [Google Scholar] [CrossRef]
- Jenkins, D.; Dobert, R.; Atanassova, A.; Pavely, C. Impacts of the regulatory environment for gene editing on delivering beneficial products. In Vitro Cell. Dev. Biol. Plant 2021, 57, 609–626. [Google Scholar] [CrossRef]
- Nonaka, S.; Arai, C.; Takayama, M.; Matsukura, C.; Ezura, H. Efficient increase of ɣ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 2019, 70, 667–697. [Google Scholar] [CrossRef]
- Cable, J.; Ronald, P.C.; Voytas, D.; Zhang, F.; Levy, A.A.; Takatsuka, A.; Arimura, S.; Jacobsen, S.E.; Toki, S.; Toda, E.; et al. Plant genome engineering from lab to field—A Keystone Symposia report. Ann. New York Acad. Sci. 2021, 1506, 35–54. [Google Scholar] [CrossRef]
- Foster, T.M.; Bassil, N.V.; Dossett, M.; Leigh Worthington, M.; Graham, J. Genetic and genomic resources for Rubus breeding: A roadmap for the future. Hortic. Res. 2019, 6, 116. [Google Scholar] [CrossRef]
- Wan, L.; Wang, Z.; Tang, M.; Hong, D.; Sun, Y.; Ren, J.; Zhang, N.; Zeng, H. CRISPR-Cas9 gene editing for fruit and vegetable crops: Strategies and prospects. Horticulturae 2021, 7, 193. [Google Scholar] [CrossRef]
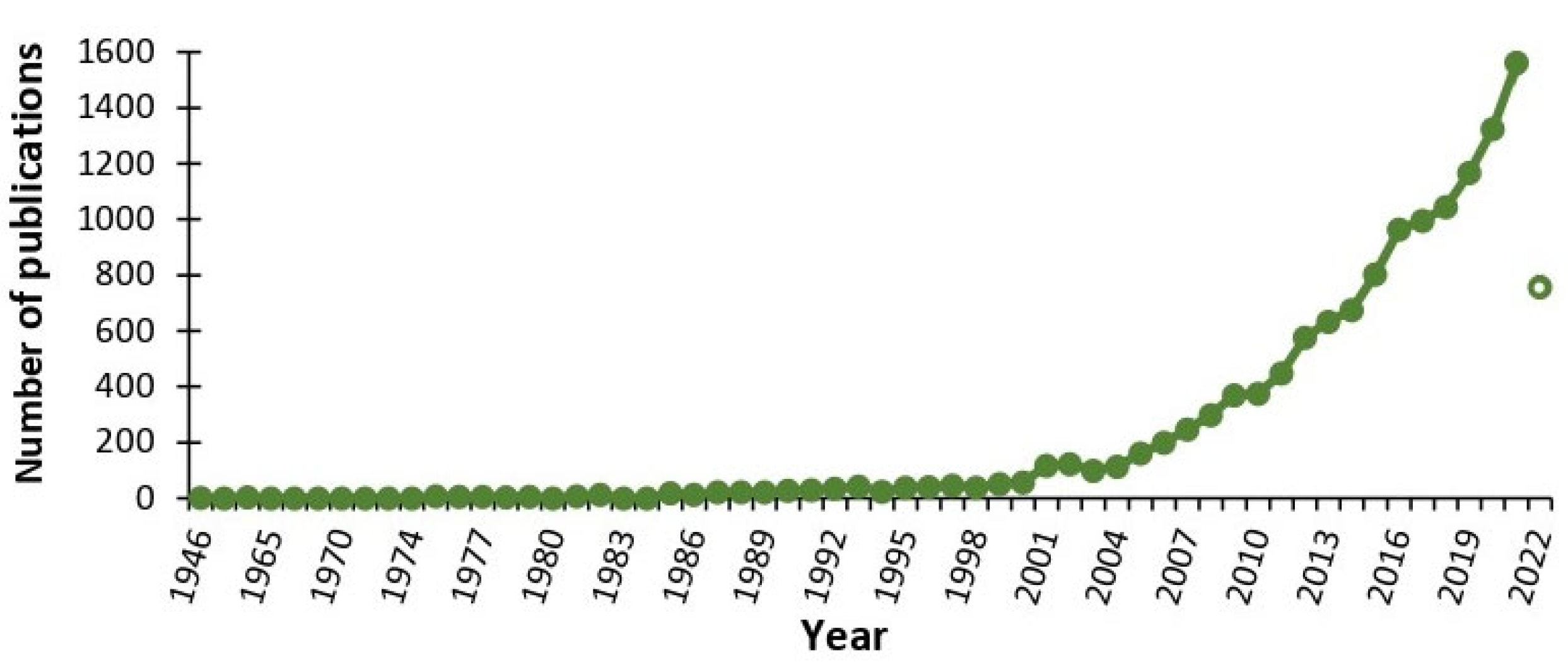
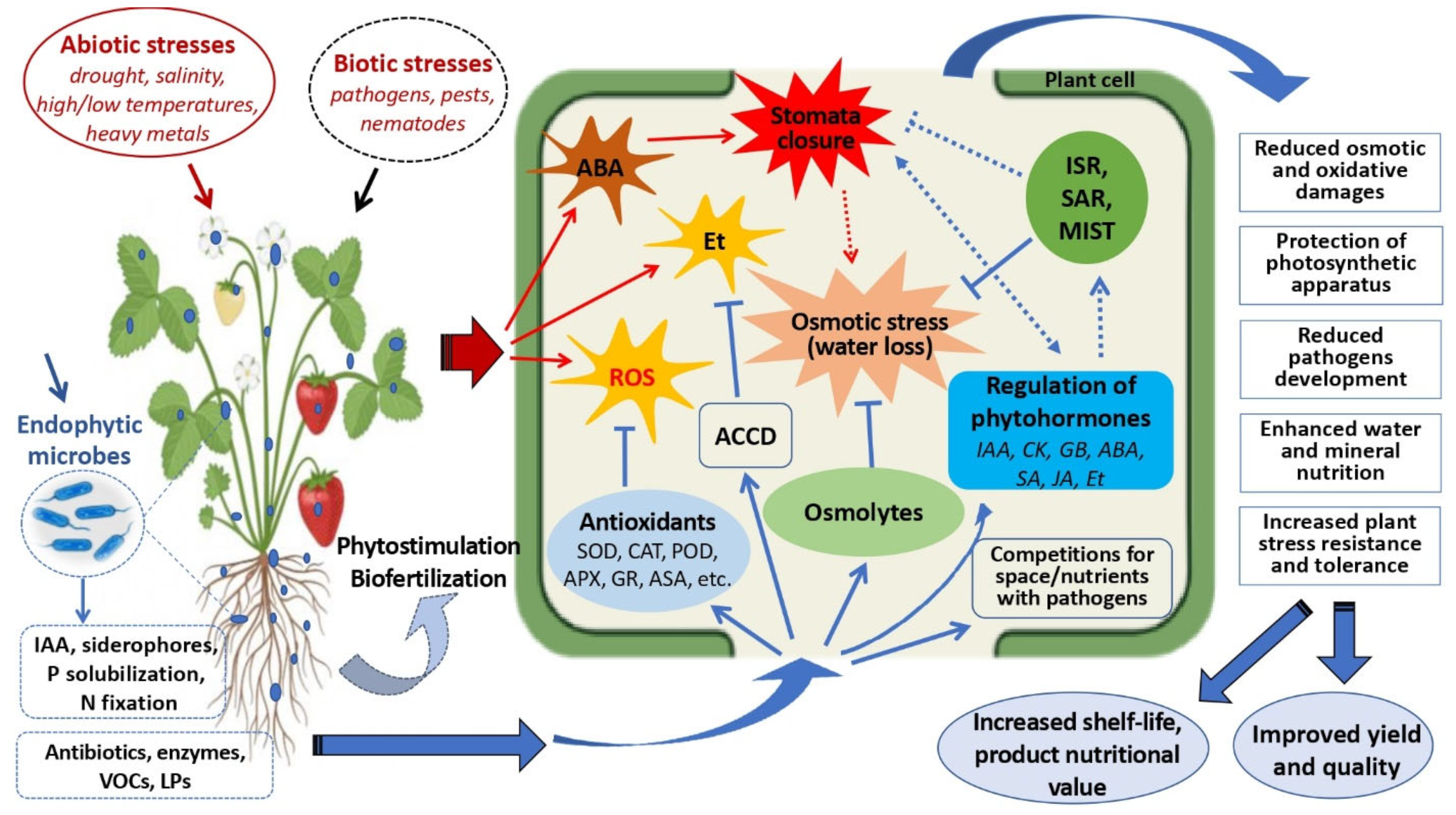
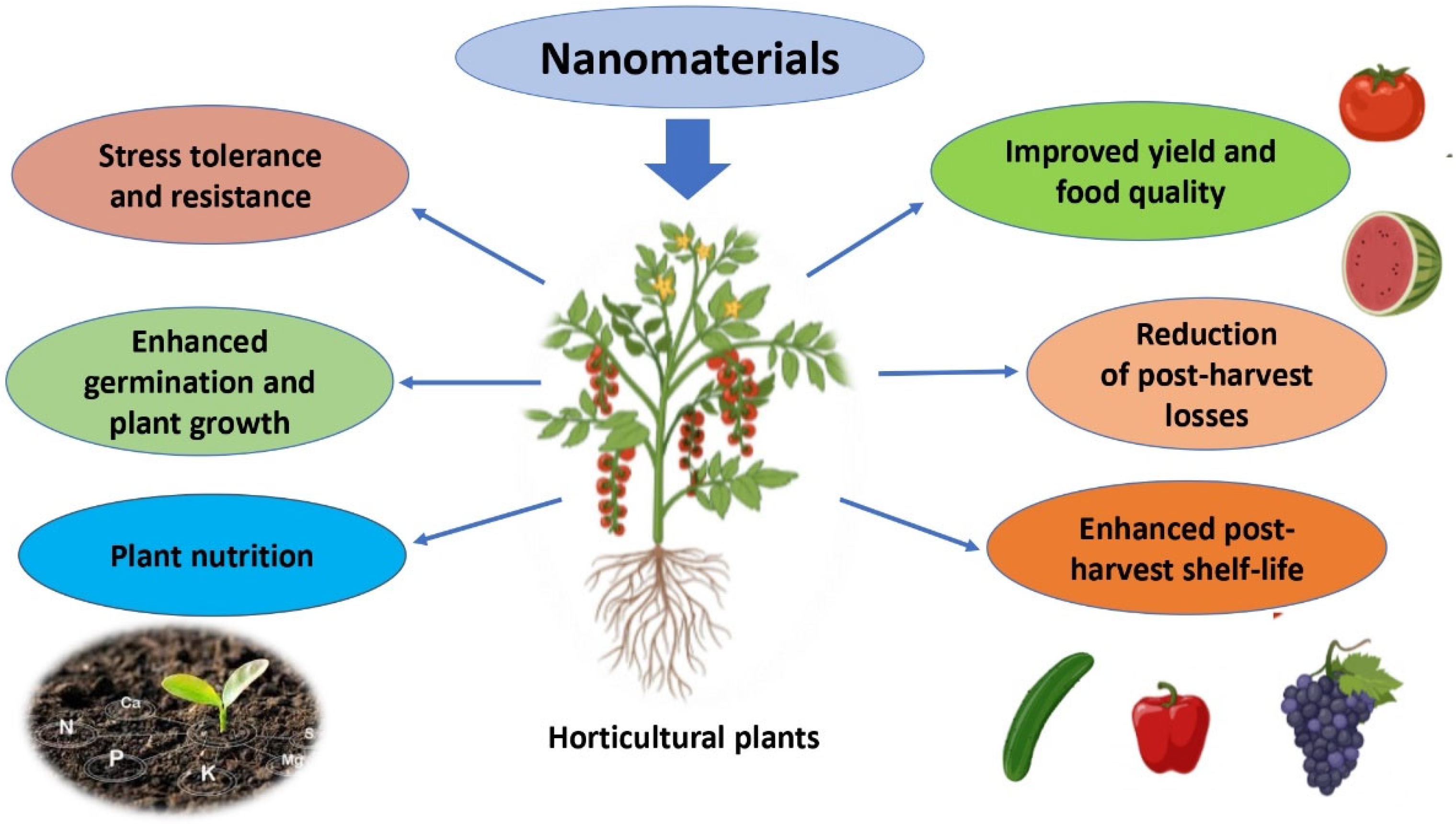
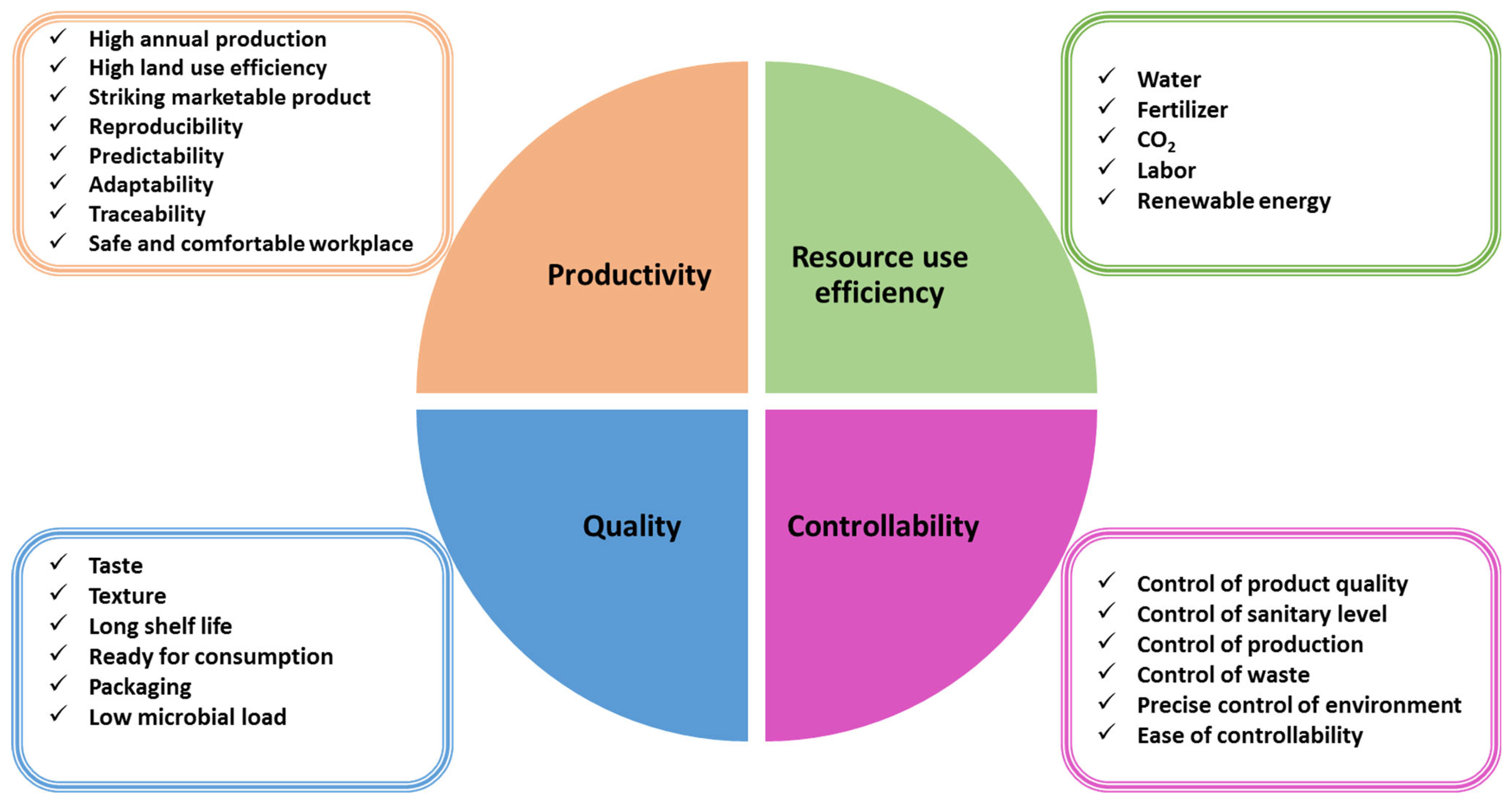
| Host Plant | Endophytes | Beneficial Effects/Possible Mechanisms | Effect on Yield and Quality | Reference |
|---|---|---|---|---|
| Newhall navel orange | Piriformospora indica | Improved soil properties | Increased fruit yield enriched with Fe and Zn | [125] |
| Potato | Streptomyces spp. | Growth promotion, biocontrol | Increased productivity, reduced disease infestations | [126] |
| Serratia plymuthica, Pseudomonas putida | Growth promotion, biocontrol/Antibiotic 2,4-diacetyl-phloroglucinol production | [127] | ||
| Burkholderia phytofirmans PsJN | IAA production, ACC-deaminase activity | N/A | [128] | |
| Tomato | Bacillus subtilis | Biocontrol of A. solani, Ph. infestans | [129] | |
| Sphingomonas sp. | IAA and GB production | [130] | ||
| Bacillus sp., Burkholderia sp., Enterobacter sp., Pseudomonas sp., Rhizobium sp., Staphylococcus sp., Stenotrophomonas sp. | Growth enhancement | [131] | ||
| Trichoderma sp. | Higher expression of swolenin gene in roots, increased Bioaccumulation Index (BI) for Fe and Cr, and decreased BI for heavy metals Ni and Pb in fruits | Increased fruit yield, total flavonoids content, decreased starch | [89] | |
| Bacillus pumilus | Improved growth/N uptake under N fertilization N2 fixation | Increased yield, improved quality | [98] | |
| B. subtilis 26D, B. subtilis Ttl2 | Biocontrol of viral diseases (PVX, d PVY)/Production of ribonucleases, phytohormones (CKs, IAA), expression of PR genes | N/A | [33] | |
| B. subtilis SR22 | Growth promotion, disease (Rhizoctonia solani) reduction/Production of chlorogenic acid, pyrrolo [1.2-a]pyrazine-1.4-dione, hexahydro, propyl thioglycolic acid, phthalic acid, 2.3-butanediol; upregulation JERF3 and POD, PR1 gene expression; increased phenolic content, POD, PPO activities | [34] | ||
| Tomato (susceptible and tolerant cultivars) | S. williamsii, S. herbamans, S. indica, or S. vermifera | Cultivar-specific responces to Fusarium wilt (Fusarium oxysporum f. sp. lycopersici) Fusarium wilt reduced only by S. herbamans and S. vermifera | [132] | |
| Citrus species | Bacillus sp. | IAA production, P solubilization | [133] | |
| B. velezensis EB-39 | Reduced (by 38%) incidence of canker (Xanthomonas citri subsp. citri) on the infected leaves | [82] | ||
| Coffee | Escherichia fergusonii, Acinetobacter calcoaceticus, Salmonella enterica, Brevibacillus choshinensis, Pectobacterium carotovorum, Bacillus megaterium, Microbacterium testaceum, Cedecea davisae | Biocontrol of leaf rust (Hemileia vastatrix)/IAA and phosphatase production | [134] | |
| Grapevine | Bacillus pumilus, Paenibacillus sp. | Biocontrol (Phaeomoniella chlamydospore) | [135] | |
| Bacillus subtilis, Curtobacterium sp. | Biocontrol (Agrobacterium vitis) | [136] | ||
| Bacillus, Staphylococcus, Microbacterium, Paenibacillus, Curtobacterium, Stenotrophomonas, Variovorax, Micrococcus, Agrococcus | Growth promotion, biocontrol | [137] | ||
| Banana | Pseudomonas aeruginosa | Biocontrol | [138] | |
| Streptomyces malaysiensis 8ZJF-21 | Growth promotion, biocontrol of Fusarium oxysporum f. sp. cubense tropical race 4 (Foc TR4)/Enhancment the expression of defense-related and antioxidant enzyme genes, production of extracellular enzymes, metabolites, VOCs | [29] | ||
| Pochonia chlamydosporia 123 | Increased root, corm and leaf length and leaf weight | [88] | ||
| Chickpea | B. subtilis NUU4 | Improve plant growth, suppression of root rot caused by Fusarium solani under salt stress/decreased H2O2 and increased proline contents | [139] | |
| Periwinkle | Streptomyces sp. | Growth promotion/Increased N, P, K, carotenoids, ascorbic acid and alkaloid | Enhancing plant biomass, phytopharmaceuticals accumulation | [21] |
| Strawberry | Bacillus velezensis IALR308, IALR585, and IALR619 | Disease reduction (C. gloeosporioides)/Auxin production, P solubilization, antibiotics surfactin and iturin production | Increased marketuable fruit yield | [19] |
| Bacillus sp., Pantoea sp. | Reduced gray mold disease (B. cinerea)/Production of diffusible and volatile antifungal compounds | [140] | ||
| Pitaya | Penicillium rolfsii Y17 | Reduction of disease (Neoscytadium dimidiatum)/Increased POD, CAT, PPO activities and total antioxidant capacity | N/A | [141] |
| Ginseng | Bacterial endophytes | Growth promotion/IAA, siderophore production, P solubilization, N fixation, and production of bioactive metabolites | [20] | |
| Apple | Trichoderma asperellum 6S-2 | Biocontrol (−52.41%) of disease (Fusarium proliferatum f. sp. malus domestica MR5), plant growth promotion/Reduced oxidative damages, increased protease, amylase, cellulase and laccase activities, secretion of Fe carriers, auxin, ammonia and P solubilization | [123] | |
| Pak choi, chinese amaranth, lettuces | Burkholderia seminalis 869T2 | Increased plant growth | Increased flower and fruit production | [93] |
| Olive | Aureobasidium pullulans, Sarocladium summerbellii | Biocontrol of anthracnose (Colletotrichum acutatum)/Production of VOCs (Z-3-hexen-1-ol, benzyl alcohol, nonanal) | N/A | [142] |
| Eggplants | Endophytic bacteria SaMR12 | Improved plant growth Phytoremediation of Cd-contaminated soil | Increased yield with reduces Cd content | [143] |
| Blueberry | Antarctic fungal endophytes (AFE) Penicillium rubens and P. bialowienzense | Protection against cold events in combination with drought under controlled conditions/Higher gene expression of LEA1 protein, higher photochemical efficiency, low oxidative stress | Increased yield, improved fruit diameter and fruit fresh weight | [90] |
| Methylobacterium sp. CP3, Kineococcus endophyticus CP19 | Increased plant growth and tolerance in polluted soils (Zn, Cd)/IAA production, P solubilization, enhanced Mg uptake | Increased yield with improved nutritional vallue | [94] | |
| Pea | Pseudomonas thivervalensis, Paenibacillus amylolyticus, P. polymyxa, Paenibacillus sp., Peribacillus simplex | Shoot growth promotion under greenhouse condition | Increased yield | [28] |
| [92] | ||||
| Tomato | Colletotrichum tofieldiae Ct0861 | Growth promotion in greenhouse and field conditions | [144] | |
| Bradyrhizobium, Trichoderma, Bradyrhizobium + Trichoderma | Growth promotion (increased biomass, 100 seed weight, shelling percentage, seed and pod HI)/Increased chlorophyll | Increased yield | [23] | |
| Peanut | Bacillus siamensis EB.CP6, B. velezensis EB.KN12, and B. methylotrophycus EB.KN13 | Reduction of disease (Phytophthora) (8.45–11.21%) and lower fatal rate (11.11–15.55%), increased plant height, length of roots and fresh biomass | N/A | [145] |
| Black pepper | Bacillus megaterium DS9 | Biocontrol of root-knot nematodes (Meloidogyne spp.), plant growth promotion | [62] | |
| Watermelon, melon | Trichoderma | Biocontrol against the main soil-borne diseases | [95] |
| Endophyte | Fruit/Vegetable | Effects/Possible Mechanisms | Influences on Postharvest Quality and Marketing Life | Reference |
|---|---|---|---|---|
| Lactobacillus spp. | Apple | Biocintrol of grey mould, soft rot (P. expansum, X. campestris, M. laxa, B. cinerea, E. carotovora) | Reduced foodborne human pathogens in ready-to-eat fresh fruits | [212] |
| Serratia plymuthica | Biocintrol of ring rot (Botryosphaeria dothidea) (−84.64%)/Expressions of genes related to membrane, catalytic activity, oxidation-reduction, metabolisms of tyrosine, glycolysis/gluconeogenesis, and glycerolipid | Reduced fruits titratable acidity (TA), enhanced soluble sugar (SS), vitamin C, SS/TA ratio, maintained firmness | [218] | |
| Bacillus velezensis P2-1 | Biocontrol of ring rot (Botryosphaeria dothidea)/Biosynthesis of antifungal LPs and polyketides, enhanced expression of MdPR1 and MdPR5 genes | Did not affect fruit qualities (firmness, TA, ascorbic acid, SS) but reduced postharvest decay | [219] | |
| B. velezensis | Grape berries | Biocontrol of grey mould (B. cinerea) | Reduced postharvest decay | [30] |
| Trichoderma afroharzianum, T. afroharzianum | Chili | Biocontrol of Fusarium infections (F. oxysporum and F. proliferatum) | Prevented significant market losse, reduced health hazards caused by Fusarium-associated mycotoxin | [220] |
| B. velezensis | Banana | Biocintrol of anthracnose (C. musae) | Reduced postharvest decay | [221] |
| Pseudomonas synxantha DLS65 | Peach | Biocintrol of brown rot (Monilinia fructicola, M. fructigen)/competition for nutrients and space, production of diffusible toxic metabolites and VOCs | Reduced pathogens in ready-to-eat fresh products | [222] |
| Bacillus amyloliquefaciens | Kiwifruit | Biocontrol of soft rot (Botryosphaeria dothidea) | Improved disease resistance, delayed senescence, maintained quality during storage | [223] |
| Enterobacter sp., Bacillus sp. | Tomato | Biocontrol of rot (B. cinerea) | Reduced postharvest decay | [213] |
| Pseudomonas putida BP25 | Mango | Biocontrol of anthracnose (Colletotrichum gloeosporioides)/Production of VOCs, proline, total-soluble solids, phenols, carotenoid, flavonoid | Increased fruit phytonutrient quality and firmness | [224] |
| Bacillus safensis B3 | Strawberry | Biocintrol of grey mold (B. cinerea Str5)/Enzymes (chitinase, hydrolytic lipase, protease) production | Reduced disease severity in fruit products and reduced postharvest decay | [215] |
| Daldinia eschscholtzii MFLUCC 19-0493 | Biocontrol of anthracnose (Colletotrichum acutatum)/Production of VOCs (elemicin, benzaldehyde dimethyl acetal, ethyl sorbate, methyl geranate, trans-sabinene hydrate, 3.5-dimethyl-4-heptanone) | [225] | ||
| B. subtilis L1-21 | Citrus Fruits | Biocintrol of citrus green mold (Penicillium digitatum)/Antifungal compounds surfactin, fengycin, bacillaene and bacilysin production | Reduced infestation of products with pathogen | [226] |
| B. subtilis 10-4, B. subtilis 26D | Potato | Biocontrol of Ph. infestans and F. oxysporum/Modulation of enzyme production (proteases, hydrolases), ascorbic acid, glykoalkaloids (solanine, chakonine), starch, reducing sugars | Prolonged shelf-life, increased vitamin C, reduced glykoalkaloids | [61,83] |
| NPs | Plants | Influence on Plants | Reference |
|---|---|---|---|
| Silver NPs (Ag NPs) | Tomato | Alleviated salt stress effects, germination percentage, improved germination rate, root length and seedling fresh and dry weight of tomato under NaCl stress | [276] |
| Decreased fungal spores (48.57%), SOD (39.59%), proline (28.57%) in Alternara alternata infected plants, not variation in terms of soil pH, cultured population, carbon source utilization pattern and soil enzymes (dehydrogenase, urease, protenase, and β-glucosidase), increased photosynthesis | [277] | ||
| Multiwalled carbon nanotubes (MWCNTs) | Tomato | Production of two times more flowers and fruit compared to control plants | [249] |
| Grape | Increased root length and germination rate (at 90 μg/mL of MWCNTs), decreased MDA and increased antioxidant capacity (SOD, CAT, POD, DHAR, APX, GST, GR) under salinity | [251] | |
| Carbon nanotubes (CNTs) | Tomato, radish, onion | Increased dry weight, improved germination percentage and rate | [237] |
| Turnip | No effect on germination and growth | [237] | |
| Carbon dots (CDs) nanocapsules | Lettuce | Increased photosynthesis rate, production yield, soluble sugar and soluble protein concentration | [250] |
| Zinc oxide NPs (ZnO NPs) | Eggplant | Increased relative water content, membrane stability, photosynthetic efficiency, improved stem and leaf anatomical structures, increased fruit yield (by 12.2–22.6%) | [256] |
| Tomato | Increased shoot and root lengths, biomass, leaf area, chlorophyll content, photosynthetic attributes of plants in the presence/absence of salt stress, enhanced protein content and antioxidative enzyme activity (POD, SOD, CAT) under salt stress, Alleviated of NaCl toxicity in plants | ||
| NPK + CeO₂ NPs | Cabbage | Increased leaf chlorophyll, cabbage head weight increased three times more than control | [257] |
| Metal NPs (CuO, ZnO, and FeO) | Tomato | Reduced incidence of bacterial wilt (Ralstonia solanacearum) disease, improved morphological and physiological parameters of plants, increase the Chao1 and Shannon index | [258] |
| Chitosan/Titanium Dioxide Nanoparticles/Sodium Tripolyphosphate (CS-TiO2-STP) | Cucumber | Enhanced post-harvest shelf-life (reduced decay after 21-day storage period) | [267] |
| CuO NPs and TiO2 NPs | Onion | Reduced mitotic index (MI) by 28% and 17%, increased ROS activity in roots | [272] |
| Al2O3 NPs | Augmented MI by 13%, increased ROS activity in roots | ||
| AgNO3 NPs and Ag NPs | Grape | Ag NPs and AgNO3 NPs enhanced grape brunches’ longevity and quality of up to 30 days Ag NPs produced the best results for soluble solids content, titratable acidity, MDA content, polyphenol oxidase, POD, and pectin methylestraese activity | [279] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lastochkina, O.; Aliniaeifard, S.; SeifiKalhor, M.; Bosacchi, M.; Maslennikova, D.; Lubyanova, A. Novel Approaches for Sustainable Horticultural Crop Production: Advances and Prospects. Horticulturae 2022, 8, 910. https://doi.org/10.3390/horticulturae8100910
Lastochkina O, Aliniaeifard S, SeifiKalhor M, Bosacchi M, Maslennikova D, Lubyanova A. Novel Approaches for Sustainable Horticultural Crop Production: Advances and Prospects. Horticulturae. 2022; 8(10):910. https://doi.org/10.3390/horticulturae8100910
Chicago/Turabian StyleLastochkina, Oksana, Sasan Aliniaeifard, Maryam SeifiKalhor, Massimo Bosacchi, Dilara Maslennikova, and Alsu Lubyanova. 2022. "Novel Approaches for Sustainable Horticultural Crop Production: Advances and Prospects" Horticulturae 8, no. 10: 910. https://doi.org/10.3390/horticulturae8100910
APA StyleLastochkina, O., Aliniaeifard, S., SeifiKalhor, M., Bosacchi, M., Maslennikova, D., & Lubyanova, A. (2022). Novel Approaches for Sustainable Horticultural Crop Production: Advances and Prospects. Horticulturae, 8(10), 910. https://doi.org/10.3390/horticulturae8100910








