Estimation of Heterosis and Combining Ability for Improving Yield, Sweetness, Carotenoid and Antioxidant Qualities in Pumpkin Hybrids (Cucurbita moschata Duch. Ex Poir.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Materials, Design and Location
2.2. Hybridization to Generate 42 F1 Hybrids and 7 Inbred Parental Lines
2.3. Agronomic Practices and Plant Protection
2.4. Data Collection
2.4.1. Data Collection for Yield and Yield Contributing Traits
2.4.2. Data Collection on Fruit Quality Traits
Total Soluble Solid (Sweetness) (°Brix)
Dry Matter Content (%)
Total Carotenoid Content Estimation
Methanolic Extraction and Estimation of Antioxidant (DPPH%)
2.5. Statistical Analysis
2.5.1. Calculating GCA, SCA and Reciprocal Variances
2.5.2. Estimates of Additive and Non-Additive Variances
2.5.3. Estimation of Heritability (%)
2.5.4. Genetic Ratio or Baker Ratio (BR)
2.5.5. Magnitude of Heterosis
Relative Heterosis or Mid-Parent Heterosis (MPH)
Heterobeltiosis or Better Parent Heterosis (BPH)
Control or Standard Heterosis (SH)
2.5.6. Test of Significance of Heterosis Magnitude
3. Results
3.1. Pooled ANOVA of All the Studied Traits Grown across Two Environments
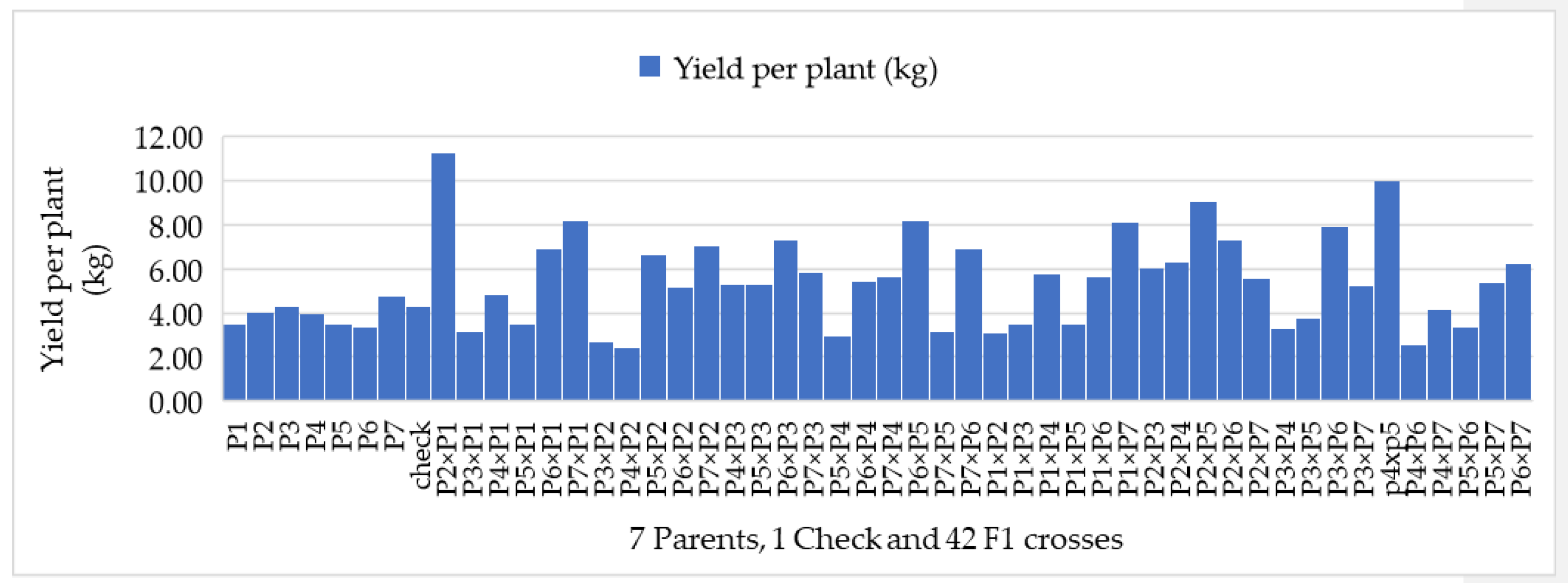
3.2. Mean Performance of F1 Hybrids with Their Parental Lines
3.3. ANOVA for Combining Ability, Maternal and Non-Maternal Variances
3.4. Genetic Variability Parameters and Gene Action
3.5. General Combining Ability (GCA) Effect of Parents and Specific Combining Ability (SCA) Effect of F1 Hybrids
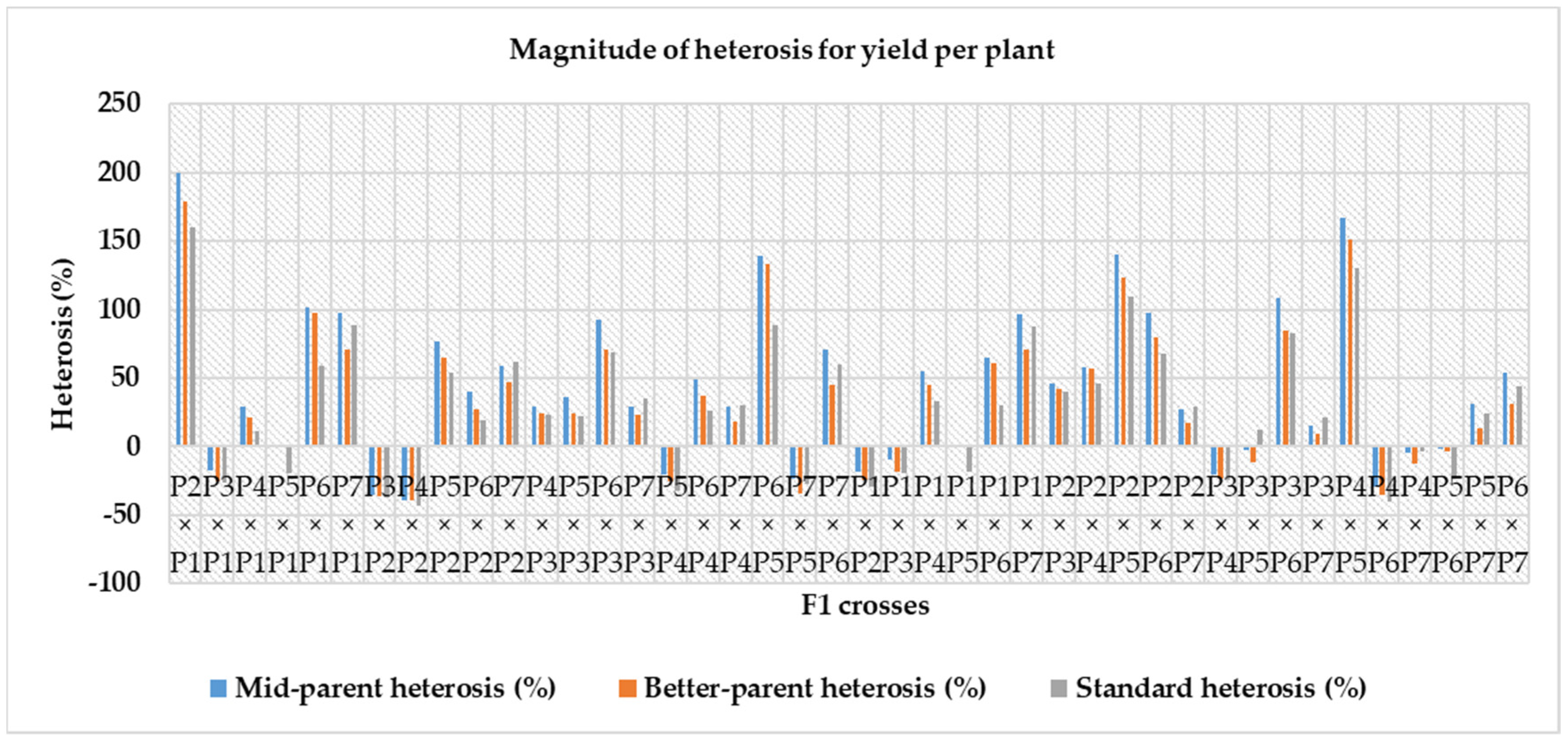
3.6. Magnitude of Heterosis over Mid-Parent, Better-Parent and Commercial Hybrids
4. Discussions
4.1. Pooled ANOVA for the Studied Traits Grown across Two Environments
4.2. Mean Performance for the Studied Traits
4.3. ANOVA Due to Combining Ability
4.4. Genetic Variability Parameters and Gene Action
4.5. General Combining Ability (GCA) and Specific Combining Ability (SCA) Effect
4.6. Magnitude of Heterosis over Mid-Parent, Better-Parent and Commercial Check Hybrid
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parental Lines | Experimental Code | Fruit Shape and Colour | Reference |
|---|---|---|---|
| Gold Butter 315 | P1 | Flattened and brown |  |
| 928 Fuxiang | P2 | Flattened and brown |  |
| Ser Bajadi | P3 | Flattened and black |  |
| Asian pumpkin | P4 | Flattened and brown |  |
| Sarawak | P5 | Pyriform and green |  |
| F3-2 | P6 | Pyriform and brown |  |
| Australia-1 | P7 | Flattened and green |  |
References
- Yadav, M.K.; Singh, D.P.; Rajiv, V.P.S.; Kumar, S. Combining ability analysis of yield and yields attributing traits of pumpkin (Cucurbita moschata Duch ex Poir). Pharma Innov. J. 2021, 10, 250–254. [Google Scholar]
- Yadav, M.; Jain, S.; Tomar, R.; Prasad, G.; Yadav, H. Medicinal and biological potential of pumpkin: An updated review. Nutr. Res. Rev. 2010, 23, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Hosen, M.; Rafii, M.Y.; Mazlan, N.; Jusoh, M.; Oladosu, Y.; Chowdhury, M.; Nazneen, F.; Muhammad, I.; Khan, M.M.H. Pumpkin (Cucurbita spp.): A Crop to Mitigate Food and Nutritional Challenges. Horticulturae 2021, 7, 352. [Google Scholar] [CrossRef]
- Adubofuor, J.; Anomah, J.W.; Amoah, I. Anti-nutritional factors and mineral composition of pumpkin pulp and functional properties of pumpkin-wheat composite flour for bread preparation. Int. J. Food Sci. Technol. 2018, 1, 1–9. [Google Scholar] [CrossRef]
- Kakamari, G.S.; Jagadeesha, R.C. Estimation of combining ability for growth, yield and its components in pumpkin (Cucurbita moschata Duch. Ex. Poir). Res. Environ. Life Sci. 2017, 10, 280–283. [Google Scholar]
- Hashash, M.M.; El-Sayed, M.M.; Abdel-Hady, A.A.; Hady, H.A.; Morsi, E.A. Nutritional potential, mineral composition and antioxidant activity squash (Curcurbita pepo L.) fruits grown in Egypt. Inflammation 2017, 9, 11–12. [Google Scholar]
- Nemeskéri, E. Breeding strategy for improvement of colour quality and carotenoid levels in dry pea seeds. Commun. Biometry Crop Sci. 2006, 1, 49–55. [Google Scholar]
- Seymen, M.; Uslu, N.; Türkmen, Ö.; Al Juhaimi, F.; Özcan, M.M. Chemical compositions and mineral contents of some hull-less pumpkin seed and oils. J. Am. Oil Chem. Soc. 2016, 93, 1095–1099. [Google Scholar] [CrossRef]
- Rani, R.; Kumar, S.; Yadav, S. Pumpkin and chia seed as dietary fibre source in meat products: A review. J. Pharm. Innov. 2021, 10, 477–485. [Google Scholar]
- Seymen, M. Seed Yield and Characteristics in a Half-Diallel Pumpkin Population. Selcuk J. Agric. Food Sci. 2020, 34, 200–206. [Google Scholar] [CrossRef]
- Caili, F.U.; Huan, S.; Quanhong, L.I. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum. Nutr. 2006, 61, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef]
- Nisha, S.K.; Veeraragavathatham, D. Heterosis and combining ability for fruit yield and its component traits in pumpkin (Cucurbita moschata Duch. ex Poir.). Adv. Appl. Sci. Res. 2014, 6, 158. [Google Scholar] [CrossRef]
- Norman, A.; Taylor, J.; Edwards, J.; Kuchel, H. Optimising genomic selection in wheat: Effect of marker density, population size and population structure on prediction accuracy. G3 Genes Genomes Genet. 2018, 8, 2889–2899. [Google Scholar] [CrossRef]
- Falconer, D.S. Introduction to Quantitative Genetics; Pearson Education India: Noida, India, 1996; ISBN 8131727408. [Google Scholar]
- Virmani, S.S. Heterosis in Rice. In Monogr. Theoretical and Applied Genetics; Virmani, S.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Fasahat, P.; Rajabi, A.; Rad, J.M.; Derera, J. Principles and utilization of combining ability in plant breeding. Biom. Biostat. Int. J. 2016, 4, 1–24. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Sugiyama, M.; Sakata, Y. Combining ability analysis of fruit texture traits in cucumber by mechanical measurement. Breed. Sci. 2010, 60, 65–70. [Google Scholar] [CrossRef]
- Ferreira, M.G.; de Almeida, G.Q.; Pessoa, H.P.; Dariva, F.D.; Dias, F.d.O.; Nick, C. Selection of squash “Menina Brasileira” carrying the allele “Bush” with high yield potential. Hortic. Bras. 2019, 37, 35–39. [Google Scholar] [CrossRef]
- Kearsey, M.J.; Pooni, H. Genetical Analysis of Quantitative Traits; Garland Science: New York, NY, USA, 2020; ISBN 1000144178. [Google Scholar]
- Griffing, B. Concept of general and specific combining ability in relation to diallel crossing systems. Aust. J. Biol. Sci. 1956, 9, 463–493. [Google Scholar] [CrossRef]
- Abdein, M.A.E.; Hassan, H.M.F.; Dalia, H.M. General performance, combining abilities and heritability of yield and yield component traits in pumpkin (Cucurbita moschata poir.) at different conditions. Curr. Appl. Sci. Technol. 2017, 17, 121–129. [Google Scholar]
- Díez, M.J.; Dooijeweert, W.; Maggioni, L.; Lipman, E. Minimum Descriptor lists for Cucurbit; cucumber, melon and watermelon. In Proceedings of the Peport of a Working Group on Cucurbits First Meeting, Plovdiv, Bulgaria, 1–2 September 2005; pp. 10–11. [Google Scholar]
- Kumar, V.; Mishra, D.; Yadav, G.; Yadav, S.; Kumar, S. Determining relationships between yield and biochemical traits in pumpkin. Pharm. Innovation. 2018, 7, 14–18. [Google Scholar]
- Lee, H.S.; Castle, W.S. Seasonal changes of carotenoid pigments and color in Hamlin, Earlygold, and Budd Blood orange juices. J. Agric. Food Chem. 2001, 49, 877–882. [Google Scholar] [CrossRef]
- Talcott, S.T.; Howard, L.R. Phenolic autoxidation is responsible for color degradation in processed carrot puree. J. Agric. Food Chem. 1999, 47, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Addai, Z.R.; Abdullah, A.; Mutalib, S.A. Influence of ripening stages on antioxidant properties of papaya fruit (Carica papaya L.). In Proceedings of the American Institute of Physics Proceedings; American Institute of Physics: College Park, MD, USA, 2013; Volume 1571, pp. 696–701. [Google Scholar]
- Musa, K.H.; Abdullah, A.; Jusoh, K.; Subramaniam, V. Antioxidant activity of pink-flesh guava (Psidium guajava L.): Effect of extraction techniques and solvents. Food Anal. Methods 2011, 4, 100–107. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, M.S.; Lamkey, K.R. DIALLEL-SAS05: A comprehensive program for Griffing’s and Gardner–Eberhart analyses. Agron. J. 2005, 97, 1097–1106. [Google Scholar] [CrossRef]
- Singh, R.K.; Chaudhary, B.D. Biometrical Method in Quantitative Genetic Analysis; Kalyani Publishers: New Delhi, India, 1985; pp. 127–140. [Google Scholar]
- Feyzian, E.; Dehghani, H.; Rezai, A.M.; Javaran, M.J. Diallel cross analysis for maturity and yield-related traits in melon (Cucumis melo L.). Euphytica 2009, 168, 215–223. [Google Scholar] [CrossRef]
- Baker, R.J. Issues in diallel analysis. Crop Sci. 1978, 18, 533–536. [Google Scholar] [CrossRef]
- Ene, C.O.; Ogbonna, P.E.; Agbo, C.U.; Chukwudi, U.P. Heterosis and combining ability in cucumber (Cucumis sativus L.). Inf. Process. Agric. 2019, 6, 150–157. [Google Scholar] [CrossRef]
- Mohsin, G.M.; Doullah, M.A.U.; Hasanuzzaman, M.; Biswas, B.K.; Islam, M.S.; Rahman, S.; Islam, A.; Dinajpur, B. Combining ability analysis in pumpkin (Cucurbita moschata Duch Ex Poir). Contemp. Res. India 2017, 7, 176–181. [Google Scholar]
- Darrudi, R.; Nazeri, V.; Soltani, F.; Shokrpour, M.; Ercolano, M.R. Evaluation of combining ability in Cucurbita pepo L. and Cucurbita moschata Duchesne accessions for fruit and seed quantitative traits. J. Appl. Res. Med. Aromat. Plants 2018, 9, 70–77. [Google Scholar] [CrossRef]
- Kumar, R.; Rajasree, V.; Praneetha, S.; Rajeswari, S.; Khuntia, S. Heterosis breeding in pumpkin (Cucurbita moschata Duch. ex Poir.) for small size, thick flesh with high yield and β-carotene. Int. J. Chem. Stud. 2018, 6, 81–85. [Google Scholar]
- Hatwal, P.K.; Yadav, V.S.; Thakur, R.; Mahawar, A.K. Estimation of heterosis in relation to combining ability for earliness, yield and quality attributes in pumpkin (Cucurbita moschata Duch. ex Poir). Indian J. Agric. Res. 2018, 52, 548–553. [Google Scholar]
- Marxmathi, P.; Krishnamoorthy, V. Per se performance of pumkin (Cucurbita moschata Duch ex Poir) hybrids for yield and quality. Asian J. Hortic. 2017, 12, 260–266. [Google Scholar] [CrossRef]
- Du, X.; Sun, Y.; Li, X.; Zhou, J.; Li, X. Genetic divergence among inbred lines in Cucurbita moschata from China. Sci. Hortic. (Amst.) 2011, 127, 207–213. [Google Scholar] [CrossRef]
- Seroczynska, A.; Antczak, A.; Korytowska, M.; Kaminska, K.; Radomski, A.; Korzeniewska, A.; Zawadzki, J.; Niemiriwicz-Szczytt, K. Evaluation of the selected forms of winter squash (Cucurbita maxima Duch.) for the content of free sugars and polysaccharides. Pol. J. Agron. 2014, 16, 69–73. [Google Scholar]
- Selvi, N.A.T.; Jansirani, P.; Pugalendhi, L. Studies on heterosis in pumpkin (Cucurbita moschata Duch. ex Poir). J. Hortic. Sci. 2014, 9, 131–140. [Google Scholar]
- Sharma, M.; Bhat, R. Extraction of carotenoids from pumpkin peel and pulp: Comparison between innovative green extraction technologies (ultrasonic and microwave-assisted extractions using corn oil). Foods 2021, 10, 787. [Google Scholar] [CrossRef]
- El-Tahawey, M.; Kandeel, A.M.; Youssef, S.M.S.; Abd El-Salam, M.M.M. Heterosis, potence ratio, combining ability and correlation of some economic traits in diallel crosses of pumpkins. Egypt. J. Plant Breed. 2015, 19, 419–439. [Google Scholar] [CrossRef]
- Hussien, A.; Hamed, A. Diallel Analysis for Studying Heterosis and Combining Ability of Some Economical Yield Traits in Pumpkin. Int. J. Plant Prod. 2015, 6, 261–270. [Google Scholar] [CrossRef]
- Gvozdanović-Varga, J.; Vasić, M.; Milić, D.; Červenski, J. Diallel cross analysis for fruit traits in watermelon. Genetika 2011, 43, 163–174. [Google Scholar] [CrossRef]
- Bahari, M.; Rafii, M.Y.; Saleh, G.B.; Latif, M.A. Combining ability analysis in complete diallel cross of watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai). Sci. World J. 2012, 543158, 6. [Google Scholar]
- Kundu, S.; Mandal, A.R.; Mukherjee, D.; Banerjee, S.; Ghosh, T.; Mandal, A.K.; Chattopadhyay, A. Breeding bitter gourd (Momordica charantia L.) for simultaneous improvement in yield, nutritional quality and downy mildew disease tolerance. Ann. Plant Soil Res. 2022, 24, 250–258. [Google Scholar]
- Golabadi, M.; Golkar, P.; Eghtedary, A. Combining ability analysis of fruit yield and morphological traits in greenhouse cucumber (Cucumis sativus L.). Can. J. Plant Sci. 2015, 95, 377–385. [Google Scholar] [CrossRef]
- Shafin, M.S.; Haque, M.E.; Parvin, M.S.; Akhter, F. Heterosis and Combining Ability in Pumpkin Inbreds (Cucurbita moschata Duch. ex Poir.). bioRxiv 2022, 9, 37–56. [Google Scholar]
- El-Gazzar, T.M.; Tartoura, E.A.; Nada, M.M. Evaluation of new inbred lines and their hybrids in balady squash variety (Cucurbita pepo L.). Int. J. Plant Prod. 2015, 6, 135–143. [Google Scholar] [CrossRef]
- El-hadi, A.H.A.; Fathy, M.; Abdein, M.A. Manifestation of heterosis and the role of the genetic parameters associated with it for some vegetative traits in squash (Cucurbita pepo, L.). Alexandria Sci. Exch. J. 2014, 35, 190–202. [Google Scholar]
- Marxmathi, P.; Krishnamoorthy, V.; Thankaraj, P. Combining ability studies in pumpkin (Cucurbita moschata Duch ex Poir). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3033–3039. [Google Scholar] [CrossRef][Green Version]
- Kaur, S.; Sharma, S.P.; Sarao, N.K.; Deol, J.K.; Gill, R.; Abd-Elsalam, K.A.; Alghuthaymi, M.A.; Hassan, M.M.; Chawla, N. Heterosis and Combining Ability for Fruit Yield, Sweetness, β-Carotene, Ascorbic Acid, Firmness and Fusarium Wilt Resistance in Muskmelon (Cucumis melo L.) Involving Genetic Male Sterile Lines. Horticulturae 2022, 8, 82. [Google Scholar] [CrossRef]
- Irshad, M.; Ahmad, I.; Mehdi, S.J.; Goel, H.C.; Rizvi, M.M.A. Antioxidant capacity and phenolic content of the aqueous extract of commonly consumed cucurbits. Int. J. Food Prop. 2014, 17, 179–186. [Google Scholar] [CrossRef]
- Karmakar, P.; Munshi, A.; Behera, T.; Kumar, R.; Kaur, C.; Singh, B. Hermaphrodite inbreds with better combining ability improve antioxidant properties in ridge gourd [Luffa acutangula (Roxb.) L.]. Euphytica 2013, 191, 75–84. [Google Scholar] [CrossRef]
- Patil, S.A.; Salimath, P.M.; Dharmatti, P.R.; Byadgi, A.S.; Nirmala, Y. Heterosis and combining ability analysis for productivity traits in bitter gourd (Momordica charantia L.). Karnataka J. Agric. Sci. 2012, 25, 9–13. [Google Scholar]
- Singh, A.K.; Pan, R.S.; Bhavana, P. Heterosis and combining ability analysis in bittergourd (Momordica charantia L.). Bioscan 2013, 8, 1533–1536. [Google Scholar]
- Gharib, A.H.A.M.; El Sayed, A.A.; El Tahawey, M.A.; Khafagi, E.Y. Breeding for fusarium wilt resistance and some economic characters in cucumber. J. Appl. Hortic. 2020, 22, 255–264. [Google Scholar]
- Hayes, J.D.; Foster, C.A. Heterosis in self pollinated crops with particular reference to barley, Heterosis in plant breeding. In Proceedings of the 7th Congress, European Association for Research on Plant Breeding, Budapest, Hungary, 24–29 June 1976; pp. 239–256. [Google Scholar]
- Khattak, G.S.S.; Ashraf, M.; Zamir, R. Gene action for synchrony in pod maturity and indeterminate growth habit in mungbean (Vigna radiata (L.) Wilczek). Pak. J. Bot. 2004, 36, 589–594. [Google Scholar]
- Kumar, V.; Mishra, D.P.; Yadav, G.C.; Yadav, S. Exploitation of heterobeltiosis and economic heterosis for horticultural yield, and its attributes and biochemical traits in pumpkin (Cucurbita moschata Duch. ex. Poir) under salt affected soil. Curr. Sci. 2018, 115, 1550–1556. [Google Scholar] [CrossRef]
- Jansi, V. Heterosis and inbreeding depression studies in pumpkin (Cucurbita moschata Duch. ex Poir.). Electron. J. Plant Breed. 2018, 9, 1031–1037. [Google Scholar] [CrossRef]
- El-Hadi, A.; El-Aziz, A.; Abd Alla, M.A.; Ashak, M.G. Genetic Evalution of some Economical Traits in Summer Squash. J. Agric. Chem. Biotechnol. 2020, 11, 147–153. [Google Scholar] [CrossRef]
- Bernardo, R. Breeding for quantitative traits in plants. In Woodbury; Stemma Press: Woodbury, MN, USA, 2014; Volume 1, p. 369. [Google Scholar]




| Month | Maximum Temperature (°C) | Minimum Temperature (°C) | Average Temperature (°C) | Precipitation (mm) |
|---|---|---|---|---|
| Season 1 | ||||
| October, 20 | 33.65 | 23.61 | 27.36 | 183.00 |
| November, 20 | 33.53 | 23.44 | 26.82 | 323.20 |
| December, 20 | 33.35 | 23.23 | 26.68 | 193.80 |
| January, 21 | 32.83 | 22.96 | 26.58 | 169.70 |
| Season 2 | ||||
| March, 21 | 35.03 | 23.42 | 27.44 | 316.40 |
| April, 21 | 34.99 | 23.64 | 27.43 | 285.40 |
| May, 21 | 34.63 | 24.02 | 27.65 | 357.00 |
| June, 21 | 34.32 | 23.41 | 27.53 | 115.00 |
| Parameters | Method of Assessment |
|---|---|
| Number of fruits per plant (NFPP, n) | From each entry until the final harvest, a record of the number of fruits collected per vine and per plant was kept. |
| Fruit flesh thickness (FT, mm) | The thickness of the fruit’s flesh at its broadest point was measured with a calliper. |
| Single fruit weight (SFW, kg) | Using the average weight of fruits per accession per replication. |
| Yield per plant (YPP, kg) | Fruits that had achieved full maturity were harvested from five plants regularly, and the total weight was kept until the final harvest. |
| Seed number per fruit (SNPF, n) | The average seed number of fruits per accessions was calculated. |
| Traits | G (df = 49) | E (df = 1) | R(E) (df = 4) | G × E (df = 49) | Error (df = 196) | CV (%) |
|---|---|---|---|---|---|---|
| NFPP | 4.38 ** | 6.05 ** | 0.04 | 0.84 ** | 0.15 | 29.93 |
| SFW | 125.00 ** | 20.83 * | 14.93 | 30.11 ** | 4.35 | 18.75 |
| FFT | 1.11 ** | 0.10 | 0.12 | 0.28 ** | 0.04 | 30.77 |
| YPP | 24.96 ** | 10.34 ** | 2.02 | 5.35 ** | 0.63 | 43.93 |
| SNPF | 53,410.09 ** | 7144.32 | 10,005.02 | 4582.28 ** | 2096.3 | 21.84 |
| TSS | 14.42 ** | 333.99 ** | 0.51 | 4.278 ** | 0.54 | 16.74 |
| DMC | 58.55 ** | 24.49 ** | 12.378 | 17.15 ** | 1.49 | 25.24 |
| TTC | 0.63 ** | 4.02 ** | 0.01 | 0.65 ** | 0.01 | 23.32 |
| DPPH | 341.59 ** | 192.03 ** | 0.96 | 21.47 ** | 1.25 | 10.79 |
| Genotypes | NFFP | FFT | SFW | YPP | SNPF | TSS | DMC | TTC | DPPH |
|---|---|---|---|---|---|---|---|---|---|
| Parents | |||||||||
| P1 | 2.50 m–q | 29.03 i–m | 1.41 m–r | 3.49 p–u | 313.50 w | 13.92 e–i | 23.36 a | 1.87 qrs | 69.71 lmn |
| P2 | 2.34 o–r | 27.83 l–q | 1.71 e–k | 4.05 o–r | 421.07 p–t | 12.23 n–s | 15.06 e–i | 1.95 o–r | 70.82 kl |
| P3 | 2.70 k–o | 26.30 n–t | 1.62 f–m | 4.28 m–q | 391.23 s–w | 11.59 rts | 11.48 o–s | 1.86 rs | 71.44 jk |
| P4 | 2.72 k–o | 29.68 h–l | 1.52 i–p | 3.97 o–s | 304.13 w | 12.49 l–r | 18.97 c | 2.70 a | 64.16 rst |
| P5 | 3.00 im | 26.23 m–t | 1.20 q–t | 3.49 p–u | 343.87 vw | 12.96 i–o | 12.15 m–r | 2.24 e–i | 75.04 gh |
| P6 | 3.56 e–h | 18.57 x | 1.00 t | 3.34 p–u | 474.43 m–q | 13.67 f–k | 10.45 s | 2.35 cde | 75.82 fgh |
| P7 | 2.71 k–o | 28.11 j–p | 1.97 de | 4.77 l–o | 456.17 n–r | 13.00 i–o | 11.99 m–s | 2.16 g–l | 78.53 e |
| Crosses | |||||||||
| P2 × P1 | 4.28 bcd | 35.18 bc | 2.85 a | 11.29 a | 585.57 b–h | 13.62 f–k | 15.55 d–h | 1.64 uv | 69.26 mno |
| P3 × P1 | 1.94 r | 26.78 m–t | 1.46 j–q | 3.19 q–u | 497.17 j–o | 10.48 uv | 12.82 l–p | 1.42 w | 92.15 a |
| P4 × P1 | 3.56 e–h | 27.34 l–s | 1.31 o–s | 4.81 l–o | 550.77 e–k | 12.35 m–s | 12.69 m–p | 2.26 d–h | 71.11 jkl |
| P5 × P1 | 2.50 m–q | 30.25 f–k | 1.46 j–r | 3.47 p–u | 388.23 tuv | 12.99 i–o | 19.66 bc | 2.09 j–o | 70.15 klm |
| P6 × P1 | 3.78 ef | 28.90 i–n | 1.94 de | 6.88 fgh | 472.97 m–q | 11.84 qrs | 11.75 n–s | 1.78 st | 67.40 pq |
| P7 × P1 | 3.57 e–h | 38.37 a | 2.39 c | 8.15 cd | 611.13 b–e | 15.13 bcd | 16.56 de | 1.80 ts | 67.87 opq |
| P3 × P2 | 2.00 qr | 24.90 r–v | 1.28 p–s | 2.71 tu | 331.37 w | 9.71 vw | 12.44 m–q | 1.52 vw | 92.68 a |
| P4 × P2 | 2.50 m–q | 21.90 w | 0.98 t | 2.45 u | 419.80 q–t | 11.55 rts | 19.82 bc | 1.52 vw | 67.46 pq |
| P5 × P2 | 5.22 a | 26.67 m–t | 1.40 m–r | 6.67 f–i | 412.83 q–t | 14.41 c–f | 10.99 qrs | 2.03 l–p | 64.39 rs |
| P6 × P2 | 5.17 a | 21.96 w | 1.09 st | 5.16 k–n | 453.20 n–s | 12.47 m–r | 11.41 o–s | 2.30 c–f | 70.41 klm |
| P7 × P2 | 3.67 efg | 33.28 cde | 1.96 de | 7.02 efg | 532.13 h–m | 13.48 f–l | 14.64 g–k | 2.53 b | 70.41 klm |
| P4 × P3 | 3.45 f–i | 28.95 i–m | 1.73 d–j | 5.33 j–m | 545.60 h–l | 12.13 n–s | 15.72 d–h | 1.91 p–s | 63.39 st |
| P5 × P3 | 3.78 ef | 25.65 o–t | 1.28 p–s | 5.30 j–m | 452.33 n–s | 11.73 rts | 16.71 de | 2.82 a | 79.10 e |
| P6 × P3 | 4.56 b | 26.60 m–t | 1.86 d–g | 7.33 def | 424.93 p–t | 14.39 c–f | 13.17 k–n | 1.70 tu | 69.18 mno |
| P7 × P3 | 3.83 def | 29.10 h–m | 1.54 h–p | 5.86 h–l | 559.47 d–j | 14.19 d–g | 13.60 i–m | 2.01 m–p | 62.88 t |
| P5 × P4 | 2.17 pqr | 26.37 m–t | 1.31 o–s | 2.95 stu | 496.03 k–o | 11.89 p–s | 8.66 t | 2.74 a | 92.94 a |
| P6 × P4 | 4.00 cde | 22.80 uvw | 1.42 l–r | 5.45 jkl | 431.40 p–t | 13.63 f–k | 12.74 m–p | 2.38 cd | 72.95 i |
| P7 × P4 | 3.50 e–i | 30.93 e–j | 1.64 f–m | 5.64 i–l | 500.60 j–o | 13.34 g–m | 14.83 f–j | 2.17 f–k | 68.49 nop |
| P6 × P5 | 5.50 a | 28.90 i–n | 1.62 f–n | 8.16 cd | 513.13 i–n | 14.06 e–h | 14.43 h–l | 2.51 b | 64.65 rs |
| P7 × P5 | 2.50 m–q | 23.90 t–w | 1.32 o–s | 3.16 r–u | 604.00 b–g | 12.87 j–p | 16.54 de | 2.12 i–n | 68.30 nop |
| P7 × P6 | 4.72 b | 22.60 vw | 1.30 o–s | 6.94 efg | 613.70 bcd | 15.20 bc | 17.01 d | 1.87 qrs | 74.71 h |
| Reciprocals | |||||||||
| P1 × P2 | 2.28 o–r | 27.82 k–r | 1.46 j–q | 3.08 r–u | 475.47 m–q | 11.62 rts | 17.13 d | 1.85 rs | 81.52 d |
| P1 × P3 | 2.56 m–p | 25.73 o–t | 1.32 o–s | 3.50 p–u | 311.80 w | 10.80 tu | 15.32 e–h | 1.61 uv | 89.29 b |
| P1 × P4 | 3.22 g–k | 27.78 k–r | 1.85 d–g | 5.77 i–l | 350.80 uvw | 14.76 be | 16.25 d–g | 2.21 f–j | 76.14 fg |
| P1 × P5 | 2.45 n–r | 26.18 m–t | 1.43 k–r | 3.52 p–u | 476.07 m–q | 11.40 st | 17.22 d | 1.78 st | 86.32 c |
| P1 × P6 | 3.39 f–j | 25.99 n–t | 1.78 d–i | 5.63 i–l | 548.60 f–k | 15.65 ab | 18.73 c | 1.99 n–s | 66.57 q |
| P1 × P7 | 3.56 e–h | 38.13 a | 2.49 bc | 8.13 cd | 640.83 b | 16.23 a | 21.13 b | 2.37 cde | 69.24 mno |
| P2 × P3 | 3.45 f–i | 24.96 r–v | 1.80 d–h | 6.07 g–k | 483.40 l–p | 13.13 h–n | 15.59 d–h | 2.29 c–g | 67.17 pq |
| P2 × P4 | 3.56 e–h | 32.56 c–f | 1.89 def | 6.34 f–j | 494.87 k–o | 12.57 l–r | 13.44 j–n | 2.29 c–g | 65.18 r |
| P2 × P5 | 3.56 e–h | 39.80 a | 2.65 ab | 9.06 c | 520.83 i–m | 12.83 k–q | 13.43 j–n | 2.08 j–o | 73.16 i |
| P2 × P6 | 4.28 bcd | 31.80 d–h | 1.73 e–j | 7.29 def | 571.60 c–i | 13.87 e–j | 14.46 h–l | 2.08 j–o | 72.93 i |
| P2 × P7 | 3.17 g–l | 31.30 e–i | 1.89 def | 5.59 jkl | 608.33 b–f | 13.59 f–k | 16.41 def | 1.78 st | 76.63 f |
| P3 × P4 | 2.39 n–r | 24.77 s–v | 1.36 m–s | 3.28 p–u | 383.30 tuv | 9.86 vw | 12.96 l–o | 2.00 m–q | 71.47 jk |
| P3 × P5 | 2.67 l–p | 26.81 m–t | 1.48 j–q | 3.79 o–t | 441.27 o–t | 9.33 w | 14.40 h–l | 2.14 h–m | 75.90 fgh |
| P3 × P6 | 4.33 bc | 30.77 e–j | 1.94 de | 7.92 de | 531.33 h–m | 13.61 f–k | 13.34 j–n | 1.93 pqr | 70.25 klm |
| P3 × P7 | 2.89 j–n | 37.52 ab | 2.02 d | 5.24 klm | 555.17 d–k | 12.15 n–s | 12.03 m–s | 2.02 m–p | 67.60 pq |
| P4 × P5 | 3.67 efg | 32.57 c–f | 2.84 a | 9.97 b | 702.13 a | 12.42 m–r | 11.32 o–s | 1.79 st | 72.35 ij |
| P4 × P6 | 2.17 pqr | 22.63 vw | 1.17 rst | 2.57 u | 525.43 h–m | 9.91 uvw | 15.66 d–h | 2.34 cde | 66.53 q |
| P4 × P7 | 3.33 f–j | 32.27 d–g | 1.33 n–s | 4.18 n–r | 443.83 o–t | 12.05 o–s | 11.42 o–s | 2.42 bc | 64.81 rs |
| P5 × P6 | 2.67 l–p | 25.43 p–u | 1.28 p–s | 3.35 p–u | 343.17 vw | 10.51 uv | 10.56 rs | 1.46 w | 76.47 f |
| P5 × P7 | 3.11 h–l | 28.37 j–o | 1.70 e–l | 5.40 jkl | 407.87 r–u | 12.52 l–r | 11.22 p–s | 2.08 j–o | 71.22 jk |
| P6 × P7 | 3.39 f–j | 34.20 cd | 1.85 d–g | 6.24 g–k | 512.83 i–n | 12.27 n–s | 13.29 j–n | 2.17 f–k | 64.67 rs |
| Check means | 2.78 k–o | 34.25 cd | 1.60 g–o | 4.33 m–p | 622.50 bc | 14.33 c–g | 20.86 b | 2.04 k–p | 68.06 op |
| Mean | 3.29 | 28.57 | 1.65 | 5.31 | 481.04 | 12.73 | 14.63 | 2.06 | 72.38 |
| Standard Error | 0.06 | 0.31 | 0.03 | 0.13 | 6.07 | 0.12 | 0.21 | 0.03 | 0.45 |
| Maximum | 6.33 | 50.20 | 3.74 | 14.10 | 870.00 | 20.45 | 24.42 | 3.35 | 94.29 |
| Minimum | 1.33 | 16.20 | 0.78 | 1.23 | 223.40 | 8.63 | 4.26 | 0.97 | 60.78 |
| Traits | GCA (df = 6) | SCA (df = 21) | REC (df = 21) | MAT (df = 6) | NONM (df = 15) | GCA × E (df = 6) | SCA × E (df = 21) | REC × E (df = 21) | MAT × E (df = 6) | NONM × E (df = 15) |
|---|---|---|---|---|---|---|---|---|---|---|
| NFPP | 8.40 ** | 3.34 ** | 4.40 ** | 4.69 ** | 4.28 ** | 2.09 ** | 0.54 ** | 0.81 ** | 1.46 ** | 0.55 ** |
| SFW | 1.18 ** | 0.99 ** | 1.25 ** | 2.33 ** | 0.82 ** | 0.03 ** | 0.19 ** | 0.45 ** | 0.21 ** | 0.55 ** |
| FFT | 257.77 ** | 99.35 ** | 111.61 ** | 191.53 ** | 79.64 ** | 20.00 ** | 18.42 ** | 44.69 ** | 32.86 ** | 49.42 ** |
| YPP | 15.68 ** | 24.29 ** | 29.20 ** | 43.48 ** | 23.49 ** | 7.80 ** | 4.03 ** | 6.20 ** | 6.63 ** | 6.03 ** |
| SNPF | 70,982.91 ** | 51,237.38 ** | 47,271.57 ** | 109,007.08 ** | 22,577.36 ** | 3375.38 | 3951.94 * | 5775.55 ** | 7870.29 ** | 4937.65 ** |
| TSS | 32.85 ** | 9.48 ** | 14.03 ** | 17.50 ** | 12.65 ** | 5.02 ** | 4.45 ** | 4.04 ** | 5.73 ** | 3.37 ** |
| DMC | 147.10 ** | 45.28 ** | 37.98 ** | 57.93 ** | 29.99 ** | 11.83 ** | 13.57 ** | 22.75 ** | 21.38 ** | 23.30 ** |
| TTC | 1.34 ** | 0.37 ** | 0.72 ** | 0.50 ** | 0.81 ** | 0.38 ** | 0.46 ** | 0.95 ** | 1.12 ** | 0.89 ** |
| DPPH | 396.94 ** | 384.98 ** | 293.22 ** | 374.58 ** | 260.68 ** | 30.20 ** | 15.07 ** | 26.33 ** | 45.13 ** | 18.81 ** |
| Traits | σ2e | σ2g | σ2s | σ2r | σ2A | σ2D | h2B | h2N | BR |
|---|---|---|---|---|---|---|---|---|---|
| NFPP | 0.14 | 0.59 | 3.2 | 2.13 | 1.18 | 3.2 | 99.47 | 26.80 | 0.27 |
| SFW | 0.04 | 0.08 | 0.95 | 0.61 | 0.16 | 0.95 | 99.40 | 14.55 | 0.15 |
| FFT | 4.44 | 18.1 | 94.91 | 53.59 | 36.19 | 94.91 | 99.44 | 27.45 | 0.28 |
| YPP | 0.63 | 1.08 | 23.66 | 14.29 | 2.15 | 23.66 | 99.59 | 8.30 | 0.08 |
| SNPF | 2106.58 | 4919.74 | 49,130.8 | 22,582.5 | 9839.48 | 49,130.8 | 99.41 | 16.59 | 0.17 |
| TSS | 0.55 | 2.31 | 8.93 | 6.74 | 4.61 | 8.93 | 99.33 | 33.84 | 0.34 |
| DMC | 1.48 | 10.4 | 43.8 | 18.25 | 20.8 | 43.8 | 99.62 | 32.08 | 0.32 |
| TTC | 0.02 | 0.09 | 0.35 | 0.35 | 0.19 | 0.35 | 99.38 | 34.80 | 0.35 |
| DPPH | 1.26 | 28.26 | 383.72 | 145.98 | 56.53 | 383.72 | 99.95 | 12.83 | 0.13 |
| NFPP | FFT | SFW | YPP | SNPF | TSS | DMC | TTC | DPPH | |
|---|---|---|---|---|---|---|---|---|---|
| GCA effects of parents | |||||||||
| P1 | −0.30 ** | 1.29 ** | 0.10 ** | −0.02 ns | −11.27 * | 0.49 ** | 2.75 ** | −0.16 ** | 2.28 ** |
| P2 | 0.11 ** | 0.67 ** | 0.09 ** | 0.44 ** | 2.67 ns | −0.04 ns | 0.17 ns | −0.07 ** | −0.12 ** |
| P3 | −0.21 ** | −0.95 ** | −0.05 ** | −0.47 ** | −28.19 ** | −0.94 ** | −0.85 ** | −0.13 ** | 2.10 ** |
| P4 | −0.23 ** | −0.58 ** | −0.09 ** | −0.57 ** | −17.24 ** | −0.46 ** | 0.03 ns | 0.19 ** | −2.38 ** |
| P5 | −0.03 ns | −0.36 ns | −0.07 ** | −0.20 ** | −17.75 ** | −0.35 ** | −0.97 ** | 0.09 ** | 2.18 ** |
| P6 | 0.63 ** | −2.76 ** | −0.15 ** | 0.35 ** | 14.07 ** | 0.49 ** | −1.11 ** | 0.03 ** | −1.87 ** |
| P7 | 0.03 ns | 2.70 ** | 0.16 ** | 0.46 ** | 57.72 ** | 0.80 ** | −0.02 ns | 0.06 ** | −2.19 ** |
| SCA effects of crosses | |||||||||
| P2 × P1 | 0.16 ns | 1.08 * | 0.31 ** | 1.42 ** | 60.96 ** | −0.54 ** | −1.09 ** | −0.07 ** | 0.76 ** |
| P3 × P1 | −0.54 ** | −2.55 ** | −0.31 ** | −1.50 ** | −34.22 ** | −1.61 ** | −2.33 ** | −0.26 ** | 13.87 ** |
| P4 × P1 | 0.62 ** | −1.60 ** | −0.09 ns | 0.55 ** | 1.14 ns | 0.82 ** | −2.81 ** | 0.15 ** | 1.26 ** |
| P5 × P1 | −0.50 ** | −1.17* | −0.24 ** | −1.61 ** | −16.98 ns | −0.64 ** | 2.16 ** | −0.05 * | 1.31 ** |
| P6 × P1 | −0.05 ns | 0.46 ns | 0.26 ** | 0.59 ** | 29.83 ** | 0.06 ns | −0.90 ** | −0.04 ns | −5.89 ** |
| P7 × P1 | 0.52 ** | 5.80 ** | 0.52 ** | 2.37 ** | −45.25 ** | 1.69 ** | 1.62 ** | 0.04 ns | 5.48 ** |
| P3 × P2 | −0.48 ** | −3.25 ** | −0.15 ns | −0.92 ** | −6.25 ns | −0.31 ns | 0.20 ns | −0.27 ** | −3.64 ** |
| P4 × P2 | −0.15 ns | −1.31 ** | −0.22 ** | −0.81 ** | 3.76 ns | −0.15 ns | 1.93 ** | −0.02 ns | −5.75 ** |
| P5 × P2 | 1.01 ** | 4.47 ** | 0.35 ** | 2.30 ** | 17.51 ns | 1.31 ** | −1.50 ** | 0.18 ** | 1.19 ** |
| P6 × P2 | 0.68 ** | 0.52 ns | −0.18 ** | 0.10 ns | 31.72 ** | 0.01 ns | −0.63 * | −0.17 ** | −4.75 ** |
| P7 × P2 | −0.03 ns | 0.46 ns | 0.02 ns | 0.07 ns | 14.58 ns | 0.07 ns | 0.88 ** | 0.45 ** | 0.75 ** |
| P4 × P3 | 0.06 ns | −0.07 ns | 0.04 ns | 0.01 ns | 14.09 ns | −0.31 ns | 0.66 * | −0.15 ** | −2.98 ** |
| P5 × P3 | 0.16 ns | −0.92 ns | −0.15 ** | −0.12 ns | 155.92 ** | −0.88 ** | 2.88 ** | −0.07 ** | 10.39 ** |
| P6 × P3 | 0.72 ** | 3.94 ** | 0.46 ** | 2.41 ** | 3.43 ns | 1.74 ** | 0.72 * | 0.09 ** | 1.5 ** |
| P7 × P3 | 0.24 ** | 3.10 ** | 0.02 ns | 0.23 ns | −46.32 ** | 0.61 ** | −0.81 ** | −0.19 ** | −2.22 ** |
| P5 × P4 | −0.12 ns | 1.95 ** | 0.58 ** | 1.90 ** | 101.38 ** | 0.27 ns | −3.57 ** | 0.13 ** | −4.00 ** |
| P6 × P4 | −0.61 ** | −2.39 ** | −0.12 * | −1.11 ** | 31.69 ** | −0.97 ** | 0.78 ** | 0.11 ** | 3.36 ** |
| P7 × P4 | 0.32 ** | 1.03 * | −0.24 ** | −0.32 ns | 49.63 ** | −0.35 ns | −1.38 ** | 0.02 ns | −7.14 ** |
| P6 × P5 | 0.18 ns | 1.83 ** | 0.02 ns | 0.27 ns | −46.42 ** | −0.56 ** | 0.01 ns | −0.01 ns | −1.24 ** |
| P7 × P5 | −0.50 ** | −4.66 ** | −0.24 ** | −1.31 ** | −12.19 ns | −0.45 * | 0.37 ns | −0.11 ** | −2.69 ** |
| P7 × P6 | 0.09 ns | 0.01 ns | −0.08 ns | 0.44 * | 13.32 ns | −0.26 ns | 1.78 ** | −0.13 ** | 1.28 ** |
| SCA effects of reciprocal crosses | |||||||||
| P1 × P2 | −1.00 ** | −3.68 ** | −0.69 ** | −4.11 ** | −55.05 ** | −1.00 ** | 0.79 * | 0.11 ** | 6.13 ** |
| P1 × P3 | 0.31 ** | −0.53 ns | −0.07 ns | 0.15 ns | −92.68 ** | 0.16 ns | 1.25 ** | 0.09 ** | −1.43 ** |
| P1 × P4 | −0.17 ns | 0.22 ns | 0.27 ** | 0.48* | −99.98 ** | 1.21 ** | 1.78 ** | −0.03 ns | 2.51 ** |
| P1 × P5 | −0.03 ns | −2.03 ** | −0.01 ns | 0.03 ns | 43.92 ** | −0.79 ** | −1.22 ** | −0.15 ** | 8.08 ** |
| P1 × P6 | −0.20 ns | −1.46 * | −0.08 ns | −0.63 ** | 37.82 ** | 1.91 ** | 3.49 ** | 0.10 ** | −0.42 ns |
| P1 × P7 | −0.01 ns | −0.12 ns | 0.05 ns | −0.01 ns | 14.85 ns | 0.55 ** | 2.29 ** | 0.28 ** | 0.69 ** |
| P2 × P3 | 0.72 ** | 0.03 ns | 0.26 ** | 1.68 ** | 76.02 ** | 1.71 ** | 1.58 ** | 0.39 ** | −12.76 * |
| P2 × P4 | 0.53 ** | 5.33 ** | 0.46 ** | 1.95 ** | 37.53 ** | 0.51 * | −3.19 ** | 0.38 ** | −1.14 ** |
| P2 × P5 | −0.83 ** | 6.57 ** | 0.62 ** | 1.20 ** | 54.00 ** | −0.79 ** | 1.22 ** | 0.02 ns | 4.38 ** |
| P2 × P6 | −0.44 ** | 4.92 ** | 0.32 ** | 1.06 ** | 59.20 ** | 0.70 ** | 1.53 ** | −0.11 ** | 1.26 ** |
| P2 × P7 | −0.25 * | −0.99 ns | −0.04 ns | −0.72 ** | 38.10 ** | 0.06 ns | 0.88 ** | −0.38 ** | 3.11 ** |
| P3 × P4 | −0.53 ** | −2.09 ** | −0.19 ** | −1.03 ** | −81.15 ** | −1.13 ** | −1.38 ** | 0.05 ns | 4.04 ** |
| P3 × P5 | −0.56 ** | 0.58 ns | 0.10 ns | −0.76 ** | −5.53 ns | −1.20 ** | −1.15 ** | −0.34 ** | −1.60 ** |
| P3 × P6 | −0.11 ns | 2.08 ** | 0.04 ns | 0.30 ns | 53.20 ** | −0.39 ns | 0.08 ns | 0.12 ** | 0.53 ns |
| P3 × P7 | −0.47 ** | 4.21 ** | 0.24 ** | −0.31 ns | −2.15 ns | −1.02 ** | −0.78 * | 0.02 ns | 2.36 ** |
| P4 × P5 | 0.75 ** | 3.10 ** | 0.76 ** | 3.51 ** | 103.05 ** | 0.26 ns | 1.33 ** | −0.47 ** | −10.30 ** |
| P4 × P6 | −0.92 ** | −0.08 ns | −0.13 * | −1.44 ** | 47.02 ** | −1.86 ** | 1.46 ** | −0.02 ns | −3.21 ** |
| P4 × P7 | −0.08 ns | 0.67 ns | −0.15 ** | −0.73 ** | −28.38 * | −0.65 ** | −1.71 ** | 0.12 ** | −1.84 ** |
| P5 × P6 | −1.42 ** | −1.73 ** | −0.17 ** | −2.40 ** | −84.98 ** | −1.78 ** | −1.93 ** | −0.53 ** | 5.91 ** |
| P5 × P7 | 0.31 ** | 2.23 ** | 0.19 ** | 1.12 ** | −98.07 ** | −0.18 ns | −2.66 ** | −0.02 ns | 1.46 ** |
| P6 × P7 | −0.67 ** | 5.80 ** | 0.27 ** | −0.35 ns | −50.43 ** | −1.47 ** | −1.86 ** | 0.15 ** | −5.02 ** |
| NFPP | FFT | SFW | YPP | |||||||||
| Crosses | MPH | BPH | SH | MPH | BPH | SH | MPH | BPH | SH | MPH | BPH | SH |
| P2 × P1 | 76.97 ** | 71.13 ** | 53.99 ** | 23.75 ** | 21.2 ** | 2.72 * | 82.28 ** | 66.05 ** | 78.93 ** | 199.32 ** | 178.46 ** | 160.46 ** |
| P3 × P1 | −25.57 ** | −28.60 ** | −30.05 ** | −3.19 ** | −7.74 ** | −21.80 ** | −3.41 ** | −9.77 ** | −7.97 ** | −17.92 ** | −25.51 ** | −26.42 ** |
| P4 × P1 | 36.12 ** | 29.27 ** | 27.96 ** | −6.86 ** | −7.88 ** | −20.17 ** | −10.38 ** | −13.53 ** | −17.61 ** | 28.97 ** | 21.17 ** | 10.96 ** |
| P5 × P1 | −9.09 ** | −16.67 ** | −10.02 ** | 9.48 ** | 4.20 ** | −11.68 ** | 11.43 ** | 3.32 ** | −8.49 ** | −0.50 ns | −0.52 ns | −19.88 ** |
| P6 × P1 | 24.86 ** | 6.33 ** | 36.05 ** | 21.44 ** | −0.45 ns | −15.62 ** | 61.44 ** | 37.75 ** | 22.01 ** | 101.56 ** | 97.23 ** | 58.77 ** |
| P7 × P1 | 36.67 ** | 30.23 ** | 28.43 ** | 34.29 ** | 32.16 ** | 12.02 ** | 41.25 ** | 21.03 ** | 50.21 ** | 97.42 ** | 70.87 ** | 88.15 ** |
| P3 × P2 | −20.90 ** | −26.52 ** | −28.01 ** | −8.00 ** | −10.5 ** | −27.30 ** | −23.04 ** | −25.39 ** | −19.29 ** | −34.96 ** | −36.68 ** | −37.46 ** |
| P4 × P2 | −1.09 ** | −9.03 ** | −9.96 ** | −23.95 ** | −26.22 ** | −36.06 ** | −39.29 ** | −42.80 ** | −38.36 ** | −39.04 ** | −39.68 ** | −43.58 ** |
| P5 × P2 | 95.81 ** | 74.11 ** | 88.00 ** | −1.35 ns | −4.19 ** | −22.14 ** | −4.00 ** | −13.67 ** | −11.95 ** | 76.71 ** | 64.43 ** | 53.81 ** |
| P6 × P2 | 75.44 ** | 45.34 ** | 85.97 ** | −5.34 ** | −21.09 ** | −35.88 ** | −19.88 ** | −28.38 ** | −31.76 ** | 39.66 ** | 27.35 ** | 19.12 ** |
| P7 × P2 | 45.02 ** | 33.82 ** | 31.98 ** | 18.97 ** | 18.38 ** | −2.84 ** | 6.24 ** | −0.76 ** | 23.16 ** | 59.17 ** | 47.19 ** | 62.08 ** |
| P4 × P3 | 26.54 ** | 25.27 ** | 24.00 ** | 3.42 ** | −2.48 * | −15.48 ** | 10.52 ** | 6.99 ** | 9.01 ** | 29.24 ** | 24.53 ** | 23.00 ** |
| P5 × P3 | 32.07 ** | 25.94 ** | 35.99 ** | −2.35 ns | −2.47 * | −25.11 ** | −9.38 ** | −20.99 ** | −19.50 ** | 36.34 ** | 23.75 ** | 22.23 ** |
| P6 × P3 | 45.14 ** | 28.13 ** | 63.95 ** | 18.57 ** | 1.14 ns | −22.34 ** | 41.78 ** | 14.51 ** | 16.67 ** | 92.34 ** | 71.18 ** | 69.08 ** |
| P7 × P3 | 40.84 ** | 39.90 ** | 37.97 ** | 6.97 ** | 3.52 ** | −15.04 ** | −14.42 ** | −22.04 ** | −3.25 ** | 29.52 ** | 22.84 ** | 35.27 ** |
| P5 × P4 | −24.28 ** | −27.78 ** | −22.01 ** | −5.69 ** | −11.17 ** | −23.02 ** | −3.49 ** | −13.70 ** | −17.50 ** | −20.89 ** | −25.66 ** | −31.92 ** |
| P6 × P4 | 27.48 ** | 12.57 ** | 44.03 ** | −5.49 ** | −23.19 ** | −33.43 ** | 13.01 ** | −6.69 ** | −10.80 ** | 49.09 ** | 37.26 ** | 25.69 ** |
| P7 × P4 | 28.56 ** | 27.74 ** | 25.98 ** | 7.05 ** | 4.21 ** | −9.68 ** | −6.26 ** | −17.01 ** | 2.83 ** | 29.10 ** | 18.23 ** | 30.19 ** |
| P6 × P5 | 67.81 ** | 54.71 ** | 97.96 ** | 29.02 ** | 10.17 ** | −15.62 ** | 47.23 ** | 34.49 ** | 1.78 ** | 138.91 ** | 133.72 ** | 88.23 ** |
| P7 × P5 | −12.61 ** | −16.67 ** | −10.02 ** | −12.0 ** | −14.98 ** | −30.22 ** | −16.79 ** | −32.91 ** | −16.87 ** | −23.62 ** | −33.88 ** | −27.19 ** |
| P7 × P6 | 50.45 ** | 32.82 ** | 69.95 ** | −3.16 ** | −19.60 ** | −34.01 ** | −12.19 ** | −33.84 ** | −18.03 ** | 71.10 ** | 45.41 ** | 60.12 ** |
| Reciprocal crosses | ||||||||||||
| P1 × P2 | −5.82 ** | −8.93 ** | −18.05 ** | −2.16 ns | −4.18 ** | −18.78 ** | −6.35 ** | −14.69 ** | −8.07 ** | −18.45 ** | −24.14 ** | −29.04 ** |
| P1 × P3 | −2.07 ** | −6.06 ** | −7.98 ** | −7.00 ** | −11.37 ** | −24.88 ** | −12.65 ** | −18.40 ** | −16.77 ** | −9.98 ** | −18.30 ** | −19.31 ** |
| P1 × P4 | 23.36 ** | 17.15 ** | 15.96 ** | −5.36 ** | −6.40 ** | −18.88 ** | 26.23 ** | 21.78 ** | 16.04 ** | 54.81 ** | 45.45 ** | 33.19 ** |
| P1 × P5 | −11.09 ** | −18.50 ** | −12.00 ** | −5.24 ** | −9.81 ** | −23.55 ** | 9.51 ** | 1.54 ** | −10.06 ** | 0.93 * | 0.91 * | −18.73 ** |
| P1 × P6 | 11.92 ** | −4.69 ** | 21.96 ** | 9.19 ** | −10.49 ** | −24.13 ** | 48.27 ** | 26.51 ** | 12.06 ** | 64.85 ** | 61.30 ** | 29.85 ** |
| P1 × P7 | 36.16 ** | 29.74 ** | 27.96 ** | 33.47 ** | 31.36 ** | 11.34 ** | 46.97 ** | 25.93 ** | 56.29 ** | 96.93 ** | 70.45 ** | 87.69 ** |
| P2 × P3 | 36.25 ** | 26.58 ** | 24.00 ** | −7.80 ** | −10.34 ** | −27.14 ** | 7.95 ** | 4.65 ** | 13.21 ** | 45.56 ** | 41.71 ** | 39.96 ** |
| P2 × P4 | 40.56 ** | 29.27 ** | 27.96 ** | 13.21 ** | 9.69 ** | −4.94 ** | 17.29 ** | 10.51 ** | 19.08 ** | 58.07 ** | 56.42 ** | 46.31 ** |
| P2 × P5 | 33.27 ** | 18.50 ** | 27.96 ** | 47.23 ** | 43.00 ** | 16.20 ** | 81.38 ** | 63.10 ** | 66.35 ** | 140.30 ** | 123.60 ** | 109.15 ** |
| P2 × P6 | 45.27 ** | 20.35 ** | 53.99 ** | 37.07 ** | 14.25 ** | −7.15 ** | 27.63 ** | 14.08 ** | 8.70 ** | 97.25 ** | 79.85 ** | 68.23 ** |
| P2 × P7 | 25.18 ** | 15.51 ** | 13.92 ** | 11.90 ** | 11.35 ** | −8.61 ** | 2.35 ** | −4.39 ** | 18.66 ** | 26.69 ** | 17.15 ** | 29.00 ** |
| P3 × P4 | −12.21 ** | −13.09 ** | −13.98 ** | −11.5 ** | −16.56 ** | −27.69 ** | −13.39 ** | −16.15 ** | −14.57 ** | −20.55 ** | −23.44 ** | −24.38 ** |
| P3 × P5 | −6.79 ** | −11.11 ** | −4.02 ** | 2.05 ns | 1.92 ns | −21.74 ** | 4.90 ** | −8.54 ** | −6.81 ** | −2.57 ** | −11.57 ** | 12.65 ** |
| P3 × P6 | 38.08 ** | 21.89 ** | 55.97 ** | 37.15 ** | 16.98 ** | −10.17 ** | 48.41 ** | 19.86 ** | 22.12 ** | 107.97 ** | 85.09 ** | 82.81 ** |
| P3 × P7 | 6.12 ** | 5.41 ** | 3.96 ** | 37.90 ** | 33.46 ** | 9.54 ** | 12.28 ** | 2.28 ** | 26.94 ** | 15.71 ** | 9.74 ** | 20.85 ** |
| P4 × P5 | 28.07 ** | 22.17 ** | 31.92 ** | 16.49 ** | 9.72 ** | −4.91 ** | 108.59 ** | 86.51 ** | 78.30 ** | 167.44 ** | 151.32 ** | 130.15 ** |
| P4 × P6 | −30.98 ** | −39.05 ** | −22.01 ** | −6.18 ** | −23.75 ** | −33.92 ** | −6.91 ** | −23.14 ** | −26.52 ** | −29.70 ** | −35.28 ** | −40.73 ** |
| P4 × P7 | 22.44 ** | 21.65 ** | 19.98 ** | 11.66 ** | 8.70 ** | −5.80 ** | −23.56 ** | −32.32 ** | −16.14 ** | −4.46 ** | −12.50 ** | −3.65 ** |
| P5 × P6 | −18.59 ** | −24.94 ** | −3.96 ** | 13.54 ** | −3.05 * | −25.74 ** | 16.45 ** | 6.37 ** | −19.50 ** | −1.83 ** | −3.96 ** | −22.65 ** |
| P5 × P7 | 8.77 ** | 3.72 ** | 12.00 ** | 4.40 ** | 0.91 ns | −17.18 ** | 6.82 ** | −13.87 ** | 6.71 ** | 30.68 ** | 13.13 ** | 24.58 ** |
| P6 × P7 | 7.96 ** | −4.69 ** | 21.96 ** | 46.54 ** | 21.66 ** | −0.15 ns | 24.43 ** | −6.26 ** | 16.14 ** | 53.84 ** | 30.74 ** | 43.96 ** |
| CD (5%) | 0.43 | 0.43 | 0.43 | 2.40 | 2.40 | 2.40 | 0.23 | 0.23 | 0.23 | 0.9 | 0.9 | 0.9 |
| CD (1%) | 0.56 | 0.56 | 0.56 | 3.16 | 3.16 | 3.16 | 0.3 | 0.3 | 0.3 | 1.19 | 1.19 | 1.19 |
| TSS | DMC | TCC | DPPH | |||||||||
| Crosses | MPH | BPH | SH | MPH | BPH | SH | MPH | BPH | SH | MPH | BPH | SH |
| P2 × P1 | 4.19 ** | −2.11 ** | −4.95 ** | −19.07 ** | −33.45 ** | −25.48 ** | −14.15 ** | −15.77 ** | −19.49 ** | −1.44 * | −2.21 ** | 1.76 ** |
| P3 × P1 | −17.80 ** | −14.32 ** | −26.87 ** | −26.38 ** | −14.84 ** | −38.53 ** | −23.97 ** | −24.31 ** | −30.38 ** | 30.57 ** | 28.99 ** | 35.40 ** |
| P4 × P1 | −6.48 ** | 6.56 ** | −13.86 ** | −40.03 ** | 10.55 ** | −39.16 ** | −1.06 ** | −16.23 ** | 11.14 ** | 6.24 ** | 2.01 ** | 4.48 ** |
| P5 × P1 | −3.34 ** | 4.00 ** | −9.39 ** | 10.75 ** | 3.66 ** | −5.74 ** | 1.38 ** | −6.99 ** | 2.46 ** | −3.07 ** | −6.51 ** | 3.08 ** |
| P6 × P1 | −14.20 ** | −8.65 ** | −17.43 ** | −30.48 ** | −3.29 ** | −43.66 ** | −15.57 ** | −24.09 ** | −12.53 ** | −7.37 ** | −11.10 ** | −0.97 ** |
| P7 × P1 | 12.41 ** | 10.68 ** | 5.56 ** | −6.32 ** | −29.11 ** | −20.62 ** | −10.87 ** | −16.82 ** | −11.71 ** | −8.44 ** | −13.58 ** | −0.29 ns |
| P3 × P2 | −18.44 ** | −20.60 ** | −32.23 ** | −6.22 ** | −17.38 ** | −40.35 ** | −20.26 ** | −22.11 ** | −25.55 ** | 30.30 ** | 29.73 ** | 36.18 ** |
| P4 × P2 | −6.53 ** | −7.48 ** | −19.40 ** | 16.51 ** | 4.50 ** | −4.98 ** | −34.55 ** | −43.70 ** | −25.31 ** | −0.04 ns | −4.74 ** | −0.88 ns |
| P5 × P2 | 14.40 ** | 11.22 ** | 0.52 ns | −19.26 ** | −27.06 ** | −47.34 ** | −3.03 ** | −9.44 ** | −0.25 ** | −11.71 ** | −14.19 ** | −5.39 ** |
| P6 × P2 | −3.74 ** | −8.80 ** | −13.02 ** | −10.54 ** | −24.24 ** | −45.30 ** | 7.23 ** | −1.92 ** | 13.02 ** | −3.97 ** | −7.14 ** | 3.45 ** |
| P7 × P2 | 6.84 ** | 3.68 ** | −5.94 ** | 8.25 ** | −2.78 ** | −29.81 ** | 23.35 ** | 17.21 ** | 24.41 ** | −5.72 ** | −10.34 ** | 3.45 ** |
| P4 × P3 | 0.75 ns | −2.88 ** | −15.39 ** | 3.22 ** | −26.77 ** | −24.66 ** | −16.36 ** | −29.44 ** | −6.39 ** | −6.50 ** | −11.27 ** | −6.86 ** |
| P5 × P3 | −4.45 ** | −9.49 ** | −18.20 ** | 41.39 ** | 37.48 ** | −19.91 ** | 37.75 ** | 25.87 ** | 38.66 ** | 8.00 ** | 5.41 ** | 16.22 ** |
| P6 × P3 | 13.94 ** | 5.25 ** | 0.38 ns | 20.12 ** | 14.74 ** | −36.86 ** | −19.13 ** | −27.58 ** | −16.54 ** | −6.04 ** | −8.75 ** | 1.65 ** |
| P7 × P3 | 15.40 ** | 9.11 ** | −1.0 * | 15.83 ** | 13.35 ** | −34.83 ** | 0.29 ** | −6.79 ** | −1.06 ** | −16.14 ** | −19.93 ** | −7.61 ** |
| P5 × P4 | −6.51 ** | −8.20 ** | −17.02 ** | −44.34 ** | −54.34 ** | −58.49 ** | 10.76 ** | 1.36 ** | 34.48 ** | 33.54 ** | 23.86 ** | 36.56 ** |
| P6 × P4 | 4.23 ** | −0.28 ns | −4.89 ** | −13.43 ** | −0.06 ns | −38.95 ** | −5.58 ** | −11.79 ** | 17.04 ** | 4.24 ** | −3.78 ** | 7.19 ** |
| P7 × P4 | 4.64 ** | 2.56 ** | −6.95 ** | −4.18 ** | −21.80 ** | −28.89 ** | −10.56 ** | −19.51 ** | 6.80 ** | −4.01 ** | −12.79 ** | 0.62 ns |
| P6 × P5 | 5.64 ** | 2.88 ** | −1.88 ** | 27.65 ** | 18.72 ** | −30.84 ** | 9.52 ** | 7.11 ** | 23.42 ** | −14.30 ** | −14.74 ** | −5.02 ** |
| P7 × P5 | −0.82 ns | −1.00 *** | −10.19 ** | 36.96 ** | 36.07 ** | −20.73 ** | −3.52 ** | −1.70 ** | 4.34 ** | −11.05 ** | −13.02 ** | 0.35 ns |
| P7 × P6 | 14.00 ** | 11.21 ** | 6.07 ** | 51.57 ** | 41.83 ** | −18.46 ** | −17.06 ** | −13.50 ** | −8 .11 ** | −3.19 ** | −4.8 ** | 9.77 ** |
| Reciprocal crosses | ||||||||||||
| P1 × P2 | −11.14 ** | −16.52 ** | −18.94 ** | −10.82 ** | −26.66 ** | −17.88 ** | −2.88 ** | −4.71 ** | −8.93 ** | 16.02 ** | 15.11 ** | 19.78 ** |
| P1 × P3 | −15.31 ** | −11.72 ** | −24.65 ** | −12.07 ** | 1.72* | −26.57 ** | −13.86 ** | −14.25 ** | −21.13 ** | 26.52 ** | 24.99 ** | 31.20 ** |
| P1 × P4 | 11.79 ** | 27.37 ** | 2.97 ** | −23.24 ** | −30.45 ** | −22.12 ** | −3.54 ** | −18.33 ** | 8.35 ** | 13.75 ** | 9.22 ** | 11.87 ** |
| P1 × P5 | −15.14 ** | −8.69 ** | −20.45 ** | −3.04 ** | −9.24 ** | −17.47 ** | −13.53 ** | −20.67 ** | −12.61 ** | 19.27 ** | 15.04 ** | 26.83 ** |
| P1 × P6 | 13.47 ** | 20.82 ** | 9.20 ** | 10.78 ** | −19.83 ** | −10.23 ** | −5.69 ** | −15.21 ** | −2.29 ** | −8.52 ** | −12.20 ** | −2.19 ** |
| P1 × P7 | 20.60 ** | 18.75 ** | 13.26 ** | 19.56 ** | −9.53 ** | 1.31 ns | 17.32 ** | 9.49 ** | 16.22 ** | −6.58 ** | −11.82 ** | 1.74 ** |
| P2 × P3 | 10.20 ** | 7.29 ** | −8.43 ** | 17.52 ** | 3.54 ** | −25.25 ** | 20.70 ** | 17.91 ** | 12.69 ** | −5.57 ** | −5.98 ** | −1.31 *** |
| P2 × P4 | 1.66 ** | 0.63 ns | −12.34 ** | −21.02 ** | −29.16 ** | −35.59 ** | −1.54 ** | −15.31 ** | 12.37 ** | −3.42 ** | −7.96 ** | −4.23 ** |
| P2 × P5 | 1.83 ** | −1.00 * | −10.52 ** | −1.32 ns | −10.86 ** | −35.64 ** | −0.88 ** | −7.44 ** | 1.97 ** | 0.31 ns | −2.51 ** | 7.49 ** |
| P2 × P6 | 7.09 ** | 1.46 ** | −3.23 ** | 13.39 ** | −3.96 ** | −30.66 ** | −3.26 ** | −11.51 ** | 1.97 ** | −0.54 ns | −3.81 ** | 7.15 ** |
| P2 × P7 | 7.73 ** | 4.54 ** | −5.16 ** | 21.30 ** | 8.94 ** | −21.35 ** | −13.44 ** | −17.75 ** | −12.69 ** | 2.61 ** | −2.42 ** | 12.59 ** |
| P3 × P4 | −18.10 ** | −21.05 ** | −31.22 ** | −14.90 ** | −49.49 ** | −37.90 ** | −12.11 ** | −25.86 ** | −1.64 ** | 5.41 ** | 0.04 ns | 5.01 ** |
| P3 × P5 | −24.01 ** | −28.02 ** | −34.94 ** | 21.86 ** | 18.49 ** | −30.98 ** | 4.39 ** | −4.61 ** | 5.08 ** | 3.63 ** | 1.14 ** | 11.51 ** |
| P3 × P6 | 7.77 ** | −0.44 ** | −5.05 ** | 21.62 ** | 16.17 ** | −36.07 ** | −8.02 ** | −17.63 ** | −5.08 ** | −4.59 ** | −7.35 ** | 3.21 ** |
| P3 × P7 | −1.18 ** | −6.56 ** | −15.23 ** | 2.47 ** | 0.28 ns | −42.35 ** | 0.46 ** | −6.64 ** | −0.90 ** | −9.85 ** | −13.9 ** | −0.68 ns |
| P4 × P5 | −2.35 ** | −4.12 ** | −13.34 ** | −27.28 ** | −40.35 ** | −45.76 ** | −27.42 ** | −33.58 ** | −11.88 ** | 3.96 ** | −3.58 ** | 6.31 ** |
| P4 × P6 | −24.21 ** | −27.49 ** | −30.85 ** | 6.47 ** | −17.43 ** | −24.92 ** | −7.17 ** | −13.27 ** | 15.07 ** | −4.95 ** | −12.25 ** | −2.25 ** |
| P4 × P7 | −5.49 ** | −7.37 ** | −15.96 ** | −26.26 ** | −39.82 ** | −45.28 ** | −0.62 ** | −10.56 ** | 18.67 ** | −9.16 ** | −17.46 ** | −4.77 ** |
| P5 × P6 | −21.09 ** | −23.15 ** | −26.71 ** | −6.56 ** | −13.10 ** | −49.38 ** | −36.26 ** | −37.67 ** | −28.17 ** | 1.38 ** | 0.86 ns | 12.35 ** |
| P5 × P7 | −3.56 ** | −3.74 ** | −12.67 ** | −7.08 ** | −7.68 ** | −46.22 ** | −5.72 ** | −3.94 ** | 1.97 ** | −7.24 ** | −9.30 ** | 4.64 ** |
| P6 × P7 | −8.00 ** | −10.24 ** | −14.40 ** | 18.39 ** | 10.78 ** | −36.31 ** | −3.81 ** | 0.31 ** | 6.47 ** | −16.20 ** | −17.65 ** | −4.99 ** |
| CD (5%) | 0.84 | 0.84 | 0.84 | 1.38 | 1.38 | 1.38 | 0.16 | 0.16 | 0.16 | 1.28 | 1.28 | 1.28 |
| CD (1%) | 1.11 | 1.11 | 1.1 | 1.83 | 1.83 | 1.83 | 0.21 | 0.21 | 0.21 | 1.68 | 1.68 | 1.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosen, M.; Rafii, M.Y.; Mazlan, N.; Jusoh, M.; Chowdhury, M.F.N.; Yusuff, O.; Ridzuan, R.; Karim, K.M.R.; Halidu, J.; Ikbal, M.F. Estimation of Heterosis and Combining Ability for Improving Yield, Sweetness, Carotenoid and Antioxidant Qualities in Pumpkin Hybrids (Cucurbita moschata Duch. Ex Poir.). Horticulturae 2022, 8, 863. https://doi.org/10.3390/horticulturae8100863
Hosen M, Rafii MY, Mazlan N, Jusoh M, Chowdhury MFN, Yusuff O, Ridzuan R, Karim KMR, Halidu J, Ikbal MF. Estimation of Heterosis and Combining Ability for Improving Yield, Sweetness, Carotenoid and Antioxidant Qualities in Pumpkin Hybrids (Cucurbita moschata Duch. Ex Poir.). Horticulturae. 2022; 8(10):863. https://doi.org/10.3390/horticulturae8100863
Chicago/Turabian StyleHosen, Monir, Mohd Y. Rafii, Norida Mazlan, Mashitah Jusoh, Mst. Farhana Nazneen Chowdhury, Oladosu Yusuff, Raihana Ridzuan, K. M. Rezaul Karim, Jamilu Halidu, and Mohammad Ferdous Ikbal. 2022. "Estimation of Heterosis and Combining Ability for Improving Yield, Sweetness, Carotenoid and Antioxidant Qualities in Pumpkin Hybrids (Cucurbita moschata Duch. Ex Poir.)" Horticulturae 8, no. 10: 863. https://doi.org/10.3390/horticulturae8100863
APA StyleHosen, M., Rafii, M. Y., Mazlan, N., Jusoh, M., Chowdhury, M. F. N., Yusuff, O., Ridzuan, R., Karim, K. M. R., Halidu, J., & Ikbal, M. F. (2022). Estimation of Heterosis and Combining Ability for Improving Yield, Sweetness, Carotenoid and Antioxidant Qualities in Pumpkin Hybrids (Cucurbita moschata Duch. Ex Poir.). Horticulturae, 8(10), 863. https://doi.org/10.3390/horticulturae8100863





