Rapeseed as an Ornamental
Abstract
:1. Introduction
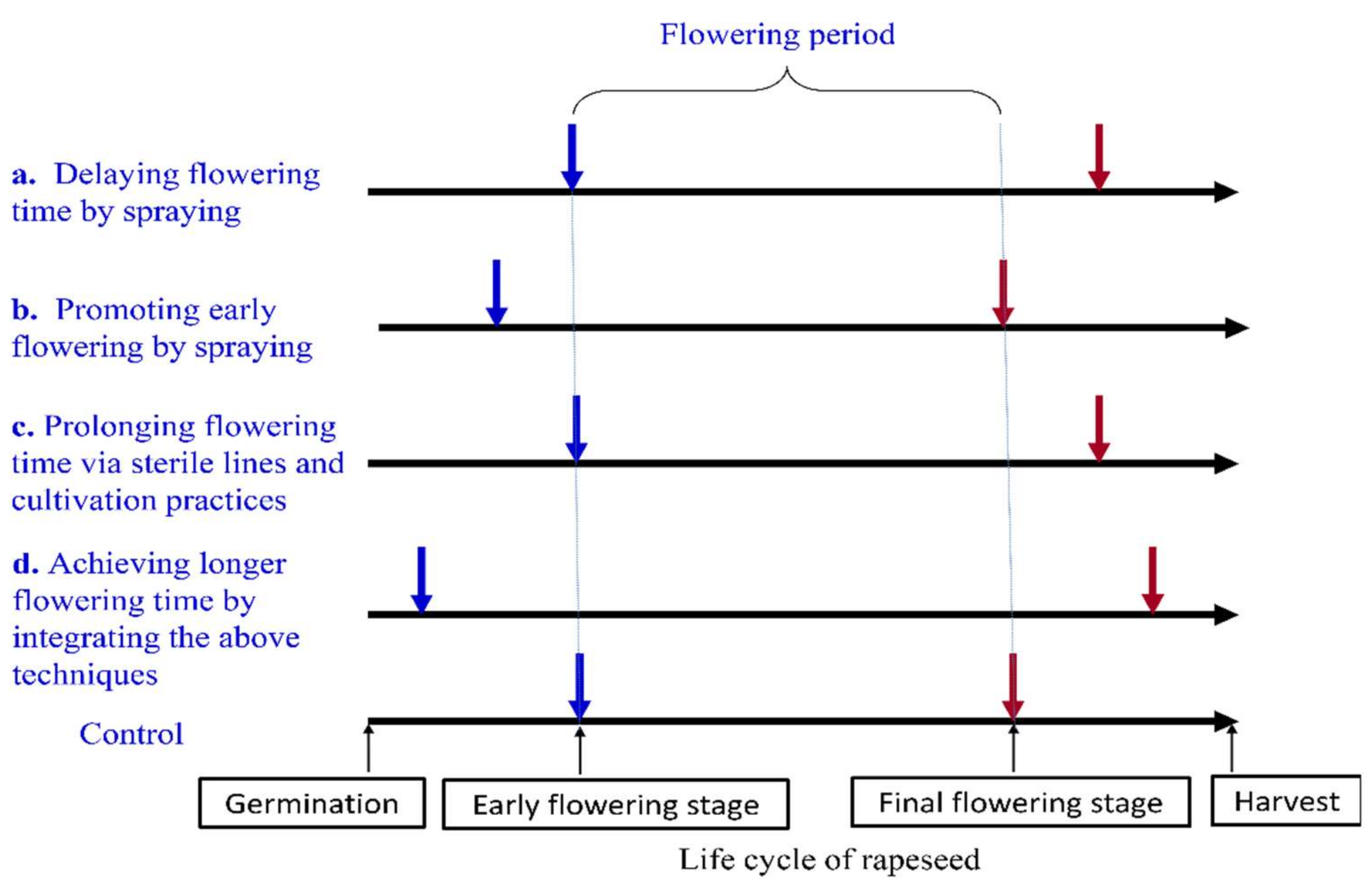
2. Relatively Short Flowering Period
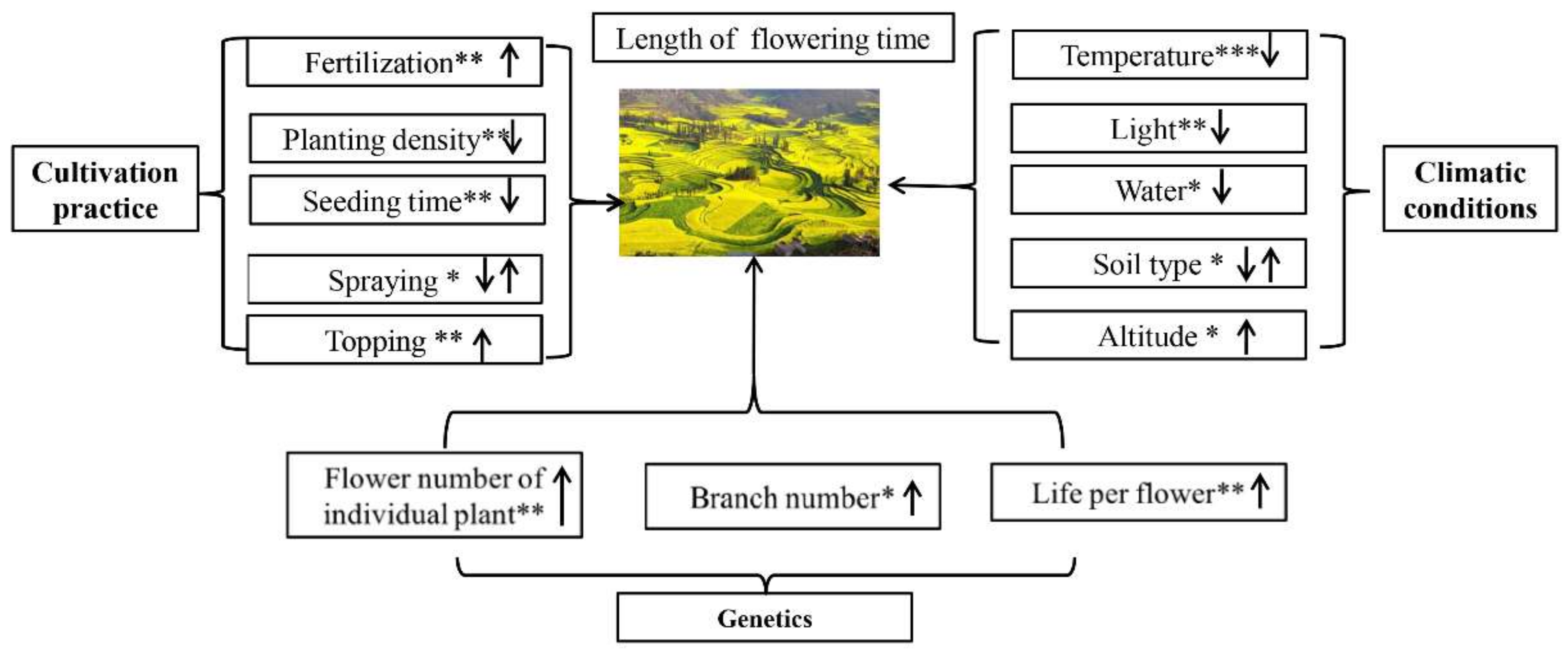
2.1. Problem
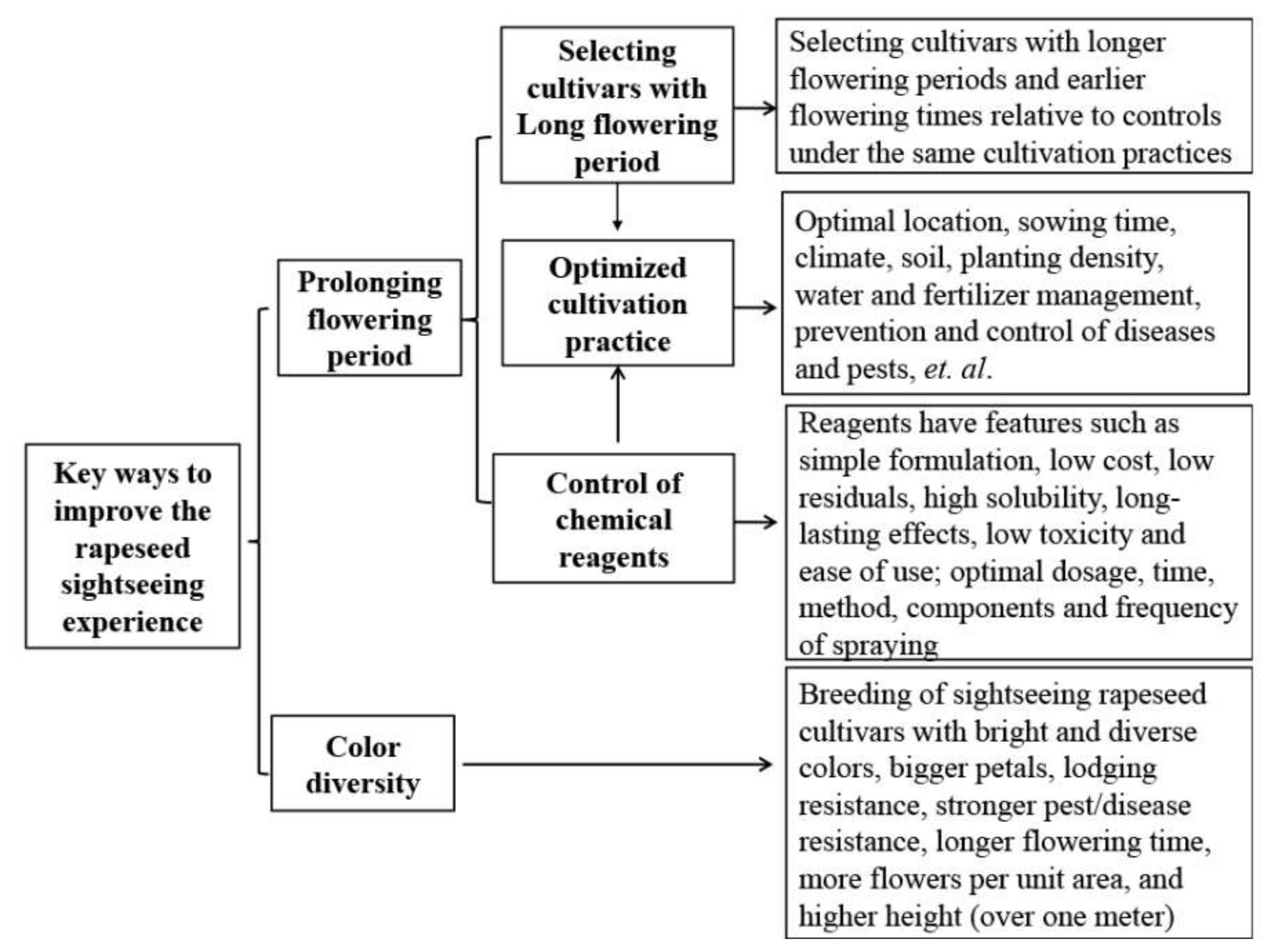
2.2. Factors Affecting Flowering Period and the Corresponding Countermeasures
- (1)
- Natural environmental conditions
- (2)
- Cultivation practices
- (3)
- Hormonal control of flowering
2.3. Prolonging the Flowering Period by Chemical Reagents
- (1)
- Ethylene
- (2)
- 8-HQC
- (3)
- Citric acid
- (4)
- 6-BA and sucrose
3. Development of a Range of Flower Colors in Rapeseed
3.1. Problem
3.2. The Molecular Mechanisms of Flower Color
3.3. How to Breed Rapeseed of Different Colors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pu, G.; Zheng, M.; Lu, S.; Huang, J. Study on the Use of Cooking Oil in Chinese Dishes. Int. J. Environ. Res. Public Health 2019, 16, 3367. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Huang, J.; Leng, B.; Feng, Z.; Li, J. Current situation, development difficulties and suggestions of Chinese rape industry. J. China Agric. Univ. 2017, 22, 203–210. [Google Scholar]
- Wigge, P.A. Ambient temperature signalling in plants. Curr. Opin. Plant Biol. 2013, 16, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Suzuki, J.-I.; Hara, T. Latitudinal variation in plant size and relative growth rate in Arabidopsis thaliana. Oecologia 1998, 115, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Moharekar Lokhande, S.; Moharekar, S.; Kobayashi, T.; Ishii, H.; Sumida, A.; Hara, T. Phenotypic plasticity and ecotypic variations in growth and flowering time of Arabidopsis thaliana (L.) under different light and temperature conditions. Indian J. Exp. Biol. 2014, 52, 344–351. [Google Scholar]
- Stavang, J.A.; Gallego-Bartolome, J.; Gomez, M.D.; Yoshida, S.; Asami, T.; Olsen, J.E.; Garcia-Martinez, J.L.; Alabadi, D.; Blazquez, M.A. Hormonal regulation of temperature-induced growth in Arabidopsis. Plant J. 2009, 60, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Han, D.; Mason, A.S.; Zhou, C.; Zheng, W.; Li, Y.; Wu, C.; Fu, D.; Huang, Y. Earliness traits in rapeseed (Brassica napus): SNP loci and candidate genes identified by genome-wide association analysis. DNA Res. 2017, 25, 229–244. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Hu, K.; Zhang, Z.; Guan, C.; Chen, S.; Hua, W.; Li, J.; Wen, J.; Yi, B.; Shen, J.; et al. Genome-wide association study reveals the genetic architecture of flowering time in rapeseed (Brassica napus L.). DNA Res. 2016, 23, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobell, D.B.; Cahill, K.N.; Field, C.B. Historical effects of temperature and precipitation on California crop yields. Clim. Chang. 2007, 81, 187–203. [Google Scholar] [CrossRef]
- Ausin, I.; Alonso-Blanco, C.; Martinez-Zapater, J.M. Environmental regulation of flowering. Int. J. Develop. Biol. 2005, 49, 689–705. [Google Scholar] [CrossRef] [Green Version]
- Pigliucci, M.; Kolodynska, A. Phenotypic plasticity and integration in response to flooded conditions in natural accessions of Arabidopsis thaliana (L.) Heynh (Brassicaceae). Ann. Botany 2002, 90, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pigliucci, M.; Whitton, J.; Schlichting, C.D. Reaction norms of Arabidopsis. I. Plasticity of characters and correlations across water, nutrient and light gradients. J. Evolut. Biol. 1995, 8, 421–438. [Google Scholar] [CrossRef]
- Kang, D.-J.; Futakuchi, K. Effect of Moderate Drought-Stress on Flowering Time of Interspecific Hybrid Progenies (Oryza sativa L. × Oryza glaberrima Steud.). J. Crop Sci. Biotechnol. 2019, 22, 75–81. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.S.; Ahn, J.H. Ambient temperature signaling in plants: An emerging field in the regulation of flowering time. J. Plant Biol. 2008, 51, 321–326. [Google Scholar] [CrossRef]
- Liang, M.; Xiao, S.; Cai, J.; Ow, D.W. OXIDATIVE STRESS 3 regulates drought-induced flowering through APETALA 1. Biochem. Biophys. Res. Commun. 2019, 519, 585–590. [Google Scholar] [CrossRef]
- Zeng, Y. Effects of different fertilizer and density on the growth and yield of rapeseed. Huazhong Agric. Univ. 2011, 6, 26–30. [Google Scholar]
- Yuan, S.; Zhang, Z.W.; Zheng, C.; Zhao, Z.Y.; Wang, Y.; Feng, L.Y.; Niu, G.; Wang, C.Q.; Wang, J.H.; Feng, H.; et al. Arabidopsis cryptochrome 1 functions in nitrogen regulation of flowering. Proc. Nat. Acad. Sci. USA 2016, 113, 7661–7666. [Google Scholar] [CrossRef] [Green Version]
- Shao, L. Effects of planting density of the development and yield of rapessed (Brassica napus L.) under different sowing dates. Huazhong Agric. Univ. 2009, 4, 12–14. [Google Scholar]
- Yantai, G.; Harker, K.N.; Kutcher, H.R.; Gulden, R.H.; Irvine, B.; May, W.E.; O’Donovan, J.T. Canola seed yield and phenological responses to plant density. Can. J. Plant Sci. 2016, 96, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Helland, S.J.J.D.; Gradworks, T. Effects of Environment and Planting Density on Plant Stature, Flowering Time, and Ear Set in IBM Populations of Maize; Theses and Dissertation; Iowa State University,: Ames, IA, USA, 2012. [Google Scholar]
- Gao, W. A study of stalk picking to postpone the flowering period of Brassica napus. Guizhou Agric. Sci. 1990, 7, 37–40. [Google Scholar]
- Fu, W.; Chen, D.; Pan, Q.; Li, F.; Zhao, Z.; Ge, X.; Li, Z. Production of red-flowered oilseed rape via the ectopic expression of Orychophragmus violaceus OvPAP2. Plant Biotechnol. J. 2017, 16, 367–380. [Google Scholar] [CrossRef] [Green Version]
- Rubinstein, B. Regulation of cell death in flower petals. Plant Mol. Biol. 2000, 44, 303–318. [Google Scholar] [CrossRef]
- Taylor, J.E.; Whitelaw, C.A. Signals in abscission. New Phytol. 2001, 151, 323–340. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Tuteja, N. Integrated signaling in flower senescence: An overview. Plant Signal. Behav. 2007, 2, 437–445. [Google Scholar] [CrossRef] [Green Version]
- van Doorn, W.G. Effect of ethylene on flower abscission: A survey. Ann. Botany 2002, 89, 689–693. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, K.; Shimizu, K.; Niki, T.; Ichimura, K. Identification of a NAC transcription factor, EPHEMERAL1, that controls petal senescence in Japanese morning glory. Plant J. 2014, 79, 1044–1051. [Google Scholar] [CrossRef]
- Yin, J.; Chang, X.; Kasuga, T.; Bui, M.; Reid, M.S.; Jiang, C.Z. A basic helix-loop-helix transcription factor, PhFBH4, regulates flower senescence by modulating ethylene biosynthesis pathway in petunia. Hortic. Res. 2015, 2, 15059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, L.; Hegelund, J.N.; Olsen, A.; Lutken, H.; Muller, R. A natural frameshift mutation in Campanula EIL2 correlates with ethylene insensitivity in flowers. BMC Plant Biol. 2016, 16, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aida, R.; Yoshida, T.; Ichimura, K.; Goto, R.; Shibata, M. Extension of flower longevity in transgenic torenia plants incorporating ACC oxidase transgene. Plant Sci. 1998, 138, 91–101. [Google Scholar] [CrossRef]
- Shaw, J.-F.; Chen, H.-H.; Tsai, M.-F.; Kuo, C.-I.; Huang, L.-C. Extended flower longevity of Petunia hybrida plants transformed with boers, a mutated ERS gene of Brassica oleracea. Mol. Breed. 2002, 9, 211–216. [Google Scholar] [CrossRef]
- Bradley, D.; Ratcliffe, O.; Vincent, C.; Carpenter, R.; Coen, E. Inflorescence commitment and architecture in Arabidopsis. Science 1997, 275, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Ratcliffe, O.J.; Amaya, I.; Vincent, C.A.; Rothstein, S.; Carpenter, R.; Coen, E.S.; Bradley, D.J. A common mechanism controls the life cycle and architecture of plants. Development 1998, 125, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Prinsi, B.; Negri, A.S.; Quattrocchio, F.M.; Koes, R.E.; Espen, L. Proteomics of red and white corolla limbs in petunia reveals a novel function of the anthocyanin regulator ANTHOCYANIN1 in determining flower longevity. J. Proteom. 2016, 131, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Trivellini, A.; Ferrante, A.; Vernieri, P.; Serra, G. Effects of abscisic acid on ethylene biosynthesis and perception in Hibiscus rosa-sinensis L. flower development. J. Exp. Botany 2011, 62, 5437–5452. [Google Scholar] [CrossRef]
- Trivellini, A.; Cocetta, G.; Vernieri, P.; Mensuali-Sodi, A.; Ferrante, A. Effect of cytokinins on delaying petunia flower senescence: A transcriptome study approach. Plant Mol. Biol. 2015, 87, 169–180. [Google Scholar] [CrossRef]
- Salleh, F.M.; Mariotti, L.; Spadafora, N.D.; Price, A.M.; Picciarelli, P.; Wagstaff, C.; Lombardi, L.; Rogers, H. Interaction of plant growth regulators and reactive oxygen species to regulate petal senescence in wallflowers (Erysimum linifolium). BMC Plant Biol. 2016, 16, 77. [Google Scholar] [CrossRef] [Green Version]
- Stead, A.D. Pollination-induced flower senescence: A review. Plant Growth Regul. 1992, 11, 13–20. [Google Scholar] [CrossRef]
- Fu, D.; Zhu, L.; Xiao, M.; Zhou, Q. Methods, Medicament and Preparation Methods for Prolonging the Flowering Period of Ornamental Rapeseed. Patent CN201610209467.6, 6 April 2016. [Google Scholar]
- Serek, M.; Sisler, E.C.; Reid, M.S. 1-Methylcyclopropene, a novel gaseous inhibitor of ethylene action, improves the life of fruits, cut flowers and potted plants. Plant Bioregul. Hortic. 1995, 394, 337–346. [Google Scholar] [CrossRef]
- Hashemabadi, D.; Liavali, M.H.; Kaviani, B.; Mousavi, M.; Keyghobadi, S.; Zahiri, S. Effect of nano-silver and boric acid on extending the vase life of cut rose (Rosa hybrida L.). J. Environ. Biol. 2014, 35, 833–838. [Google Scholar] [PubMed]
- Safa, Z.; Hashemabadi, D.; Kaviani, B.; Nikchi, N.; Zarchini, M. Studies on quality and vase life of cut Gerbera jamesonii cv. ‘Balance’ flowers by silver nanoparticles and chlorophenol. J. Environ. Biol. 2015, 36, 425–431. [Google Scholar]
- Solgi, M.; Kafi, M.; Taghavi, T.S.; Naderi, R. Essential oils and silver nanoparticles (SNP) as novel agents to extend vase-life of gerbera (Gerbera jamesonii cv. ‘Dune’) flowers. Postharvest Biol. Technol. 2009, 53, 155–158. [Google Scholar] [CrossRef]
- Hashemabadi, D. The role of silver nano-particles and silver thiosulfate on the longevity of cut carnation (Dianthus caryophyllus) flowers. J. Environ. Biol. 2014, 35, 661–666. [Google Scholar] [PubMed]
- Rahman, M.M.; Ahmad, S.H.; Lgu, K.S. Psidium guajava and Piper betle leaf extracts prolong vase life of cut carnation (Dianthus caryophyllus) flowers. Sci. World J. 2012, 2012, 102805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemabadi, D.; Torkashvand, A.M.; Kaviani, B.; Bagherzadeh, M.; Rezaalipour, M.; Zarchini, M. Effect of Mentha pulegium extract and 8-hydroxy quinoline sulphate to extend the quality and vase life of rose (Rosa hybrid) cut flower. J. Environ. Biol. 2015, 36, 215–220. [Google Scholar]
- Elhindi, K.M. Evaluation of several holding solutions for prolonging vase-life and keeping quality of cut sweet pea flowers (Lathyrus odoratus L.). Saudi J. Biolog. Sci. 2012, 19, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Darandeh, N.; Shoor, E.H.M. Extention of post-harvest vase life of Lilium cv. Brunello by Citric Acid sprays during plant growth [Conference Abstract]. In Proceedings of the Symplitaly, Pesica, Italy, 30 July 2010. [Google Scholar]
- Darandeh, N.; Hadavi, E. Effect of Pre-Harvest Foliar Application of Citric Acid and Malic Acid on Chlorophyll Content and Post-Harvest Vase Life of Lilium cv. Brunello. Front. Plant Sci. 2012, 2, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dar, R.A.; Tahir, I.; Ahmad, S.S. Sugars and sugar alcohols have their say in the regulation of flower senescence in Dianthus chinensis L. Sci. Hortic. 2014, 174, 24–28. [Google Scholar] [CrossRef]
- Arrom, L.; Munne-Bosch, S. Sucrose accelerates flower opening and delays senescence through a hormonal effect in cut lily flowers. Plant Sci. 2012, 188–189, 41–47. [Google Scholar] [CrossRef]
- Fu, D.; Zhu, L.; Xiao, M.; Zhou, Q. A Method of Prolonging the Flowering Period of Rapeseed and the Seed Production Method of the Sterile Line Rapeseed. Patent CN201610250303.8, 20 April 2016. [Google Scholar]
- Fu, D.; Zhu, L.; Xiao, M.; Zhou, Q. A Reagent of Prolonging the Flowering Period and the Corresponding Usage Method. Patent CN201610249992.0, 27 March 2016. [Google Scholar]
- Zhu, L.; Liu, B.; Xiao, M.; Zhou, Q.; Fu, D. A Study on Chemical Regulation of Flowering in Brassica napus. Acta Agric. Univ. Jiangxiensis 2017, 39, 1057–1066. [Google Scholar]
- Zhao, C.; Safdar, L.B.; Xie, M.; Shi, M.; Liu, S.J.T.C.J. Mutation of the PHYTOENE DESATURASE 3 gene causes yellowish-white petals in Brassica napus. Crop J. 2021, 9, 1124–1134. [Google Scholar] [CrossRef]
- Wan, H.; Yu, C.; Luo, L.; Han, Y.; Pan, H.; Zhang, Q.J.M.P.B. Extraction and Determination of Flavonoids and Carotenoids in Petals of Roses (Rosa spp.). Mol. Plant Breeding 2019, 17, 20–24. [Google Scholar]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids Handbook; Birkhäuser: Basel, Switzerland, 2004. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Ann. Review Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Wessinger, C.A. A genetic route to yellow flowers. New Phytol. 2015, 206, 1193–1195. [Google Scholar] [CrossRef]
- Zhao, D.; Tao, J. Recent advances on the development and regulation of flower color in ornamental plants. Front. Plant Sci. 2015, 6, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Wang, X.; Chen, W.; Dong, M.; Yuan, W.; Liu, X.; Shang, F. Differential expression of carotenoid-related genes determines diversified carotenoid coloration in flower petal of Osmanthus fragrans. Tree Genet. Genomes 2014, 10, 329–338. [Google Scholar] [CrossRef]
- Lysanne, A.; Dietmar, K.; Florian, S.; Otmar, S.J.I.J.o.M.S. Comparative Metabolite Profiling of Triterpenoid Saponins and Flavonoids in Flower Color Mutations of Primula veris L. Int. J. Mol. Sci. 2017, 18, 153. [Google Scholar]
- Sun, W.; Meng, X.; Liang, L.; Jiang, W.; Huang, Y.; He, J.; Hu, H.; Almqvist, J.; Gao, X.; Wang, L. Molecular and Biochemical Analysis of Chalcone Synthase from Freesia hybrid in flavonoid biosynthetic pathway. PLoS ONE 2015, 10, e0119054. [Google Scholar] [CrossRef] [Green Version]
- Tai, D.; Tian, J.; Zhang, J.; Song, T.; Yao, Y. A Malus crabapple chalcone synthase gene, McCHS, regulates red petal color and flavonoid biosynthesis. PLoS ONE 2014, 9, e110570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Nakayama, T. Achievements and perspectives in biochemistry concerning anthocyanin modification for blue flower coloration. Plant Cell Physiol. 2015, 56, 28–40. [Google Scholar] [CrossRef]
- Tanaka, Y.; Brugliera, F. Flower colour and cytochromes P450. Philos. Trans. R. Soc. B Biolog. Sci. 2013, 368, 20120432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsumoto, Y.; Fukuchi-Mizutani, M.; Fukui, Y.; Brugliera, F.; Holton, T.A.; Karan, M.; Nakamura, N.; Yonekura-Sakakibara, K.; Togami, J.; Pigeaire, A.; et al. Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol. 2007, 48, 1589–1600. [Google Scholar] [CrossRef]
- Boase, M.R.; Lewis, D.H.; Davies, K.M.; Marshall, G.B.; Patel, D.; Schwinn, K.E.; Deroles, S.C. Isolation and antisense suppression of flavonoid 3’, 5’-hydroxylase modifies flower pigments and colour in cyclamen. BMC Plant Biol. 2010, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakatsuka, T.; Mishiba, K.; Kubota, A.; Abe, Y.; Yamamura, S.; Nakamura, N.; Tanaka, Y.; Nishihara, M. Genetic engineering of novel flower colour by suppression of anthocyanin modification genes in gentian. J. Plant Physiol. 2010, 167, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Bordignon-Luiz, M.T.; Gauche, C.; Gris, E.F.; Falcao, L.D. Colour stability of anthocyanins from Isabel grapes (Vitis labrusca L.) in model systems. LWT-Food Sci. Technol. 2007, 40, 594–599. [Google Scholar] [CrossRef]
- Zhao, X.; Sheng, F.; Zheng, J.; Liu, R. Composition and stability of anthocyanins from purple Solanum tuberosum and their protective influence on Cr (VI) targeted to bovine serum albumin. J. Agric. Food Chem. 2011, 59, 7902–7909. [Google Scholar] [CrossRef] [PubMed]
- Momonoi, K.; Yoshida, K.; Mano, S.; Takahashi, H.; Nakamori, C.; Shoji, K.; Nitta, A.; Nishimura, M. A vacuolar iron transporter in tulip, TgVit1, is responsible for blue coloration in petal cells through iron accumulation. Plant J. 2009, 59, 437–447. [Google Scholar] [CrossRef]
- Verweij, W.; Spelt, C.; Di Sansebastiano, G.P.; Vermeer, J.; Reale, L.; Ferranti, F.; Koes, R.; Quattrocchio, F. An H+ P-ATPase on the tonoplast determines vacuolar pH and flower colour. Nat. Cell Biol. 2008, 10, 1456–1462. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, C.; Wang, Y.; Yao, X.; Wang, F.; Wu, J.; King, G.J.; Liu, K. Disruption of a CAROTENOID CLEAVAGE DIOXYGENASE 4 gene converts flower colour from white to yellow in Brassica species. New Phytol. 2015, 206, 1513–1526. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, S.; Yuan, G.; Ma, X.; Heng, S.; Yi, B.; Ma, C.; Shen, J.; Tu, J.; Fu, T.; et al. Gene silencing of BnaA09.ZEP and BnaC09.ZEP confers orange color in Brassica napus flowers. Plant J. 2020, 104, 932–949. [Google Scholar] [CrossRef]
- Luo, P.; Ning, G.; Wang, Z.; Shen, Y.; Jin, H.; Li, P.; Huang, S.; Jian, Z.; Bao, M.J.F.i.P.S. Disequilibrium of Flavonol Synthase and Dihydroflavonol-4-Reductase Expression Associated Tightly to White vs. Red Color Flower Formation in Plants. Front. Plant Sci. 2015, 6, 1257–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Brugliera, F.; Kalc, G.; Senior, M.; Chandler, S.J.B.B.B. Flower Color Modification by Engineering of the Flavonoid Biosynthetic Pathway: Practical Perspectives. Biosci. Biotechnol. Biochem. 2010, 74, 1760–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuraini, L.; Ando, Y.; Kawai, K.; Tatsuzawa, F.; Tanaka, K.; Ochiai, M.; Suzuki, K.; Aragones, V.; Daros, J.A.; Nakatsuka, T. Anthocyanin regulatory and structural genes associated with violet flower color of Matthiola incana. Planta 2020, 251, 61. [Google Scholar] [CrossRef] [PubMed]
- Tatsuzawa, F.; Aiba, Y.; Morino, T.; Saito, N.; Shinoda, K.; Kato, K.; Toki, K.; Honda, T.J.J.-J.S.f.H.S. Copigmentation with Acylated Anthocyanin and Kaempferol Glycosides in Violet and Purple Flower Cultivars of Aubrieta × cultorum (Brassicaceae). J. Jpn. Soc. Hortic. Sci. 2012, 81, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Saito, N.; Tatsuzawa, F.; Toki, K.; Shinoda, K.; Shigihara, A.; Honda, T. The blue anthocyanin pigments from the blue flowers of Heliophila coronopifolia L. (Brassicaceae). Phytochemistry 2011, 72, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.H.; Kang, H.H.; Hui, Y.J.; Soe, M.T.; Chang, K.K.J.M.B. Overexpression of the Raphanus sativus RsMYB1 Using the Flower-specific Promoter (InMYB1) Enhances Anthocyanin Accumulation in Flowers of Transgenic Petunia and Their Hybrids. Mol. Breed. 2020, 40, 97–107. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, M.; Wang, H.; Li, X.; Mason, A.S.; Fu, D. Rapeseed as an Ornamental. Horticulturae 2022, 8, 27. https://doi.org/10.3390/horticulturae8010027
Xiao M, Wang H, Li X, Mason AS, Fu D. Rapeseed as an Ornamental. Horticulturae. 2022; 8(1):27. https://doi.org/10.3390/horticulturae8010027
Chicago/Turabian StyleXiao, Meili, Huadong Wang, Xiaonan Li, Annaliese S. Mason, and Donghui Fu. 2022. "Rapeseed as an Ornamental" Horticulturae 8, no. 1: 27. https://doi.org/10.3390/horticulturae8010027
APA StyleXiao, M., Wang, H., Li, X., Mason, A. S., & Fu, D. (2022). Rapeseed as an Ornamental. Horticulturae, 8(1), 27. https://doi.org/10.3390/horticulturae8010027





