Particulate Matter (PM) Adsorption and Leaf Characteristics of Ornamental Sweet Potato (Ipomoea batatas L.) Cultivars and Two Common Indoor Plants (Hedera helix L. and Epipremnum aureum Lindl. & Andre)
Abstract
:1. Introduction
2. Materials and Methods
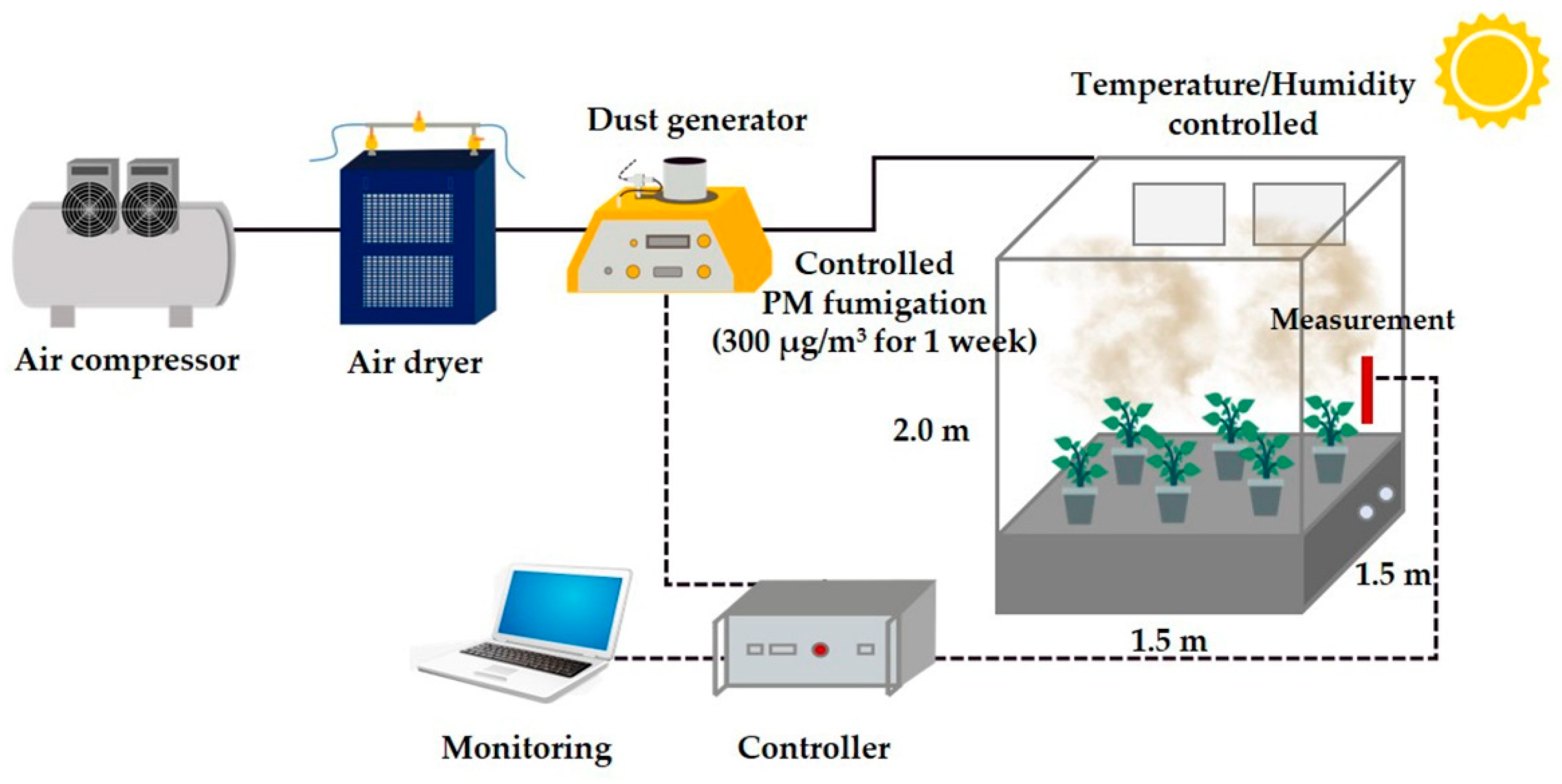
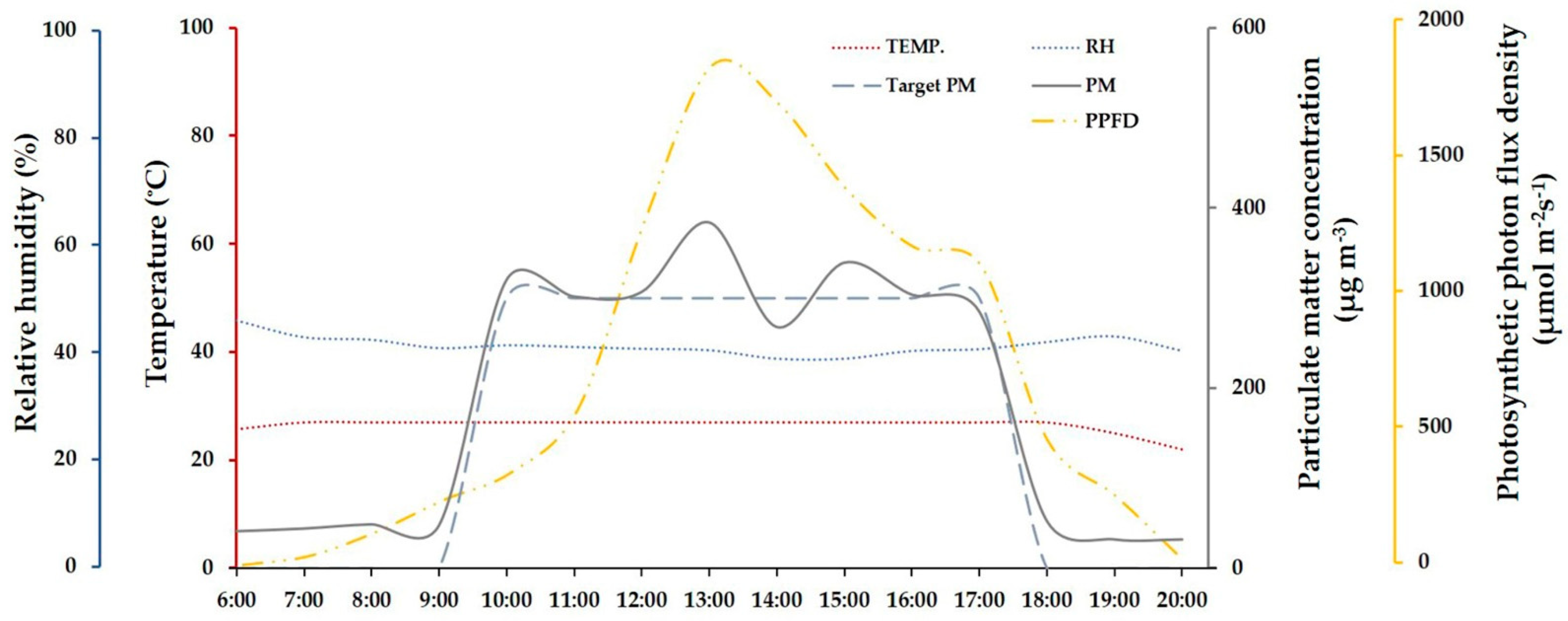
2.1. Plant Materials and the PM Fumigation Chamber
2.2. Gas Exchange Measurements
2.3. Morphological and Stomatal Characteristics
2.4. Measurement of PM Adsorption on the Leaf Surfaces
2.5. Statistical Analysis
3. Results
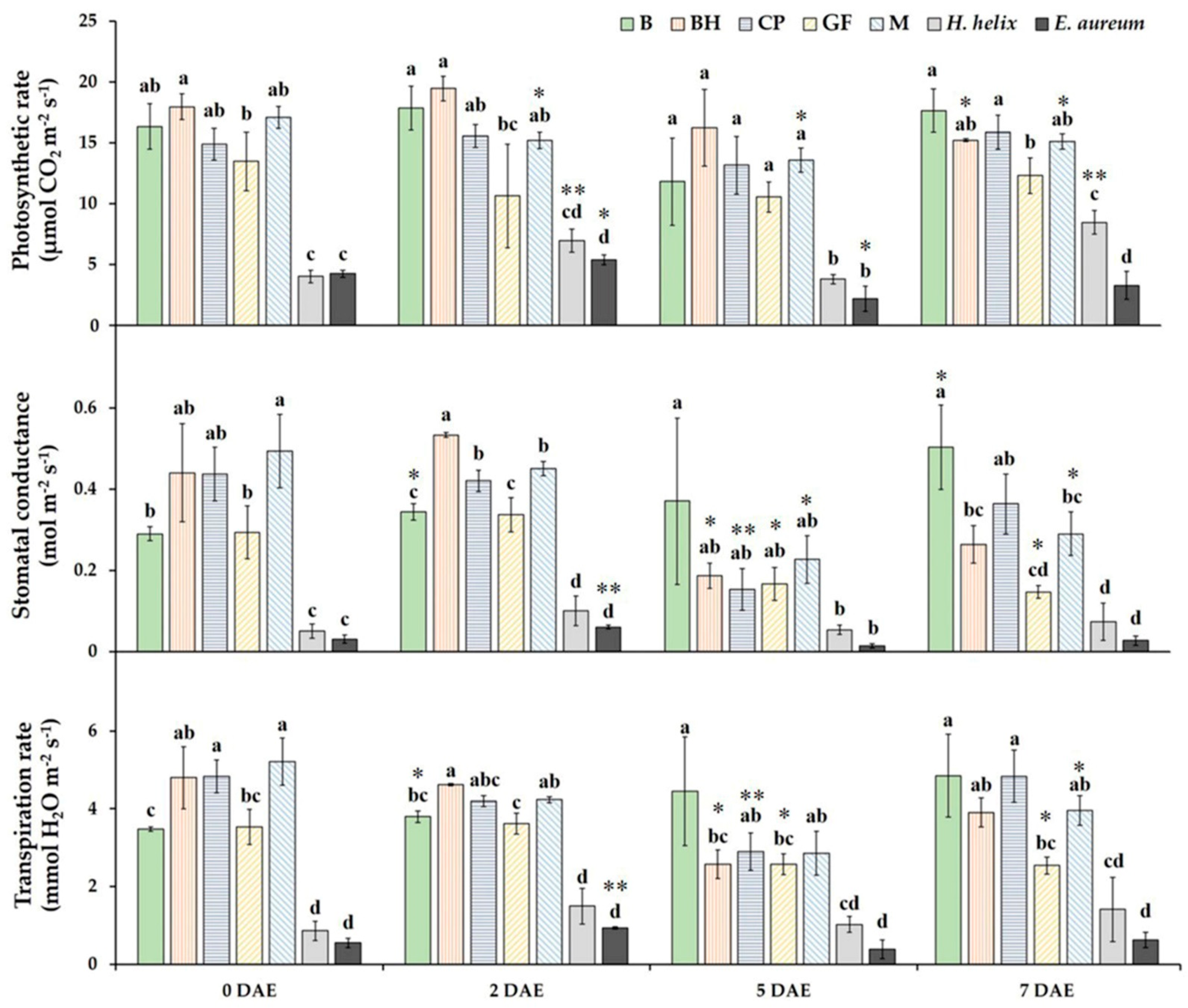
3.1. Gas Exchange Characteristics
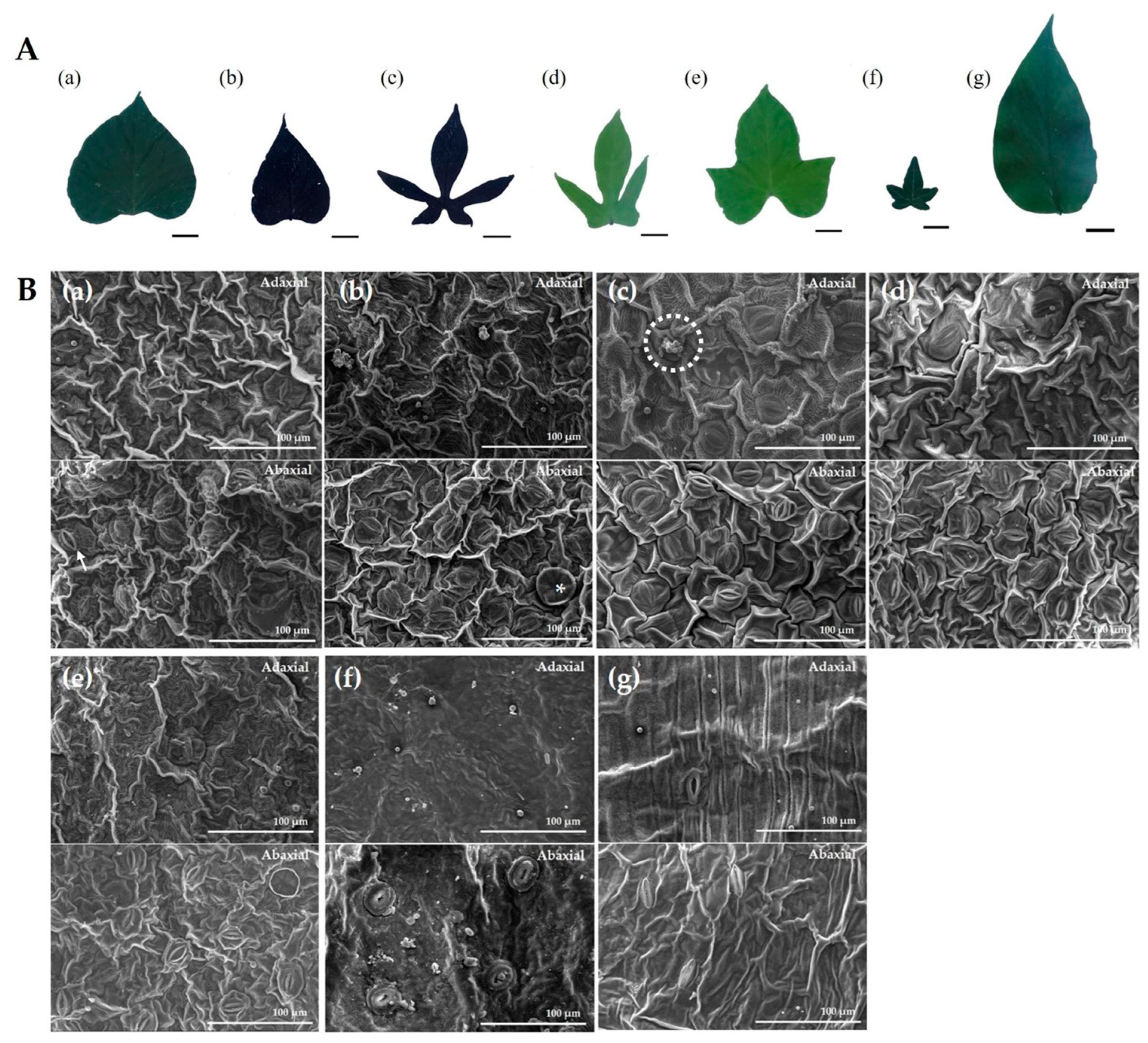
3.2. Morphological and Stomatal Characteristics
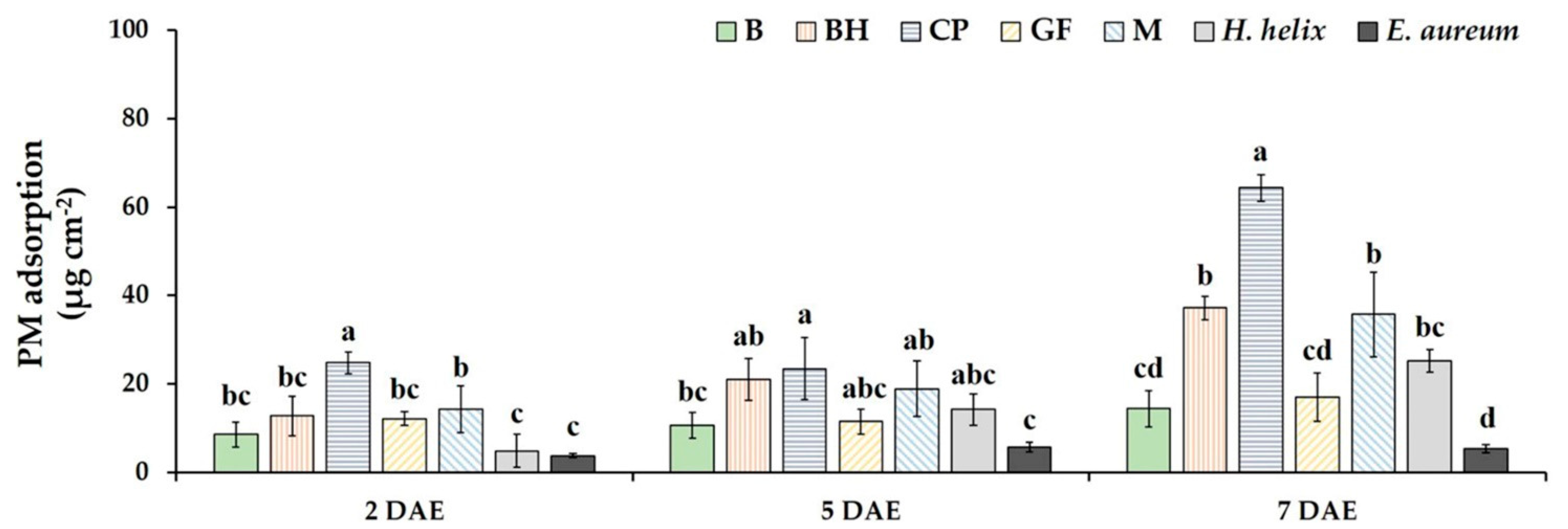
3.3. PM Adsorption on the Leaf Surfaces
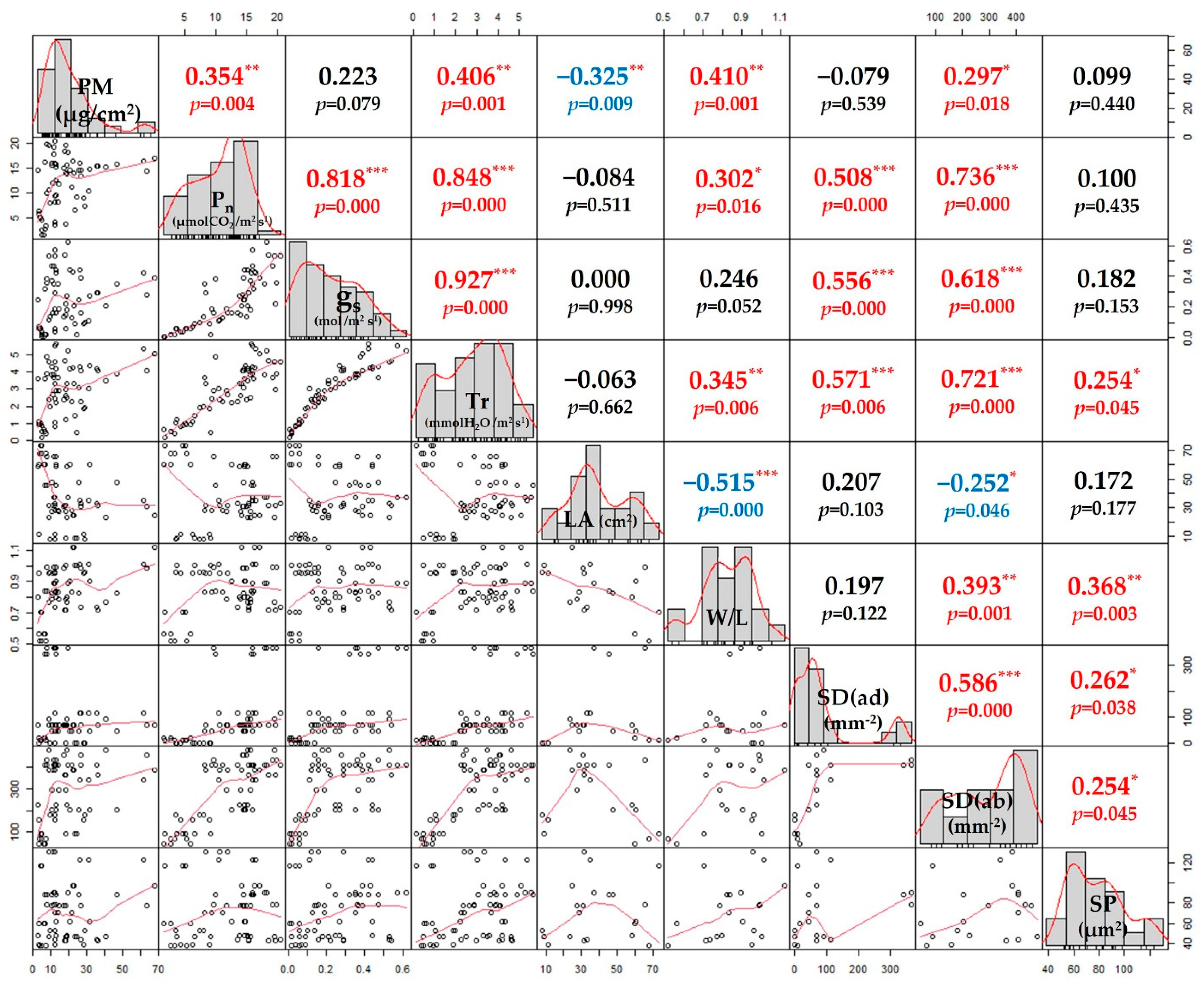
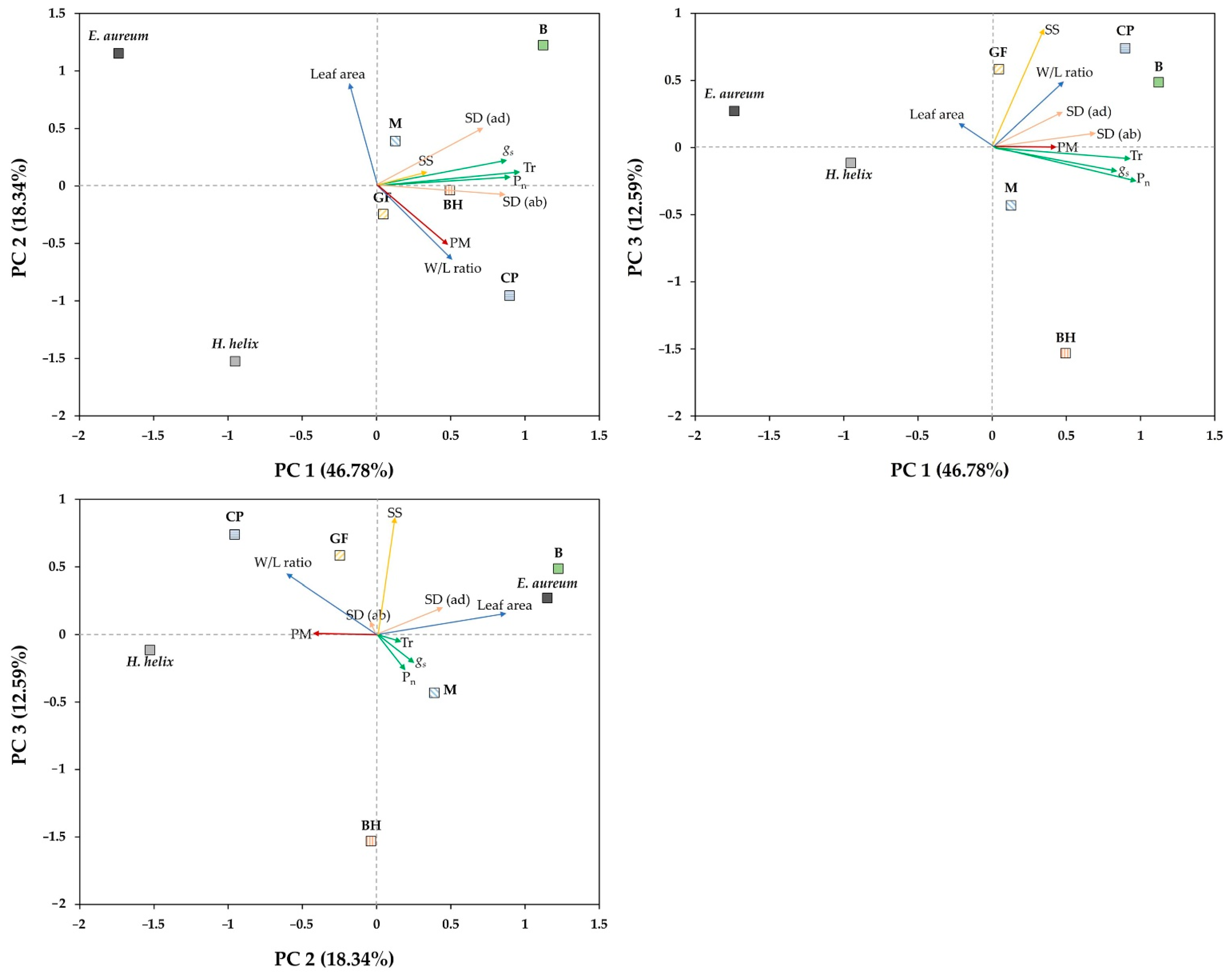
3.4. Factors Influencing PM Adsorption on the Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Chang, Y.; Yan, P. Ranking the suitability of common urban tree species for controlling PM2.5 pollution. Atmos. Pollut. Res. 2015, 6, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Zhang, R.; Khalizov, A.; Wang, L.; Hu, M.; Xu, W. Nucleation and Growth of Nanoparticles in the Atmosphere. Chem. Rev. 2012, 112, 1957–2011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, G.; Guo, S.; Zamora, M.L.; Ying, Q.; Lin, Y.; Wang, W.; Hu, M.; Wang, Y. Formation of Urban Fine Particulate Matter. Chem. Rev. 2015, 115, 3803–3855. [Google Scholar] [CrossRef] [PubMed]
- Pettit, T.; Irga, P.; Abdo, P.; Torpy, F. Do the plants in functional green walls contribute to their ability to filter particulate matter? Build. Environ. 2017, 125, 299–307. [Google Scholar] [CrossRef]
- Guo, H.; Morawska, L.; He, C.; Zhang, Y.; Ayoko, G.; Cao, M. Characterization of particle number concentrations and PM2.5 in a school: Influence of outdoor air pollution on indoor air. Environ. Sci. Pollut. Res. 2010, 17, 1268–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morawska, L.; Afshari, A.; Bae, G.N.; Buonanno, G.; Chao, Y.H.C.; Hänninen, O.; Hofmann, W.; Isaxon, C.; Jayaratne, E.R.; Pasanen, P.; et al. Indoor aerosols: From personal exposure to risk assessment. Indoor Air 2013, 23, 462–487. [Google Scholar] [CrossRef] [Green Version]
- Han, K.-T.; Ruan, L.-W. Effects of indoor plants on air quality: A systematic review. Environ. Sci. Pollut. Res. 2020, 27, 16019–16051. [Google Scholar] [CrossRef]
- Sahu, V.; Elumalai, S.P.; Gautam, S.; Singh, N.K.; Singh, P. Characterization of indoor settled dust and investigation of indoor air quality in different micro-environments. Int. J. Environ. Heal. Res. 2018, 28, 419–431. [Google Scholar] [CrossRef]
- Smith, A.; Pitt, M. Healthy workplaces: Plantscaping for indoor environmental quality. Facilities 2011, 29, 169–187. [Google Scholar] [CrossRef] [Green Version]
- Yoo, M.H.; Kwon, Y.J.; Son, K.-C.; Kays, S.J. Efficacy of Indoor Plants for the Removal of Single and Mixed Volatile Organic Pollutants and Physiological Effects of the Volatiles on the Plants. J. Am. Soc. Hortic. Sci. 2006, 131, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Irga, P.; Torpy, F.; Burchett, M. Can hydroculture be used to enhance the performance of indoor plants for the removal of air pollutants? Atmos. Environ. 2013, 77, 267–271. [Google Scholar] [CrossRef]
- Bealey, W.; McDonald, A.; Nemitz, E.; Donovan, R.; Dragosits, U.; Duffy, T.; Fowler, D. Estimating the reduction of urban PM10 concentrations by trees within an environmental information system for planners. J. Environ. Manag. 2007, 85, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Janhäll, S. Review on urban vegetation and particle air pollution—Deposition and dispersion. Atmos. Environ. 2015, 105, 130–137. [Google Scholar] [CrossRef]
- Freer-Smith, P.H.; El-Khatib, A.A.; Taylor, G. Capture of Particulate Pollution by Trees: A Comparison of Species Typical of Semi-Arid Areas (Ficus Nitida and Eucalyptus Globulus) with European and North American Species. Water Air Soil Pollut. 2004, 155, 173–187. [Google Scholar] [CrossRef]
- Dzierżanowski, K.; Popek, R.; Gawrońska, H.; Saebø, A.; Gawroński, S. Deposition of Particulate Matter of Different Size Fractions on Leaf Surfaces and in Waxes of Urban Forest Species. Int. J. Phytoremediation 2011, 13, 1037–1046. [Google Scholar] [CrossRef]
- Chen, L.; Liu, C.; Zou, R.; Yang, M.; Zhang, Z. Experimental examination of effectiveness of vegetation as biofilter of particulate matters in the urban environment. Environ. Pollut. 2016, 208, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Li, P. Resuspension of settled atmospheric particulate matter on plant leaves determined by wind and leaf surface characteristics. Environ. Sci. Pollut. Res. 2019, 26, 19606–19614. [Google Scholar] [CrossRef]
- Kwak, M.J.; Lee, J.K.; Park, S.; Kim, H.; Lim, Y.J.; Lee, K.-A.; Son, J.-A.; Oh, C.-Y.; Kim, I.; Woo, S.Y. Surface-Based Analysis of Leaf Microstructures for Adsorbing and Retaining Capability of Airborne Particulate Matter in Ten Woody Species. Forests 2020, 11, 946. [Google Scholar] [CrossRef]
- Hofman, J.; Bartholomeus, H.; Calders, K.; Van Wittenberghe, S.; Wuyts, K.; Samson, R. On the relation between tree crown morphology and particulate matter deposition on urban tree leaves: A ground-based LiDAR approach. Atmos. Environ. 2014, 99, 130–139. [Google Scholar] [CrossRef]
- Wang, L.; Dai, Z. Effects of the natural microstructures on the wettability of leaf surfaces. Biosurface Biotribol. 2016, 2, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, S.; Wuyts, K.; Samson, R. Immobilized atmospheric particulate matter on leaves of 96 urban plant species. Environ. Sci. Pollut. Res. 2020, 27, 36920–36938. [Google Scholar] [CrossRef] [PubMed]
- Schaubroeck, T.; Deckmyn, G.; Neirynck, J.; Staelens, J.; Adriaenssens, S.; Dewulf, J.; Muys, B.; Verheyen, K. Multilayered Modeling of Particulate Matter Removal by a Growing Forest over Time, From Plant Surface Deposition to Washoff via Rainfall. Environ. Sci. Technol. 2014, 48, 10785–10794. [Google Scholar] [CrossRef]
- Jeanjean, A.; Monks, P.; Leigh, R. Modelling the effectiveness of urban trees and grass on PM2.5 reduction via dispersion and deposition at a city scale. Atmos. Environ. 2016, 147, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, Z.; Meng, H.; Zhang, T. How Does Leaf Surface Micromorphology of Different Trees Impact Their Ability to Capture Particulate Matter? Forests 2018, 9, 681. [Google Scholar] [CrossRef] [Green Version]
- Burkhardt, J. Hygroscopic particles on leaves: Nutrients or desiccants? Ecol. Monogr. 2010, 80, 369–399. [Google Scholar] [CrossRef]
- Naidoo, G.; Chirkoot, D. The effects of coal dust on photosynthetic performance of the mangrove, Avicennia marina in Richards Bay, South Africa. Environ. Pollut. 2004, 127, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Hanslin, H.M.; Przybysz, A.; Slimestad, R.; Sæbø, A. Stress acclimation and particulate matter accumulation in Pinus sylvestris saplings affected by moderate combinations of urban stressors. Sci. Total. Environ. 2017, 593–594, 581–591. [Google Scholar] [CrossRef]
- Javanmard, Z.; Kouchaksaraei, M.T.; Bahrami, H.A.; Hosseini, S.M.; Sanavi, S.A.M.M.; Struve, D.; Ammere, C. Soil dust effects on morphological, physiological and biochemical responses of four tree species of semiarid regions. Eur. J. For. Res. 2020, 139, 333–348. [Google Scholar] [CrossRef]
- Hirano, T.; Kiyota, M.; Aiga, I. Physical effects of dust on leaf physiology of cucumber and kidney bean plants. Environ. Pollut. 1995, 89, 255–261. [Google Scholar] [CrossRef]
- Moradi, A.; Abkenar, K.T.; Mohammadian, M.A.; Shabanian, N. Effects of dust on forest tree health in Zagros oak forests. Environ. Monit. Assess. 2017, 189, 549. [Google Scholar] [CrossRef]
- Lee, J.; Kwak, M.; Park, S.; Kim, H.; Lim, Y.; Jeong, S.; Choi, Y.; Woo, S. Ozone Response of Leaf Physiological and Stomatal Characteristics in Brassica juncea L. at Supraoptimal Temperatures. Land 2021, 10, 357. [Google Scholar] [CrossRef]
- Popek, R.; Przybysz, A.; Gawrońska, H.; Klamkowski, K.; Gawroński, S.W. Impact of particulate matter accumulation on the photosynthetic apparatus of roadside woody plants growing in the urban conditions. Ecotoxicol. Environ. Saf. 2018, 163, 56–62. [Google Scholar] [CrossRef]
- Shah, K.; Amin, N.U.; Ahmad, I.; Ara, G. Impact assessment of leaf pigments in selected landscape plants exposed to roadside dust. Environ. Sci. Pollut. Res. 2018, 25, 23055–23073. [Google Scholar] [CrossRef]
- Craver, J.K.; Miller, C.T.; Williams, K.A.; Bello, N.M. Ultraviolet Radiation Affects Intumescence Development in Ornamental Sweetpotato (Ipomoea batatas). HortScience 2014, 49, 1277–1283. [Google Scholar] [CrossRef]
- Rosero, A.; Granda, L.; Pérez, J.-L.; Rosero, D.; Burgos-Paz, W.; Martínez, R.; Morelo, J.; Pastrana, I.; Burbano, E.; Morales, A. Morphometric and colourimetric tools to dissect morphological diversity: An application in sweet potato [Ipomoea batatas (L.) Lam.]. Genet. Resour. Crop. Evol. 2019, 66, 1257–1278. [Google Scholar] [CrossRef] [Green Version]
- Jo, Y.; Kim, S.-M.; Choi, H.; Yang, J.W.; Lee, B.C.; Cho, W.K. Sweet potato viromes in eight different geographical regions in Korea and two different cultivars. Sci. Rep. 2020, 10, 2588. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Buonfiglio, L.G.V.; Mudunkotuwa, I.A.; Alaiwa, M.A.; Calderón, O.G.V.; Borcherding, J.A.; Gerke, A.K.; Zabner, J.; Grassian, V.H.; Comellas, A.P. Effects of Coal Fly Ash Particulate Matter on the Antimicrobial Activity of Airway Surface Liquid. Environ. Heal. Perspect. 2017, 125, 077003. [Google Scholar] [CrossRef]
- Korea Ministry of Government Legislation. Available online: www.moleg.go.kr (accessed on 6 June 2021).
- Farquhar, G.D.; Von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Cao, Z.; Zou, S.; Liu, H.; Hai, X.; Wang, S.; Duan, J.; Xi, B.; Yan, G.; Zhang, S.; et al. An investigation of the leaf retention capacity, efficiency and mechanism for atmospheric particulate matter of five greening tree species in Beijing, China. Sci. Total. Environ. 2018, 616–617, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.J.; Lee, J.; Kim, H.; Park, S.; Lim, Y.; Kim, J.E.; Baek, S.G.; Seo, S.M.; Kim, K.N.; Woo, S.Y. The Removal Efficiencies of Several Temperate Tree Species at Adsorbing Airborne Particulate Matter in Urban Forests and Roadsides. Forests 2019, 10, 960. [Google Scholar] [CrossRef] [Green Version]
- Kuki, K.N.; Oliva, M.A.; Pereira, E.G.; Costa, A.C.; Cambraia, J. Effects of simulated deposition of acid mist and iron ore particulate matter on photosynthesis and the generation of oxidative stress in Schinus terebinthifolius Radii and Sophora tomentosa L. Sci. Total. Environ. 2008, 403, 207–214. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, B.; Wang, Y.; Yu, W. The Response of Plant Photosynthesis and Stomatal Conductance to Fine Particulate Matter (PM2.5) based on Leaf Factors Analyzing. J. Plant Biol. 2019, 62, 120–128. [Google Scholar] [CrossRef]
- Neves, N.R.; Oliva, M.A.; Centeno, D.; Costa, A.; Ribas, R.F.; Pereira, E.G. Photosynthesis and oxidative stress in the restinga plant species Eugenia uniflora L. exposed to simulated acid rain and iron ore dust deposition: Potential use in environmental risk assessment. Sci. Total. Environ. 2009, 407, 3740–3745. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Lin, X.; Chen, J.; Huang, D.; Li, M. Atmospheric particle retention capacity and photosynthetic responses of three common greening plant species under different pollution levels in Hangzhou. Glob. Ecol. Conserv. 2019, 20, e00783. [Google Scholar] [CrossRef]
- Popek, R.; Łukowski, A.; Karolewski, P. Particulate matter accumulation—Further differences between native Prunus padusand non-native P. serotina. Dendrobiology 2017, 78, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, I.J.; Rathore, D. Suspended particulate matter deposition and its impact on urban trees. Atmos. Pollut. Res. 2018, 9, 1072–1082. [Google Scholar] [CrossRef]
- Song, Y.; Maher, B.; Li, F.; Wang, X.; Sun, X.; Zhang, H. Particulate matter deposited on leaf of five evergreen species in Beijing, China: Source identification and size distribution. Atmos. Environ. 2015, 105, 53–60. [Google Scholar] [CrossRef]
- Lehndorff, E.; Urbat, M.; Schwark, L. Accumulation histories of magnetic particles on pine needles as function of air quality. Atmos. Environ. 2006, 40, 7082–7096. [Google Scholar] [CrossRef]
- Beckett, K.P.; Freer-Smith, P.H.; Taylor, G. Particulate pollution capture by urban trees: Effect of species and windspeed. Glob. Chang. Biol. 2000, 6, 995–1003. [Google Scholar] [CrossRef]
- Gong, Y.; Zhou, T.; Wang, P.; Lin, Y.; Zheng, R.; Zhao, Y.; Xu, B. Fundamentals of Ornamental Plants in Removing Benzene in Indoor Air. Atmosphere 2019, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Tomson, N.; Michael, R.N.; Agranovski, I.E. Removal of particulate air pollutants by Australian vegetation potentially used for green barriers. Atmos. Pollut. Res. 2021, 12, 101070. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, J.J.; Byeon, H.; Go, T.; Lee, S.J. Removal of fine particulate matter (PM2.5) via atmospheric humidity caused by evapotranspiration. Environ. Pollut. 2019, 245, 253–259. [Google Scholar] [CrossRef]
- Chen, L.; Liu, C.; Zhang, L.; Zou, R.; Zhang, Z. Variation in Tree Species Ability to Capture and Retain Airborne Fine Particulate Matter (PM2.5). Sci. Rep. 2017, 7, 3206. [Google Scholar] [CrossRef]
- Weerakkody, U.; Dover, J.W.; Mitchell, P.; Reiling, K. Quantification of the traffic-generated particulate matter capture by plant species in a living wall and evaluation of the important leaf characteristics. Sci. Total. Environ. 2018, 635, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Leonard, R.J.; McArthur, C.; Hochuli, D.F. Particulate matter deposition on roadside plants and the importance of leaf trait combinations. Urban For. Urban Green. 2016, 20, 249–253. [Google Scholar] [CrossRef]
- Mo, L.; Ma, Z.; Xu, Y.; Sun, F.; Lun, X.; Liu, X.; Chen, J.; Yu, X. Assessing the Capacity of Plant Species to Accumulate Particulate Matter in Beijing, China. PLoS ONE 2015, 10, e0140664. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, W.; Mo, L.; Heal, M.; Xu, X.; Yu, X. Quantifying particulate matter accumulated on leaves by 17 species of urban trees in Beijing, China. Environ. Sci. Pollut. Res. 2018, 25, 12545–12556. [Google Scholar] [CrossRef] [Green Version]
- Shao, F.; Wang, L.; Sun, F.; Li, G.; Yu, L.; Wang, Y.; Zeng, X.; Yan, H.; Dong, L.; Bao, Z. Study on different particulate matter retention capacities of the leaf surfaces of eight common garden plants in Hangzhou, China. Sci. Total. Environ. 2019, 652, 939–951. [Google Scholar] [CrossRef]
- Ouyang, W.; Struik, P.C.; Yin, X.; Yang, J. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 2017, 68, 5191–5205. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, L.; Chen, R.; Tian, G.; Li, D.; Chen, C.; Ge, X.; Ge, G. Effect of relative humidity on the deposition and coagulation of aerosolized SiO2 nanoparticles. Atmos. Res. 2017, 194, 100–108. [Google Scholar] [CrossRef]
- Roth-Nebelsick, A. Computer-based Studies of Diffusion through Stomata of Different Architecture. Ann. Bot. 2007, 100, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Roth-Nebelsick, A.; Hassiotou, F.; Veneklaas, E.J. Stomatal Crypts Have Small Effects on Transpiration: A Numerical Model Analysis. Plant Physiol. 2009, 151, 2018–2027. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Wang, H.; Liu, Q.; An, H.; Liu, C.; Xia, X.; Yin, W. 15N-labeled ammonium nitrogen uptake and physiological responses of poplar exposed to PM2.5 particles. Environ. Sci. Pollut. Res. 2017, 24, 500–508. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, H.; Wang, J.; Lu, M.; Liu, C.; Xia, X.; Yin, W.; Guo, H. PM2.5-bound SO42—absorption and assimilation of poplar and its physiological responses to PM2.5 pollution. Environ. Exp. Bot. 2018, 153, 311–319. [Google Scholar] [CrossRef]
- Liu, L.; Guan, D.; Peart, M.R. The morphological structure of leaves and the dust-retaining capability of afforested plants in urban Guangzhou, South China. Environ. Sci. Pollut. Res. 2012, 19, 3440–3449. [Google Scholar] [CrossRef]
- Zha, Y.; Shi, Y.; Tang, J.; Liu, X.; Feng, C.; Zhang, Y. Spatial-Temporal Variability and Dust-Capture Capability of 8 Plants in Urban China. Pol. J. Environ. Stud. 2018, 28, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Bakker, M.I.; Vorenhout, M.; Sijm, D.T.; Kollöffel, C. Dry deposition of atmospheric polycyclic aromatic hydrocarbons in three Plantago species. Environ. Toxicol. Chem. 1999, 18, 2289–2294. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Yin, S.; Ma, Y.; Kang, H.; Zhang, X.; Tan, H.; Meng, H.; Liu, C. Impact factor assessment of the uptake and accumulation of polycyclic aromatic hydrocarbons by plant leaves: Morphological characteristics have the greatest impact. Sci. Total. Environ. 2019, 652, 1149–1155. [Google Scholar] [CrossRef]
- Zhang, X.; Lyu, J.; Han, Y.; Sun, N.; Sun, W.; Li, J.; Liu, C.; Yin, S. Effects of the leaf functional traits of coniferous and broadleaved trees in subtropical monsoon regions on PM2.5 dry deposition velocities. Environ. Pollut. 2020, 265, 114845. [Google Scholar] [CrossRef] [PubMed]
- Neinhuis, C.; Barthlott, W. Seasonal changes of leaf surface contamination in beech, oak, and ginkgo in relation to leaf micromorphology and wettability. New Phytol. 1998, 138, 91–98. [Google Scholar] [CrossRef]
| Leaf Characteristics | Ipomoea batatas | Hedera helix | Epipremnum aureum | |||||
|---|---|---|---|---|---|---|---|---|
| B | BH | CP | GF | M | ||||
| Leaf area (cm2) | 49.5 ± 14.6 ab | 29.4 ± 5.1 bcd | 30.3 ± 5.0 bc | 29.2 ± 1.9 cd | 55.6 ± 6.7 a | 9.4 ± 1.6 d | 67.1 ± 6.8 a | |
| Width/Length ratio | 0.91 ± 0.08 abc | 0.74 ± 0.03 cd | 1.04 ± 0.07 a | 0.86 ± 0.09 abc | 0.84 ± 0.05 bc | 0.97 ± 0.03 ab | 0.60 ± 0.09 d | |
| Stomatal density (mm−2) | Adaxial | 356 ± 13 a | 91 ± 23 b | 84 ± 26 b | 61 ± 13 b | 53 ± 13 bc | - | 12 ± 4 cd |
| Abaxial | 417 ± 13 a | 416 ± 57 a | 394 ± 57 a | 364 ± 60 a | 212 ± 13 b | 144 ± 47 bc | 68 ± 23 c | |
| Stomatal pore size (μm2) | 85.7 ± 6.4 a | 45.0 ± 2.3 a | 99.1 ± 22.9 a | 93.1 ± 32.7 a | 66.1 ± 20.7 a | 46.6 ± 7.1 a | 71.6 ± 40.7 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.K.; Kim, D.Y.; Park, S.H.; Woo, S.Y.; Nie, H.; Kim, S.H. Particulate Matter (PM) Adsorption and Leaf Characteristics of Ornamental Sweet Potato (Ipomoea batatas L.) Cultivars and Two Common Indoor Plants (Hedera helix L. and Epipremnum aureum Lindl. & Andre). Horticulturae 2022, 8, 26. https://doi.org/10.3390/horticulturae8010026
Lee JK, Kim DY, Park SH, Woo SY, Nie H, Kim SH. Particulate Matter (PM) Adsorption and Leaf Characteristics of Ornamental Sweet Potato (Ipomoea batatas L.) Cultivars and Two Common Indoor Plants (Hedera helix L. and Epipremnum aureum Lindl. & Andre). Horticulturae. 2022; 8(1):26. https://doi.org/10.3390/horticulturae8010026
Chicago/Turabian StyleLee, Jong Kyu, Do Yeon Kim, Sang Hee Park, Su Young Woo, Hualin Nie, and Sun Hyung Kim. 2022. "Particulate Matter (PM) Adsorption and Leaf Characteristics of Ornamental Sweet Potato (Ipomoea batatas L.) Cultivars and Two Common Indoor Plants (Hedera helix L. and Epipremnum aureum Lindl. & Andre)" Horticulturae 8, no. 1: 26. https://doi.org/10.3390/horticulturae8010026
APA StyleLee, J. K., Kim, D. Y., Park, S. H., Woo, S. Y., Nie, H., & Kim, S. H. (2022). Particulate Matter (PM) Adsorption and Leaf Characteristics of Ornamental Sweet Potato (Ipomoea batatas L.) Cultivars and Two Common Indoor Plants (Hedera helix L. and Epipremnum aureum Lindl. & Andre). Horticulturae, 8(1), 26. https://doi.org/10.3390/horticulturae8010026







