Root Architecture, Growth and Photon Yield of Cucumber Seedlings as Influenced by Daily Light Integral at Different Stages in the Closed Transplant Production System
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
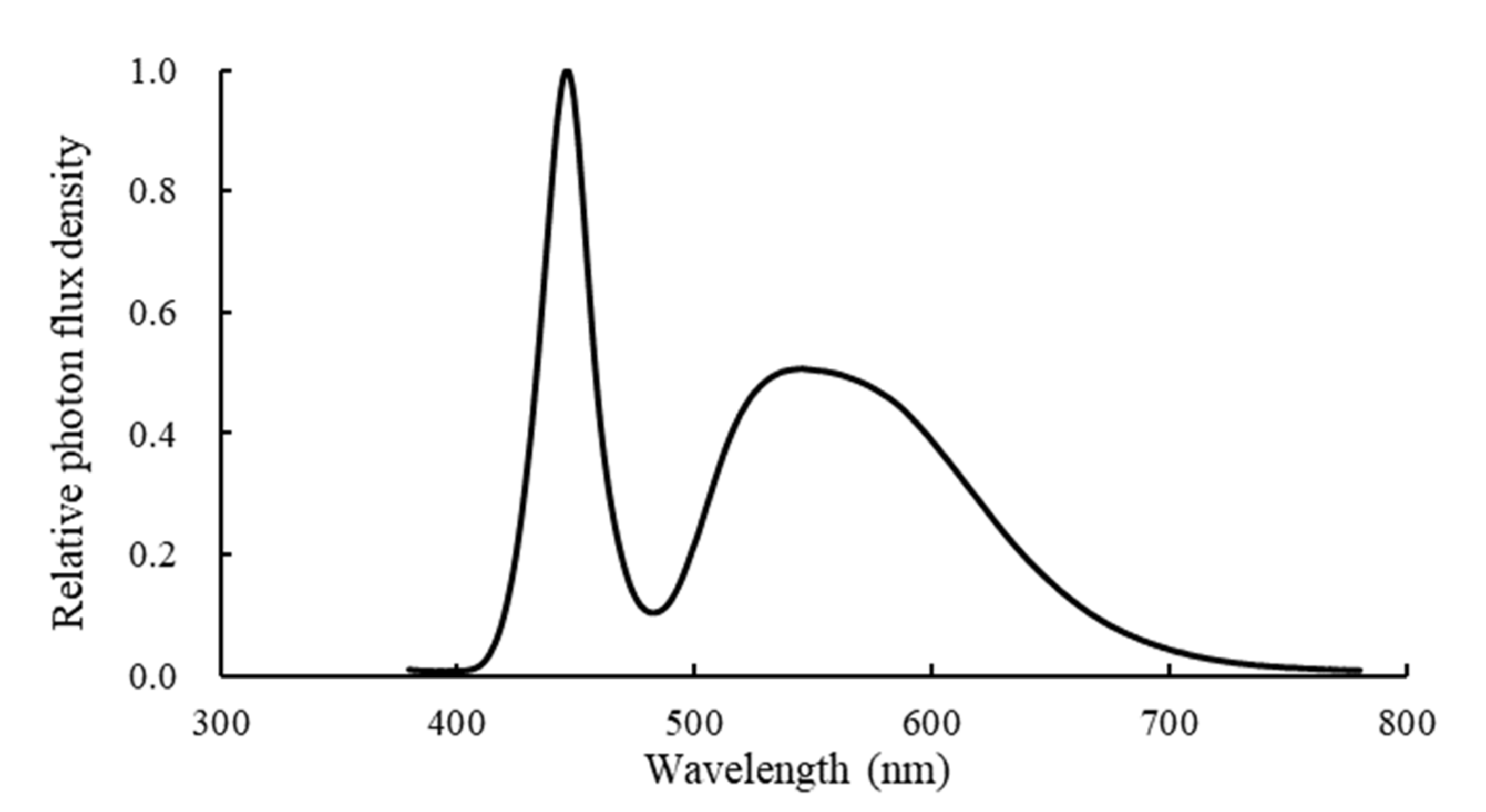
2.2. Treatment Design
2.3. Growth Measurements
2.3.1. Chlorophyll Content of Cucumber Seedlings
2.3.2. Photosynthetic Characteristic of Cucumber Seedlings
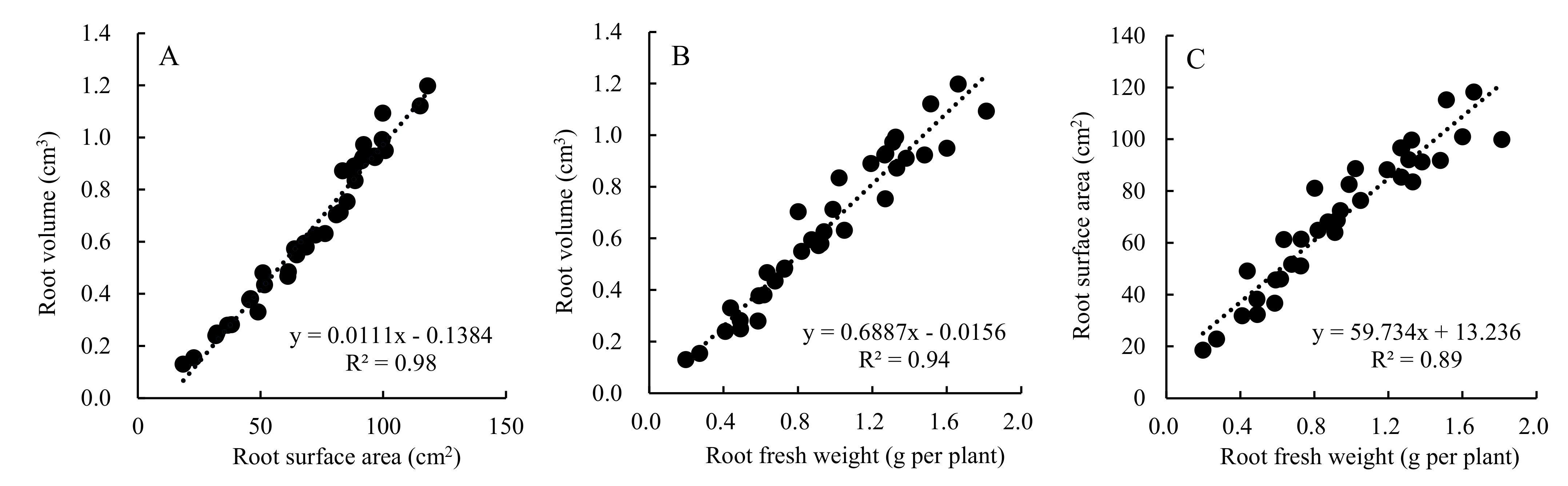
2.3.3. Plant Morphology and Root Architecture of Cucumber Seedlings
2.3.4. Biomass Accumulation and Photon Yield of Cucumber Seedlings
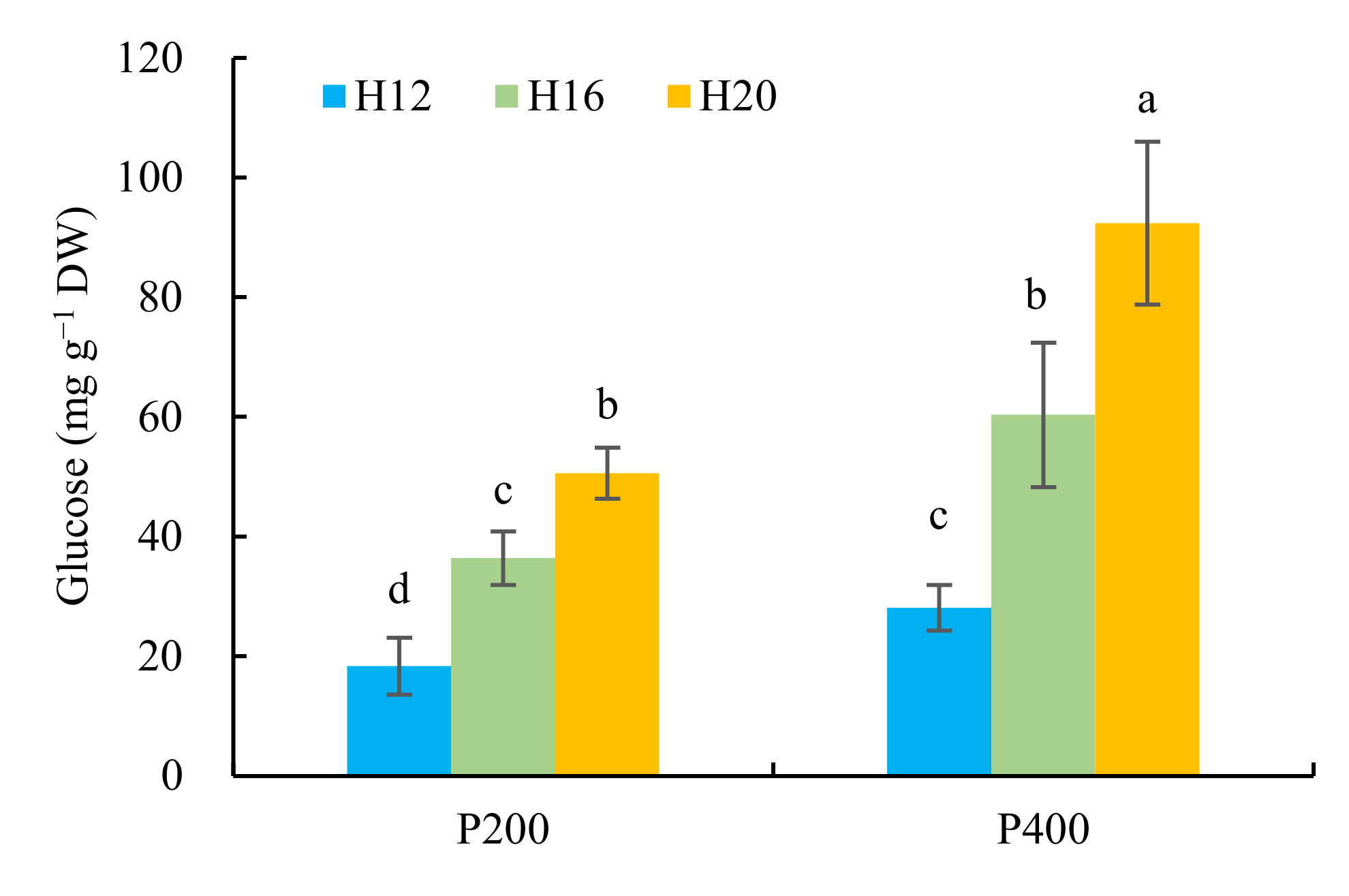
2.3.5. Glucose Content of Cucumber Seedlings
2.4. Statistical Analysis
3. Results
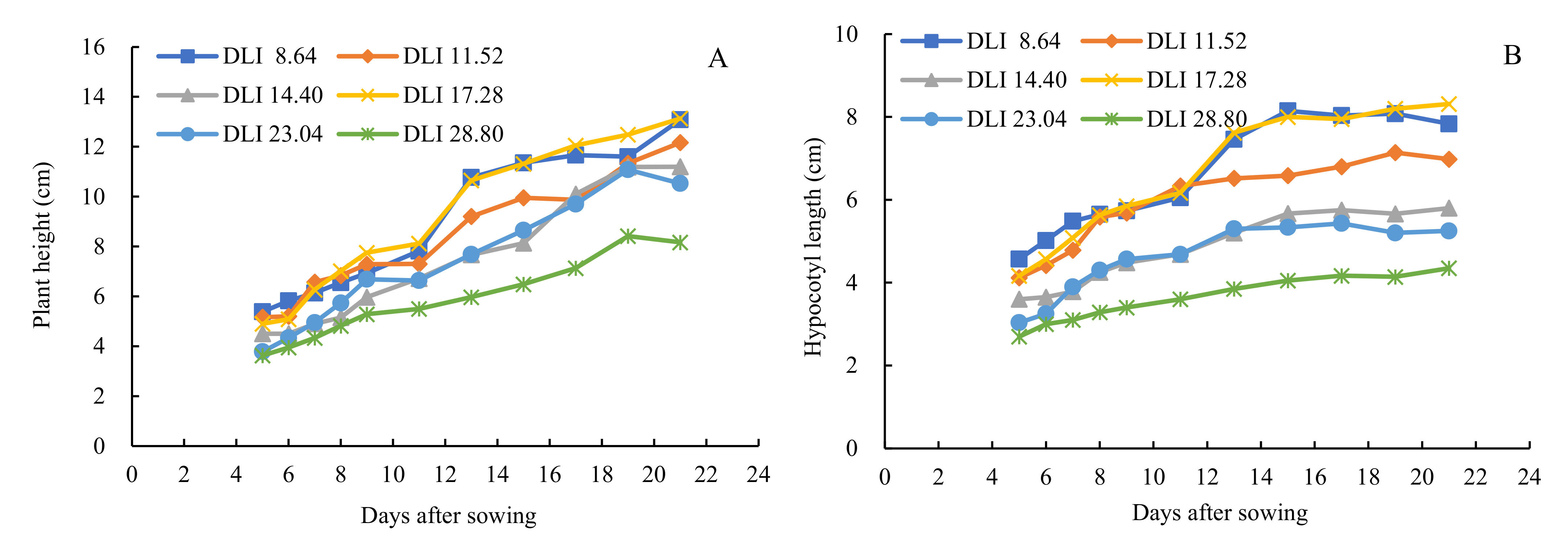
3.1. Effects of Daily Light Integral on Plant Height and Hypocotyl Length of Cucumber Seedlings Grown in a Closed Transplant System at Different Stages
3.2. Impact of Daily Light Integral on Biomass Accumulation of Cucumber Seedlings Grown in the Closed Transplant System at Different Stages
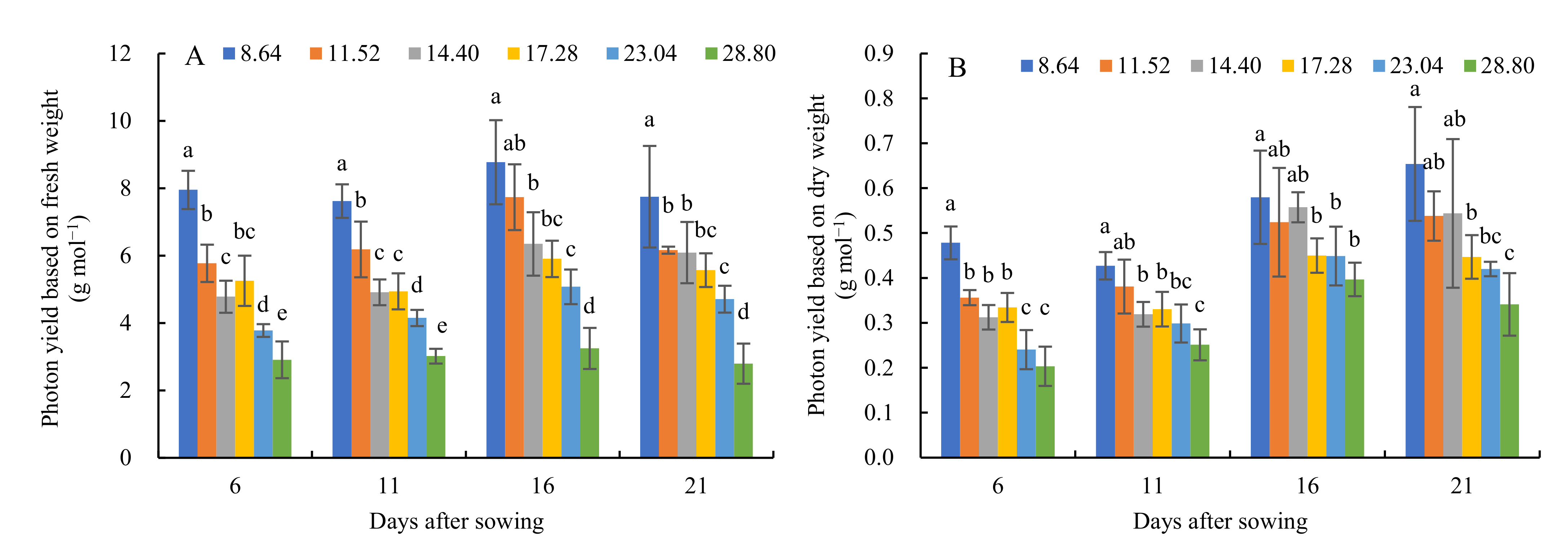
3.3. Photon Yield of Cucumber Seedlings Grown under Different Daily Light Integrals in the Closed Transplant System at Different Stages
3.4. Impact of Different Daily Light Integrals on Pigment Content and Net Photosynthetic Rate of Cucumber Seedlings at 21 Days after Sowing
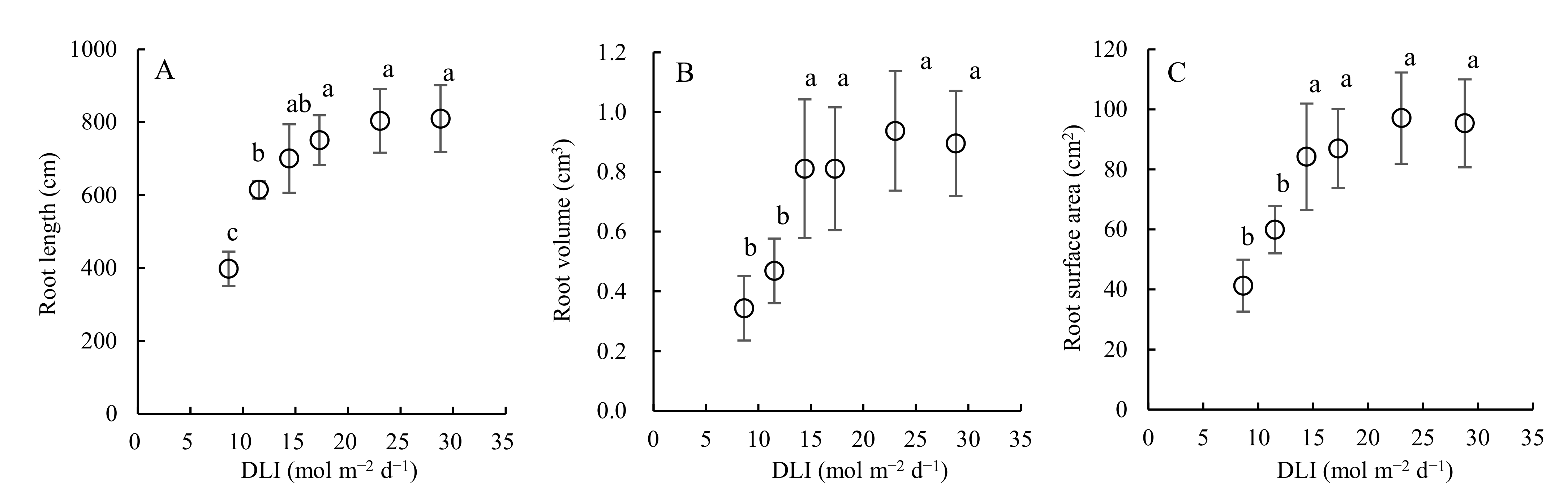
3.5. Effect of Daily Light Integral on Leaf Morphology, Root Architecture, and Biomass Accumulation of Cucumber Seedlings Grown in a Closed Transplant System
3.6. Effect of Different Combinations of Photosynthetic Photon Flux Density and Photoperiod on Glucose Content of Cucumber Seedlings at 21 Days after Sowing
4. Discussion
4.1. Influence of Daily Light Integral on Leaf Morphology and Root Architecture of Cucumber Seedling
4.2. Chlorophyll Content, Photosynthetic Parameter, Biomass Accumulation, and Glucose Content of Cucumber Seedlings Exposed to Varying Daily Light Integrals
4.3. Photon Yield of Cucumber Seedlings Grown in the Closed Transplant Seedling System as Affected by Daily Light Integral
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- He, D.X.; Yan, Z.N.; Sun, X.; Yang, P. Leaf development and energy yield of hydroponic sweetpotato seedlings using single-node cutting as influenced by light intensity and LED spectrum. J. Plant. Physiol. 2020, 254, 153274. [Google Scholar] [CrossRef]
- Liu, M.C.; Ji, Y.H.; Wu, Z.H.; He, W.M. Current suitation and development trend of vegetable seedling industry in China. China Vegetables 2018, 11, 1–7. [Google Scholar]
- Korea Rural Economic Institute (KREI). A Study on Management System and Legislation for Protection and Nurturance of Raising Seedlings Industry; Korea Rural Economic Institute (KREI): Seoul, Korea, 2014. [Google Scholar]
- Park, M.S.; Kim, H.C.; An, S.; Lee, C.W.; Choi, J.M. Influence of pre-plant root substrate nitrogen levels on growth of selected fruit bearing vegetable seedlings in cylindrical paper pots. Hortic. Environ. Biotechnol. 2021, 62, 367–375. [Google Scholar] [CrossRef]
- Miao, Y.X. Physiological Response Mechanism of Cucumber Seedlings to Red and Blue Light. Ph.D. Thesis, China Agriculture University, Beijing, China, 2015. [Google Scholar]
- Nakano, A.; Ohtake, N.; Yoneda, T.; Shinoda, A. Effects of LED alternating irradiation on tomato seedling quality and root development after transplanting. Root Res. 2017, 26, 3–9. [Google Scholar] [CrossRef]
- Chang, C.L.; Chang, K.P. The growth response of leaf lettuce at different stages to multiple wavelength-band light-emitting diode lighting. Sci. Hortic. 2014, 179, 78–84. [Google Scholar] [CrossRef]
- Yan, Z.N.; He, D.X.; Niu, G.; Zhai, H. Evaluation of growth and quality of hydroponic lettuce at harvest as affected by the light intensity, photoperiod and light quality at seedling stage. Sci. Hortic. 2019, 248, 138–144. [Google Scholar] [CrossRef]
- FAO. 2021. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 1 June 2021).
- Ji, F.; Wei, S.Q.; Liu, N.; Xu, L.J.; Yang, P. Growth of cucumber seedlings in different varieties as affected by light environment. Int. J. Agric. Biol. Eng. 2020, 13, 73–78. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant. Growth Regul. 2021, 40, 1–39. [Google Scholar] [CrossRef]
- Aguirre-Becerra, H.; Garcia-Trejo, J.F.; Vazquez-Hernandez, C.; Alvarado, A.M.; Feregrino-Perez, A.A.; Contreras-Medina, L.M.; Guevara-Gonzalez, R.G. Effect of extended photoperiod with a fixed mixture of light wavelengths on tomato seedlings. HortScience 2020, 55, 1832–1839. [Google Scholar] [CrossRef]
- Park, S.W.; An, S.; Kwack, Y. Changes in transpiration rates and growth of cucumber and tomato scions and rootstocks grown under different light intensity conditions in a closed transplant production system. Prot. Hortic. Plant Fact. 2020, 29, 399–405. [Google Scholar] [CrossRef]
- Wang, S.; Fang, H.; Xie, J.; Wu, Y.; Tang, Z.; Liu, Z.; Lv, J.; Yu, J. Physiological responses of cucumber seedlings to different supplemental light duration of red and blue LED. Front. Plant Sci. 2021, 12, 709313. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Orsini, F.; Landolfo, M.; Pistillo, A.; Crepaldi, A.; Nicola, S.; Fernández, J.A.; Marcelis, L.F.M.; Gianquinto, G. Optimal photoperiod for indoor cultivation of leafy vegetables and herbs. Eur. J. Hortic. Sci. 2020, 85, 329–338. [Google Scholar] [CrossRef]
- Dou, H.J.; Niu, G.; Gu, M.M.; Masabni, J.G. Responses of sweet basil to different daily light integrals in photosynthesis, morphology, yield, and nutritional quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Gao, W.; He, D.X.; Ji, F.; Zhang, S.; Zheng, J.F. Effects of daily light integral and LED spectrum on growth and nutritional quality of hydroponic spinach. Agronomy 2020, 10, 1082. [Google Scholar] [CrossRef]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Crepaldi, A.; Gianquinto, G.; Marcelis, L.F.M. Optimal light intensity for sustainable water and energy use in indoor cultivation of lettuce and basil under red and blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- Hwang, H.; An, S.; Pham, M.D.; Meiyan Cui, M.; Chun, C. The combined conditions of photoperiod, light intensity, and air temperature control the growth and development of tomato and red pepper seedlings in a closed transplant production system. Sustainability 2020, 12, 9939. [Google Scholar] [CrossRef]
- Korczynski, P.C.; Logan, J.; Faust, J.E. Mapping monthly distribution of daily light integral cross the contiguous United States. HortTechnology 2002, 12, 12–16. [Google Scholar] [CrossRef]
- Faust, J.E.; Logan, J. Daily Light Integral: A Research Review and High-resolution Maps of the United States. HortScience 2018, 53, 1250–1257. [Google Scholar] [CrossRef]
- Hernández, R.; Eguchi, T.; Deveci, M.; Kubota, C. Tomato seedling physiological responses under different percentages of blue and red photon flux ratios using LEDs and cool white fluorescent lamps. Sci. Hortic. 2016, 213, 270–280. [Google Scholar] [CrossRef]
- Hwang, H.; An, S.; Lee, B.; Chun, C. Improvement of growth and morphology of vegetable seedlings with supplemental far-red enriched LED lights in a plant factory. Horticulturae 2020, 6, 109. [Google Scholar] [CrossRef]
- Fraszczak, B.; Kula-Maximenko, M. The preferences of different cultivars of lettuce seedlings (Lactuca sativa L.) for the spectral composition of light. Agronomy 2021, 11, 1211. [Google Scholar] [CrossRef]
- Chen, X.L.; Xue, X.Z.; Guo, W.Z.; Wang, L.C.; Qiao, X.J.; Xue, X.Z. Growth and nutritional properties of lettuce affected by mixed irradiation of white and supplemental light provided by light-emitting diode. Sci. Hortic. 2016, 200, 111–118. [Google Scholar] [CrossRef]
- Yan, Z.N.; He, D.X.; Niu, G.; Zhou, Q.; Qu, Y.H. Growth, nutritional quality, and energy use efficiency in two lettuce cultivars as influenced by white plus red versus red plus blue LEDs. Int. J. Agric. Biol. Eng. 2020, 13, 33–40. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Spectral effects of light-emitting diodes on plant growth, visual color quality, and photosynthetic photon efficacy: White versus blue plus red radiation. PLoS ONE 2018, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Yan, Z.N.; He, D.X.; Niu, G.; Zhou, Q.; Qu, Y.H. Growth, nutritional quality, and energy use efficiency of hydroponic lettuce as influenced by daily light integrals exposed to white versus white plus red light-emitting diodes. HortScience 2019, 54, 1737–1744. [Google Scholar] [CrossRef]
- Zheng, J.F.; Ji, F.; He, D.X.; Niu, G. Effect of light intensity on rooting and growth of hydroponic strawberry runner plants in a LED plant factory. Agronomy 2019, 9, 875. [Google Scholar] [CrossRef]
- Takahashi, K.; Fujino, K.; Kikuta, Y.; Koda, Y. Involvement of the accumulation of sucrose and the synthesis of cell wall polysaccharides in the expansion of potato cells in response to jasmonic acid. Plant Sci. 1995, 111, 11–18. [Google Scholar] [CrossRef]
- Ghorbanzadeh, P.; Aliniaeifard, S.; Esmaeili, M.; Mashal, M.; Azadegan, B.; Seif, M. Dependency of growth, water use efficiency, chlorophyll fluorescence, and stomatal characteristics of lettuce plants to light intensity. J. Plant. Growth Regul. 2020, 39, 1–17. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, H.; Song, S.; Su, W.; Liu, H. Morphological and physiological responses of cucumber seedlings to supplemental led light under extremely low irradiance. Agronomy 2020, 10, 1698. [Google Scholar] [CrossRef]
- Potter, T.I.; Rood, S.B.; Zanewich, K.P. Light intensity, gibberellin content and the resolution of shoot growth in Brassica. Planta 1999, 207, 505–511. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Li, R.; Zhao, Y.; Geng, J.; Wang, G. Physiological responses of lettuce (Lactuca sativa L.) to microplastic pollution. Environ. Sci. Pollut. Res. 2020, 27, 30306–30314. [Google Scholar] [CrossRef]
- Park, J.E.; Park, Y.G.; Jeong, B.R.; Hwang, S.J. Growth of lettuce in closed-type plant production system as affected by light intensity and photoperiod under influence of white LED light. Prot. Hortic. Plant Fact. 2013, 22, 228–233. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Lin, B.; Wang, X.; Wang, T.; Zhang, K. Effects of weak light stress on growth and physiological characteristics of eggplant seedlings. Subtrop. Agric. Res. 2016, 12, 86–92. [Google Scholar]
- Hata, N.; Okazawa, A.; Morimoto, K.; Ono, E.; Satake, H.; Kobayashi, A. Effects of IBA treatment, fertigation, photoperiod, and light intensity on rooting of softwood cuttings of Forsythia suspensa. J. Sci. High Technol. Agric. 2009, 21, 15–23. [Google Scholar] [CrossRef][Green Version]
- Fredrick, C.; Ekeke, B.A.; Abere, S.A.; Chukunda, F.A. Evaluation of germination and early growth performance of Pterocarpus santalinoides seedlings at different light intensities. Afr. J. Agric. Technol. Environ. 2020, 9, 94–104. [Google Scholar]
- Yamori, W. Photosynthesis and respiration. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd ed.; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 197–206. [Google Scholar]
- Dai, Y.; Shen, Z.; Liu, Y.; Wang, L.; Hannaway, D.; Lu, H. Effects of shade treatments on the photosynthetic capcaticy, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ. Exp. Bot. 2009, 65, 177–182. [Google Scholar] [CrossRef]
- Baumgardt, C.J.; Ying, Q.; Zheng, Y.; Bozzo, G.G. The growth and morphology of microgreens is associated with modified ascorbate and anthocyanin profiles in response to the intensity of sole-source light-emitting diodes. Can. J. Plant Sci. 2020, 101, 212–228. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Long, S.P.; Bernacchi, C.J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xin, G.; Wei, M.; Shi, Q.; Yang, F.; Wang, X. Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities. Sci. Hortic. 2017, 225, 490–497. [Google Scholar] [CrossRef]
- Mengin, V.; Pyl, E.T.; Moraes, T.A.; Sulpice, R.; Krohn, N.; Encke, B.; Mark, S. Photosynthate partitioning to starch in Arabidopsis thaliana is insensitive to light intensity but sensitive to photoperiod due to a restriction on growth in the light in short photoperiods. Plant. Cell Environ. 2017, 40, 2608–2627. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Q.; Zhou, R.; Wang, X.X.; Kjær, K.H.; Rosenqvist, E.; Ottosen, C.O.; Chen, J.F. Evaluation of genotypic variation during leaf development in four cucumis genotypes and their response to high light conditions. Environ. Exp. Bot 2016, 124, 100–109. [Google Scholar] [CrossRef]
- Chung, H.Y.; Chang, M.Y.; Wu, C.C.; Fang, W. Quantitative evaluation of electric light recipes for red leaf lettuce cultivation in plant factories. HortTechnology 2018, 28, 755–763. [Google Scholar] [CrossRef]
- Jayalath, T.C.; van Iersel, M.W. Canopy size and light use efficiency explain growth differences between lettuce and mizuna in vertical farms. Plants 2021, 10, 704. [Google Scholar] [CrossRef]
- Weaver, G.; van Iersel, M.W. Photochemical characterization of greenhouse-grown lettuce (Lactuca sativa L. ‘Green Towers’) with applications for supplemental lighting control. HortScience 2019, 54, 317–322. [Google Scholar] [CrossRef]
- Lu, N.; Bernardo, E.L.; Tippayadarapanich, C.; Takagaki, M.; Kagawa, N.; Yamori, W. Growth and accumulation of secondary metabolites in perilla as affected by photosynthetic photon flux density and electrical conductivity of the nutrient solution. Front. Plant. Sci. 2017, 8, 708. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Role of the plant factory with artificial lighting (PFAL) in urban areas. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd ed.; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; p. 34. [Google Scholar]


| Treatment | Photosynthetic Photon Flux Density (μmol m−2 s−1) | Photoperiod (h d−1) | Daily Light Integral (mol m−2 d−1) |
|---|---|---|---|
| P200-H12 | 200 | 12 | 8.64 |
| P200-H16 | 16 | 11.52 | |
| P200-H20 | 20 | 14.40 | |
| P400-H12 | 400 | 12 | 17.28 |
| P400-H16 | 16 | 23.04 | |
| P400-H20 | 20 | 28.80 |
| Treatment | Chlorophyll a Content (mg g−1) | Chlorophyll b Content (mg g−1) | Total Chlorophyll Content (mg g−1) | Chlorophyll a/b |
|---|---|---|---|---|
| P200-H12 | 1.59 ± 0.22 a | 0.61 ± 0.08 a | 2.20 ± 0.30 a | 2.60 ± 0.12 b |
| P200-H16 | 1.38 ± 0.22 a | 0.51 ± 0.10 b | 1.89 ± 0.31 b | 2.70 ± 0.17 b |
| P200-H20 | 1.09 ± 0.16 b | 0.38 ± 0.05 c | 1.48 ± 0.21 c | 2.84 ± 0.04 b |
| P400-H12 | 1.13 ± 0.15 b | 0.41 ± 0.03 c | 1.55 ± 0.18 c | 2.73 ± 0.22 b |
| P400-H16 | 1.08 ± 0.15 b | 0.39 ± 0.06 c | 1.47 ± 0.21 c | 2.80 ± 0.06 b |
| P400-H20 | 0.86 ± 0.06 c | 0.28 ± 0.02 d | 1.14 ± 0.07 d | 3.10 ± 0.28 a |
| Treatment | Plant Height (cm) | Stem Diameter (cm) | Hypocotyl Length (cm) | Leaf FW (g per Plant) | Root FW (g per Plant) | Leaf DW (g per Plant) | Root DW (g per Plant) |
|---|---|---|---|---|---|---|---|
| P200-H12 | 13.08 ± 0.90 a | 3.68 ± 0.43 c | 7.83 ± 0.68 a | 2.48 ± 0.22 c | 0.53 ± 0.10 c | 0.202 ± 0.030 c | 0.026 ± 0.008 d |
| P200-H16 | 12.17 ± 1.25 a | 3.78 ± 0.25 c | 6.73 ± 0.61 b | 2.72 ± 0.22 bc | 0.62 ± 0.12 c | 0.251 ± 0.041 bc | 0.033 ± 0.002 d |
| P200-H20 | 10.32 ± 1.69 b | 4.41 ± 0.30 ab | 5.17 ± 0.92 c | 3.14 ± 0.39 b | 1.04 ± 0.27 b | 0.336 ± 0.096 b | 0.058 ± 0.016 bc |
| P400-H12 | 13.13 ± 1.00 a | 4.04 ± 0.25 bc | 8.08 ± 0.54 a | 3.56 ± 0.38 ab | 1.22 ± 0.29 ab | 0.339 ± 0.032 b | 0.050 ± 0.005 c |
| P400-H16 | 10.53 ± 0.74 b | 4.65 ± 0.29 a | 5.43 ± 0.84 c | 3.77 ± 0.40 a | 1.35 ± 0.22 a | 0.426 ± 0.041 a | 0.066 ± 0.002 b |
| P400-H20 | 8.17 ± 0.71 c | 4.20 ± 0.41 b | 4.00 ± 0.71 d | 2.95 ± 0.48 bc | 1.10 ± 0.24 ab | 0.408 ± 0.099 ab | 0.089 ± 0.009 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chu, Y.; Wan, Z.; Zhang, G.; Liu, L.; Yan, Z. Root Architecture, Growth and Photon Yield of Cucumber Seedlings as Influenced by Daily Light Integral at Different Stages in the Closed Transplant Production System. Horticulturae 2021, 7, 328. https://doi.org/10.3390/horticulturae7090328
Wang Y, Chu Y, Wan Z, Zhang G, Liu L, Yan Z. Root Architecture, Growth and Photon Yield of Cucumber Seedlings as Influenced by Daily Light Integral at Different Stages in the Closed Transplant Production System. Horticulturae. 2021; 7(9):328. https://doi.org/10.3390/horticulturae7090328
Chicago/Turabian StyleWang, Yifei, Yangyang Chu, Ze Wan, Geng Zhang, Lei Liu, and Zhengnan Yan. 2021. "Root Architecture, Growth and Photon Yield of Cucumber Seedlings as Influenced by Daily Light Integral at Different Stages in the Closed Transplant Production System" Horticulturae 7, no. 9: 328. https://doi.org/10.3390/horticulturae7090328
APA StyleWang, Y., Chu, Y., Wan, Z., Zhang, G., Liu, L., & Yan, Z. (2021). Root Architecture, Growth and Photon Yield of Cucumber Seedlings as Influenced by Daily Light Integral at Different Stages in the Closed Transplant Production System. Horticulturae, 7(9), 328. https://doi.org/10.3390/horticulturae7090328





