Weed Control, Growth, Nodulation, Quality and Storability of Peas as Affected by Pre- and Postemergence Herbicides
Abstract
:1. Introduction:
2. Materials and Methods
2.1. Experiment Layout and Treatments
2.2. Weed Data Recording
2.3. Plant Growth, Nodulation, and Yield Determination
2.4. Chlorophyll, Carotenoid, Vitamin C, Protein, and Total Sugar Contents of Pea Pods
2.5. Storage Experiment
2.6. Determination of Herbicide Residues in Green Pods
2.6.1. Standards and Reagents
2.6.2. Sample Extraction
2.7. Statistical Analysis
3. Results and Discussion
3.1. Weed Control Efficiency, Total Weed Dry Weights
3.2. Nodule Formation
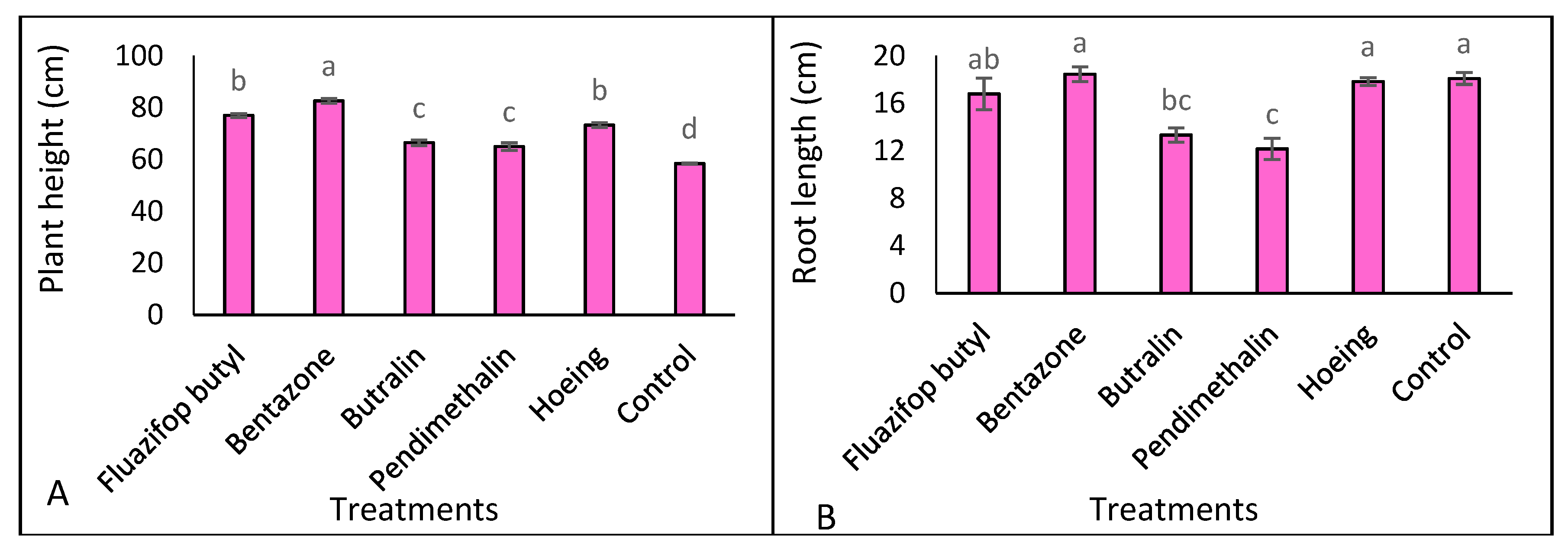
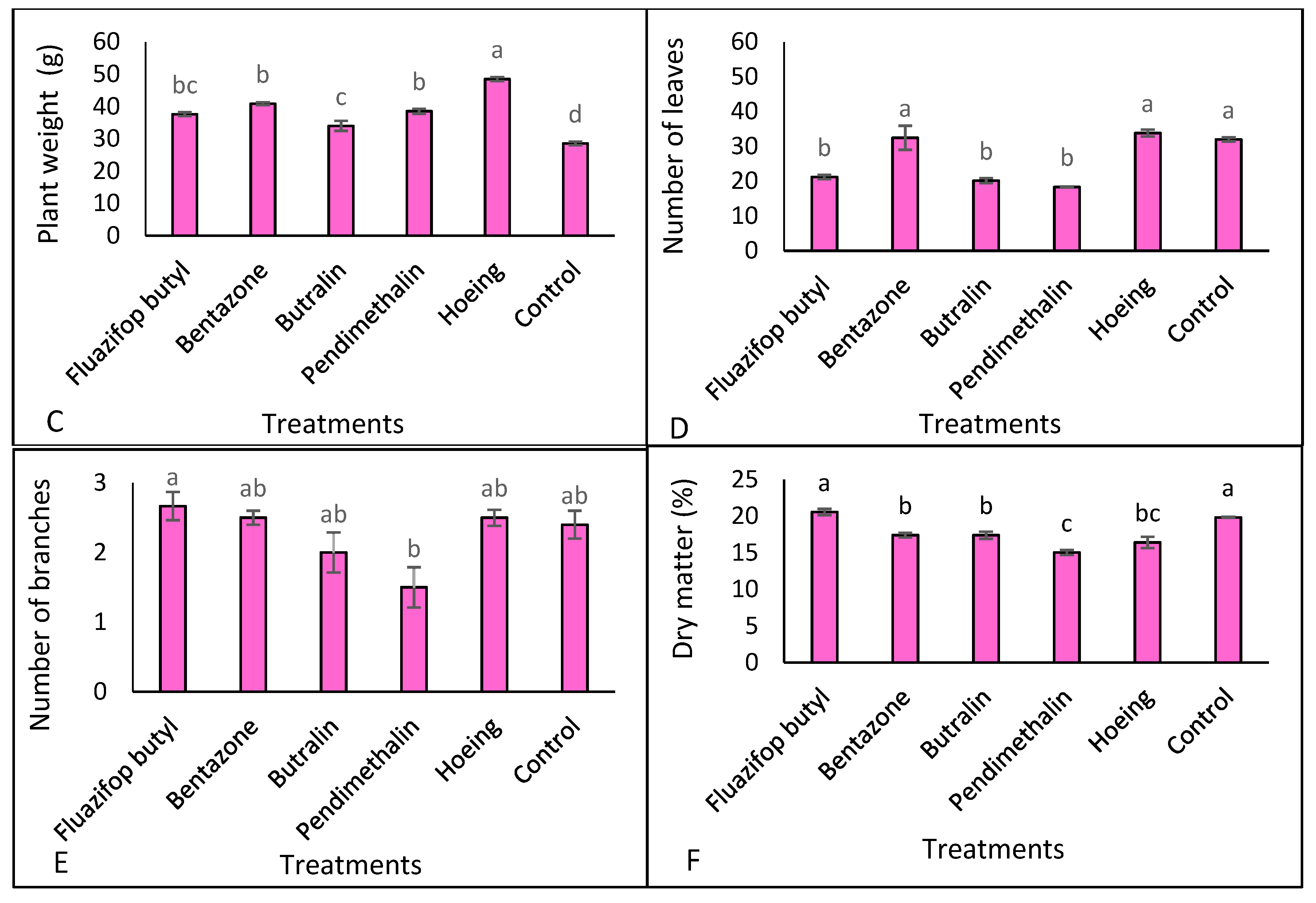
3.3. Plant Growth and Dry Matter
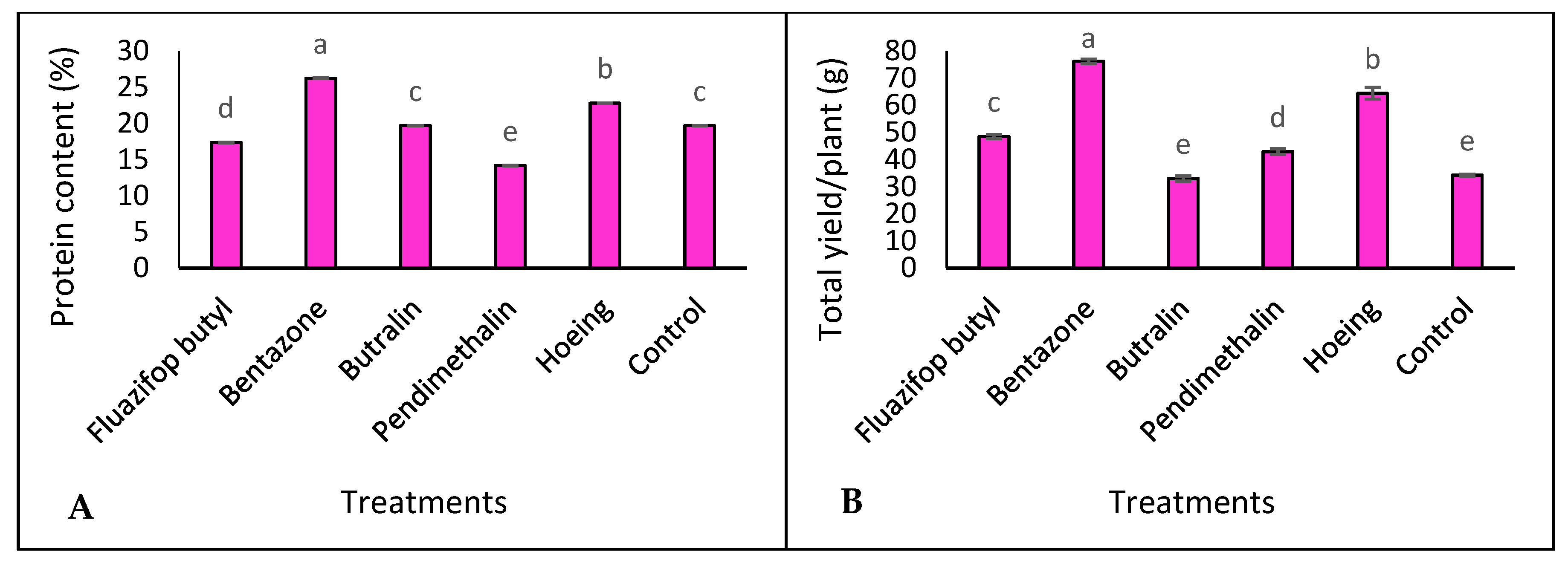
3.4. Protein Content and Total Yield
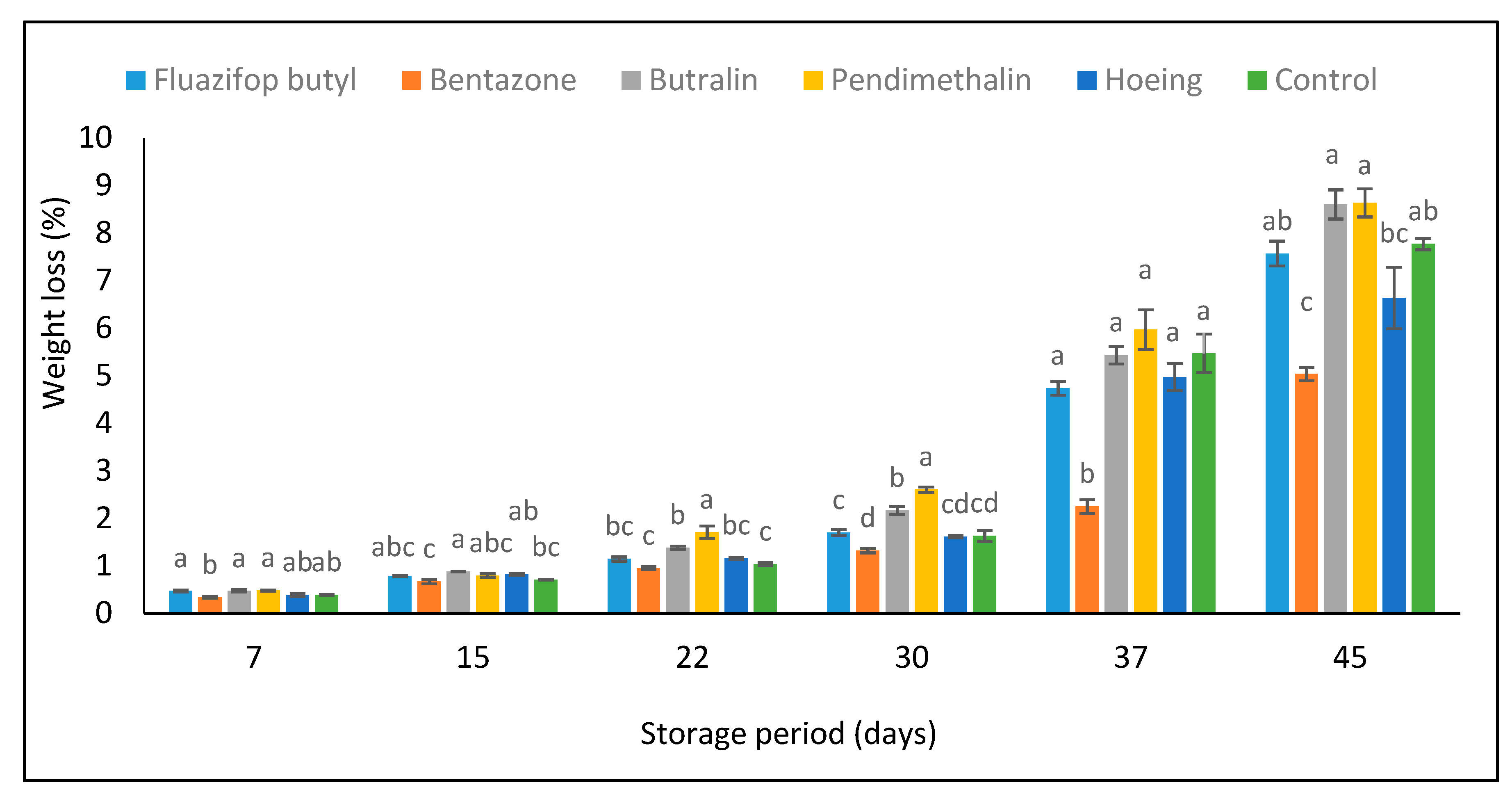
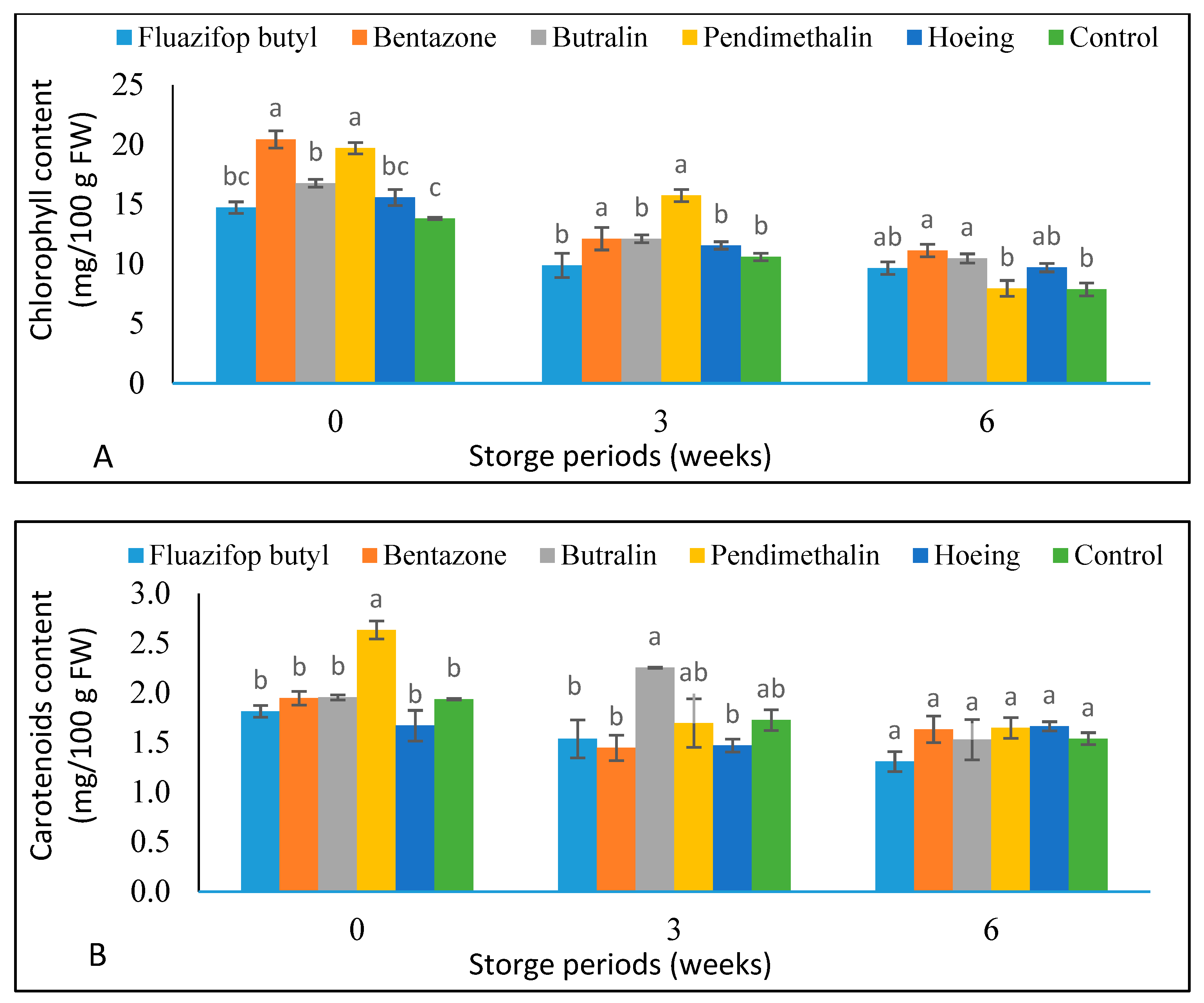
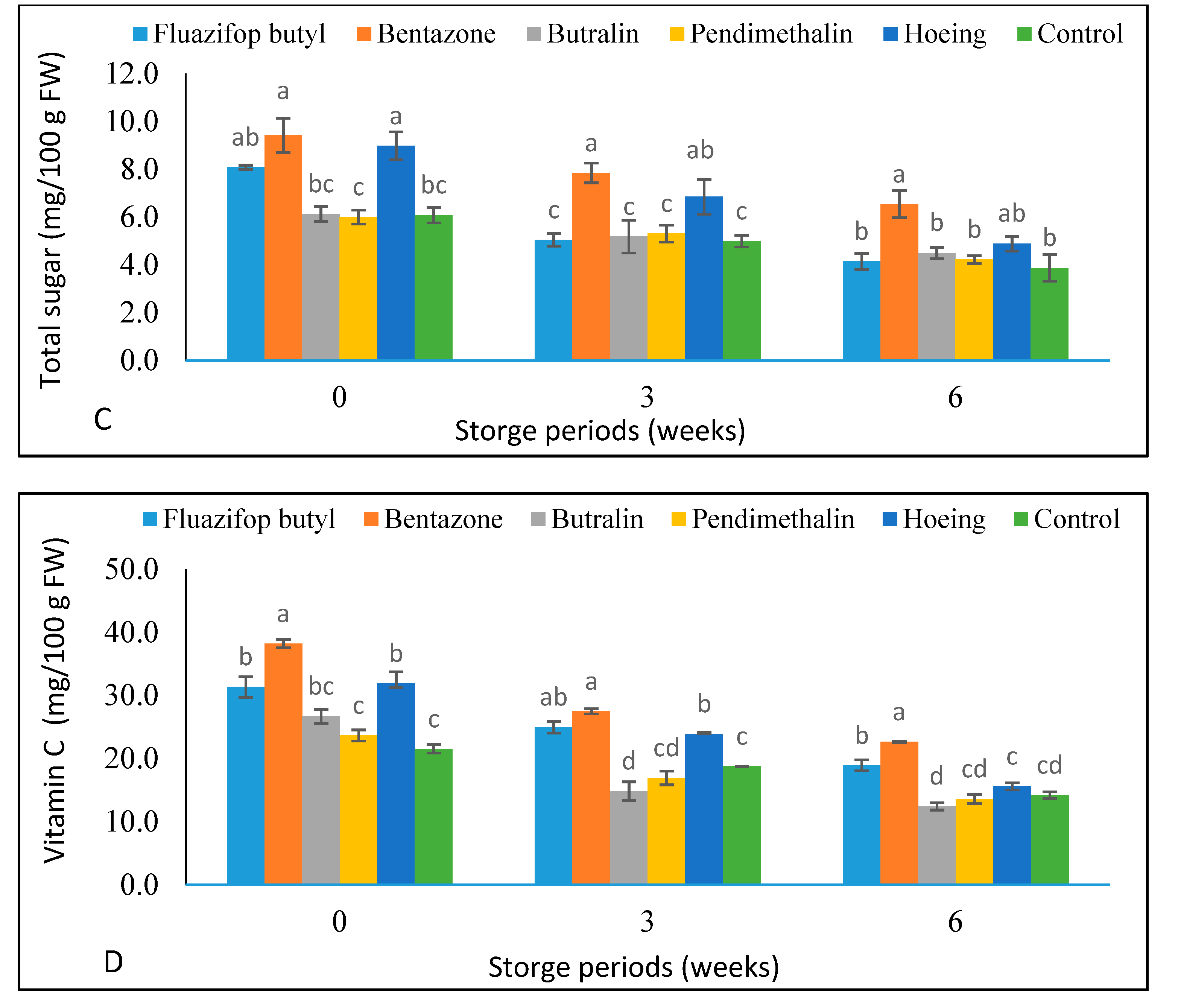
3.5. Green Pea Pods Weight Loss % and Chemical Composition during Storage
3.6. Determination of Herbicide Residues in Green Pods
4. Correlation Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Urbano, G.; Aranda, P.; Gomez-Villalva, E. Nutritional evaluation of pea (Pisum sativum) protein diets after mild hydrothermal treatment and with and without added phytase. J. Agric. Food Chem. 2003, 51, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Oram, P.A.; Agcaoili, M. Current Status and Future Trends in Supply and Demand of Cool Season Food Legumes. In Expanding the Production and Use of Cool Season Food Legumes. Current Plant Science and Biotechnology in Agriculture; Muehlbauer, F.J., Kaiser, W.J., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 3–49. [Google Scholar]
- Rana, S.; Pandita, V.; Chhokar, R.S.; Sirohi, S. Effect of pre and post emergence herbicides on weeds and seed yield of garden pea. Legume Res. Int. J. 2015, 38, 484. [Google Scholar] [CrossRef]
- Harker, K.N. Survey of yield losses due to weeds in central Alberta. Can. J. Plant Sci. 2001, 81, 339–342. [Google Scholar] [CrossRef]
- Bhyan, B.S.; Batra, V.K.; Singh, J.; Thakral, K.K. Effect of weed competition on quality of pea seed. Haryana J. Hortic. Sci. 2004, 33, 130–131. [Google Scholar]
- Singh, B. Survey and Indexing of Weeds Growing around Potato Fields for Their Role as an Inoculum Source for Potato leafroll virus (PLRV). Br. Biotechnol. J. 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Eizenberg, H.; Goldwasser, Y.; Achdary, G.; Hershenhorn, J. The Potential of Sulfosulfuron to Control Troublesome Weeds in Tomato. Weed Technol. 2003, 17, 133–137. [Google Scholar] [CrossRef]
- Das, S.K. Chemical weed management in pea (Pisum sativum L.). J. Crop Weed 2016, 12, 110–115. [Google Scholar]
- Kumar, R.; Kumar, V.; Prasad, S.K.; Verma, S.K. Effect of chemical on weed management practices on irrigated field pea (Pisum sativum L) crop yield and yield attributes. Int. J. Chem. Stud. 2019, 7, 843–847. [Google Scholar]
- Govardhan, J.D.; Dar, S.; Bharat, K.A. Effect of different weed management practices on growth and yield of field pea (Pisum sativum L.). Agric. Sci. Digest. 2007, 27, 311–312. [Google Scholar]
- Wágner, G.; Nádasy, E. Effect of pre-emergence herbicides on growth parameters of green pea. Commun. Agric. Appl. Boil. Sci. 2006, 71, 809–813. [Google Scholar]
- Flores, M.; Barbachano, M. Effects of herbicides Gramoxone, Diuron and Totacol® on growth and nodulation of three strains of villa Rhizobium meliloti. Sci. Total Environ. 1992, 123–124, 249–260. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abouziena, H.F.; Abdelgawad, K.; Elkhawaga, F.A. Weed Control Efficacy, Growth and Yield of Potato (Solanum tuberosum L.) as Affected by Alternative Weed Control Methods. Potato Res. 2019, 62, 139–155. [Google Scholar] [CrossRef]
- Somasegaran, P.; Hoben, H.J. Methods in Legume-Rhizobium Technology; NifTAL Project and MIRCEN, Department of Agronomy and Soil Science, Hawaii Institute Tropical Agriculture Human Research, University of Hawaii at Manoa: Honolulu, HI, USA, 1985; pp. 1–52. [Google Scholar]
- Morna, R. Formula for determination of chlorophylls pigments extracted with N. N. dimethyl formamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef] [Green Version]
- El-Mogy, M.M.; Salama, A.M.; Mohamed, H.F.Y.; Abdelgawad, K.F.; Abdeldaym, E.A. Responding of long green pepper plants to different sources of foliar potassium fertilizer. Agriculture 2019, 65, 59–76. [Google Scholar]
- Shehata, S.A.; El-Mogy, M.M.; Mohamed, H.F.Y. Postharvest quality and nutrient contents of long sweet pepper enhanced by supplementary potassium foliar application. Int. J. Veg. Sci. 2019, 25, 196–209. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ali, M.; Parmar, A.; Niedbała, G.; Wojciechowski, T.; El-Yazied, A.A.; El-Gawad, H.; Nahhas, N.; Ibrahim, M.; El-Mogy, M. Improved Shelf-Life and Consumer Acceptance of Fresh-Cut and Fried Potato Strips by an Edible Coating of Garden Cress Seed Mucilage. Foods 2021, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdallah, I.; Amer, A.; El-Hefny, D. Influence of herbicides under biofertilizer application on fennel (Foeniculum vulgare) yield and quality with special reference to herbicide residues. Bull. Natl. Res. Cent. 2021, 45, 1–13. [Google Scholar] [CrossRef]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S. Agroforestry Database: A Tree Reference and Selection Guide Version 4.0.; World Agroforestry Centre: Nairobi, Kenya, 2009. [Google Scholar]
- Barros, A. Efficacy and selectivity of post-emergence herbicides for control of southern sandbar (Cenchrus echinatus L.) in soyabean crops. Comun. Tec. Empresa Goiana Pesqui. Agropecu. 1989, 15, 9. [Google Scholar]
- Delchev, G.; Barakova, T. Efficacy of herbicides, herbicide combinations and herbicide tank mixtures on winter forage pea (Pisum sativum L.). Res. J. Agric. Sci. 2018, 50, 71–79. [Google Scholar]
- Cruz, L.S.P.; Novo, M.C.S.S.; Pereira, J.C.V.N.A.; Nagai, V. Herbicides applied post-emergence in groundnuts: I. Weed control and persistence in the soil. Bragantia 1991, 50, 103–114. [Google Scholar] [CrossRef]
- Mandloi, K.S.; Vyas, M.D.; Tomar, V.S. Effect of weed management methods in soybean (Glycine max) grown in vertisols of Madhya Pradesh. Indian J. Agron. 2000, 45, 158–161. [Google Scholar]
- Singh, G.; Wright, D. Effects of herbicides on and growth of two varieties of pea (Pisium sativum). Acta Agron. Hung. 2002, 50, 337–348. [Google Scholar] [CrossRef]
- Abdelhamid, M.; El-Metwally, I. Growth, nodulation, and yield of soybean and associated weeds as affected by weed management. Planta Daninha 2008, 26, 855–863. [Google Scholar] [CrossRef] [Green Version]
- Hassanein, E.E. Effect of some weed control treatments on soybean and associated weeds. Egypt. J. Agric. Res. 2000, 78, 1979–1993. [Google Scholar]
- EL-Metwally, I.M.; Saad El-Din, S.A. Response of pea (Pisium sativum L.) plants to some weed control treatments. J. Agric. Sci. 2003, 28, 947–969. [Google Scholar]
- Vaghasia, P.M.; Nadiyadhara, M.V. Influence of fluazifop-p-butyl on grassy weeds in groundnut (Arachis hypogaea L.) and its residual effect on succeeding crops. Int. J. Agric. Sci. 2014, 10, 695–699. [Google Scholar]
- Ahemad, M.; Zaidi, A.; Khan, M.S.; Oves, M. Factors affecting the variation of microbial communities in different agro-ecosystems. In Microbial Strategies for Crop Improvement; Khan, M.S., Zaidi, A., Musarrat, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 301–324. [Google Scholar]
- Walley, F.; Taylor, A.; Lupwayi, N. Herbicide Effects on Pulse Crop Nodulation and Nitrogen Fixation. FarmTech 2006 Proc. 2006, pp. 121–123. Available online: https://www.researchgate.net/profile/Fran-Walley/publication/228903508_HERBICIDE_EFFECTS _ON_PULSE_CROP_NODULATION_AND_NITROGEN_FIXATION/links/0912f50f966a57e998000000/HERBICIDE-EFFECTS-ON-PULSE-CROP-NODULATION-AND-NITROGEN-FIXATION.pdf (accessed on 19 August 2021).
- Khan, S.; Zaidi, A.; Aamil, M. Influence of herbicides on Chickpea-Mesorhizobium symbiosis. Agronomy 2004, 24, 123–127. [Google Scholar] [CrossRef]
- Ahemad, M.; Khan, M.S. Pesticides as Antagonists of Rhizobia and the Legume-Rhizobium Symbiosis: A Paradigmatic and Mechanistic Outlook. Biochem. Mol. Biol. 2013, 1, 63. [Google Scholar] [CrossRef]
- Jensen, P.J. Tolerance to foliage applied herbicides in combining peas: Effect of growth stage m cultivar type and herbicide. Crop Protection 1993, 12, 214–218. [Google Scholar] [CrossRef]
- Santos, M.; Freitas, F.; Ferreira, F.; Viana, R.; Santos, L.T.; Fonseca, D. Eficácia e persistência no solo de herbicidas utilizados em pastagem. Planta Daninha 2006, 24, 391–398. [Google Scholar] [CrossRef]
- Singh, G.; Wright, D. In vitro studies on the effects of herbicides on the growth of rhizobia. Lett. Appl. Microbiol. 2002, 35, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.P.D.R.; Jonior, A.F.C.; Reis, M.R.; Santos, G.R.; Chagas, L.F.B. Efeitos de herbicidas na biomassa e nodulaço do feio-caupi inoculado com rizobio. Rev. Caatinga 2012, 25, 44–51. [Google Scholar]
- Bollich, P.K.; Dunigan, E.P.; Kitchen, L.M.; Taylor, V. The Influence of Trifluralin and Pendimethalin on Nodulation, N2(C2H2) Fixation, and Seed Yield of Field-Grown Soybeans (Glycine max). Weed Sci. 1988, 36, 15–19. [Google Scholar] [CrossRef]
- Strandberg, M.; Scott-Fordsmand, J.J. Effects of pendimethalin at lower trophic levels—A review. Ecotoxicol. Environ. Saf. 2004, 57, 190–201. [Google Scholar] [CrossRef]
- Cruz, A.B.d.S.; de Albuquerque, J.D.A.A.; Rocha, P.R.R.; de Souza, L.T.; Cruz, D.L.d.S.; Soares, M.B.B.; Castro, T.S.; dos Santos, T.S.; Da Silva, E.S. Effect of the use of pre- and post-emergence herbicides on nodulation and production of cowpea (Vigna unguiculata L.) in the Amazonian savannah. Agron. Colomb. 2020, 38, 280–286. [Google Scholar] [CrossRef]
- Rupareliya, V.V.; Mathukia, R.K.; Gohil, B.S.; Javiya, P.P. Effect of post emergence herbicides and their mixture on growth, yield and quality of soybean (Glycine max L.). J. Pharmacogn. Phytochem. 2020, 9, 1161–1164. [Google Scholar]
- Zargar, M.; Lakzian, A.; Rasooli, R.; Izadi-Darbandi, E. Evaluation of PRE and POST Herbicides on Growth Features, Nodulation, and Nitrogen Fixation of Three Cultivars of Chickpea (Cicer aritinium L.). J. Crop. Sci. Biotechnol. 2020, 23, 157–162. [Google Scholar] [CrossRef]
- Zaid, A.M.; Mayouf, M.; Farouj, Y.S. The Effects of Post-Emergence Herbicides on Soil Microflora and Nitrogen Fixing Bacteria in Pea field. Int. J. Chem. Environ. Biol. Sci. 2014, 2, 40–42. [Google Scholar]
- Raman, R.; Krishnamoorthy, R. Nodulation and yield of mungbean (Vigna radiata L.) influenced by integrated weed management practices. Legume Res. 2005, 28, 128–130. [Google Scholar]
- Gonzalez, A.; Gonzalez-Murua, C.; Royuela, M. Influence of Imazethapyr on Rhizobium Growth and its Symbiosis with Pea (Pisum sativum). Weed Sci. 1996, 44, 31–37. [Google Scholar] [CrossRef]
- Procopio, S.D.O.; Santos, J.B.; Jacques, R.J.; Kasuya, M.C.M.; Silva, A.A.; Werlang, R.C. Crescimento de estirpes de Bradyrhizobium sob influência dos herbicidas glyphosate potassico, fomesafen, imazethapyr e carfentrazone-ethyl. Rev. Ceres 2015, 51, 294–297. [Google Scholar]
- Van Rensburg, H.J.; Strijdom, B.W. Effect of herbicides on survival of rhizobia and nodulation of peas, groundnuts and lucerne. South Afr. J. Plant Soil 1984, 1, 135–138. [Google Scholar] [CrossRef]
- Eldabaa, M.A.T.; Abouziena, H.F.; El-Desoki, E.R.; Abd El Wahed, M.S.A. Efficacy of Some Chemical Weed Control Treatments on Soybean (Glycine max L.) Plants and Associated Weeds in Sandy Soil. J. Appl. Sci. Res. 2012, 8, 4678–4684. [Google Scholar]
- El-Metwally, I.M.; Dawood, M.G. Weed management, folic acid and seaweed extract effects on Faba bean plants and associated weeds under sandy soil conditions. Agric. Eng. Int. CIGR J. 2017, 19, 27–34. [Google Scholar]
- Singh, V.P.; Singh, G.; Singh, M. Evaluation of bentazon and its ready mix formulation with blazer for weed control in soybean. Indian J. Weed Sci. 2004, 36, 207–209. [Google Scholar]
- Singh, G.; Jolly, R.S. Effect of herbicides on the weed infestation and grain yield of soybean (Glycine max (L.) Merill). Acta Agron. Hungrica 2004, 52, 199–203. [Google Scholar] [CrossRef]
- Mishra, J.; Singh, V.P. Weed dynamics and productivity of soybean (Glycine max)—Based cropping systems as influenced by tillage and weed management. Indian J. Agron. 2009, 54, 29–35. [Google Scholar]
- Meena, D.S.; Meena, B.L.; Patidar, B.K.; Chaman, J. Bio-efficacy of pendimethalin 30% EC + imazethapyr 2% SL premix against weeds of soybean. Int. J. Sci. Environ. Technol. 2018, 7, 1236–1241. [Google Scholar]
- Feruzan, D. The Effects of Fusilade (Fluazifop-p-butyl) on Root and Shoot Growth of Lentil (Lens culinaris Medik.) Seedlings. J. Appl. Biol. Sci. 2007, 1, 9–13. [Google Scholar]
- Abd El-Rhman, S.M. Effect of Bazagran herbicide and Indole Acetic Acid (IAA) on growth, yield, chemical composition and associated weeds of soybean plants. Egypt. J. Appl. Sci. 2004, 19, 79–91. [Google Scholar]
- Moldes, C.A.; Medici, L.O.; Abrahao, O.S.; Tsai, S.M.; Azevedo, R.A. Biochemical responses of glyphosate resistant and susceptible soybean plants exposed to glyphosate. Acta Physiol. Plant. 2008, 30, 469–479. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; Bonini, E.A.; De Oliveira, R.S.; Kremer, R.J.; Ferrarese-Filho, O. Glyphosate affects lignin content and amino acid production in glyphosate-resistant soybean. Acta Physiol. Plant. 2010, 32, 831–837. [Google Scholar] [CrossRef]
- Badr, A.; Zaki, H.; Germoush, M.O.; Tawfeek, A.Q.; El-Tayeb, M.A. Cytophysiological impacts of Metosulam herbicide on Vicia faba plants. Acta Physiol. Plant. 2013, 35, 1933–1941. [Google Scholar] [CrossRef]
- Chhokar, R.S.; Balyan, R.S.; Pahuja, S.S. The critical period of weed competition in soybean [Glycine max (L.) Merrill]. Indian J. Weed Sci. 1995, 27, 197–200. [Google Scholar]
- Al-Khatib, K.; Kadir, S.; Libbey, C. Effect of Adjuvants on Bentazon Efficacy in Green Pea (Pisum sativum). Weed Technol. 1995, 9, 426–431. [Google Scholar] [CrossRef]
- Mirjha, P.R.; Prasad, S.K.; Singh, M.K.; Paikra, R.H.; Patel, S.; Majumdar, M. Effect of weed control measures on weeds, nodulation, growth and yield of mungbean (Vigna radiata). Indian J. Agron. 2013, 58, 615–617. [Google Scholar]
- Czékus, Z.; Farkas, M.; Bakacsy, L.; Ördög, A.; Gallé, Á.; Poór, P. Time-Dependent Effects of Bentazon Application on the Key Antioxidant Enzymes of Soybean and Common Ragweed. Sustainability 2020, 12, 3872. [Google Scholar] [CrossRef]
- Abdallah, I.S.; Atia, M.A.M.; Nasrallah, A.K.; El-Beltagi, H.S.; Kabil, F.F.; El-Mogy, M.M.; Abdeldaym, E.A. Effect of New Pre-Emergence Herbicides on Quality and Yield of Potato and Its Associated Weeds. Sustainability 2021, 13, 9796. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M. The effect of herbicide applications on the content of ascorbic acid and glycoalkaloids in potato tubers. Plant Soil Environ. 2011, 49, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Smirnof, N.; Wheeler, G.L. Ascorbic acid in plants: Biosynthesis and function. Curr. Rev. Plant Sci. 2000, 19, 267–290. [Google Scholar] [CrossRef]
- Yaman, M.; Nalbantoğlu, B. Investigation of the Effects of the Fenoxaprop-p-Ethyl Herbicide and Salicylic Acid on the Ascorbic Acid and Vitamin B6 Vitamers in Wheat Leaves. J. Plant Growth Regul. 2020, 39, 729–737. [Google Scholar] [CrossRef]
- Çeliktaş, V.; Düzenli, S.; Otu, H. Physiological Effects of Five Herbicides on Wheat Cultivars. In Proceedings of the World Congress on Civil, Structural, and Environmental Engineering, Prague, Czech Republic, 30–31 March 2016. [Google Scholar]
- Akbulut, G.B.; Yigit, E.; Kaya, A.; Aktas, A. Effects of salicylic acid and organic selenium on wheat (Triticum aestivum L.) exposed to fenoxaprop-p-ethyl. Ecotoxicol. Environ. Saf. 2018, 148, 901–909. [Google Scholar] [CrossRef]
- Nakajima, Y.; Yoshida, S.; Ono, T.-A. Differential Effects of Urea/Triazine-type and Phenol-type Photosystem II Inhibitors on Inactivation of the Electron Transport and Degradation of the D1 Protein during Photoinhibition. Plant Cell Physiol. 1996, 37, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Kaur, P.; Bhullar, M.S. Effect of repeated application of pendimethalin on its persistence and dissipation kinetics in soil under field and laboratory conditions. Environ. Technol. 2017, 40, 997–1005. [Google Scholar] [CrossRef]
- Saha, A.; Ahammed Shabeer, T.P.; Banerjee, K.; Hingmire, S.; Bhaduri, D.; Jain, N.K.; Utture, S. Simultaneous analysis of herbicides pendimethalin, oxyfluorfen, imazethapyr and quizalofop-p-ethyl by LC–MS/MS and safety evaluation of their harvest time residues in peanut (Arachis hypogaea L.). J. Food Sci. Technol. 2014, 52, 4001–4014. [Google Scholar] [CrossRef] [Green Version]
- Hormenoo, Y.A.; Agbenorhevi, J.K.; Ekyem, S.O.; Bonsu, K.O.; Torve, V.; Voegborlo, B.R. Determination of some herbicide residues in sweet potato. Cogent Food Agric. 2021, 7. [Google Scholar] [CrossRef]
- El-Said Salem, R.E.M. Determination of the herbicide basagran residues in maize plants and soil. J. Agric. Res. Kafr El-Sheikh Univ. 2016, 42, 9–18. [Google Scholar]
- Clegg, B.S. Gas chromatographic analysis of fluazifop-butyl (Fusilade) in potatoes, soybeans, and soil. J. Agric. Food Chem. 1987, 35, 269–273. [Google Scholar] [CrossRef]
- Yang, L.; Song, X.; Zhou, X.; Zhou, Y.; Zhou, Y.; Gong, D.; Luo, H.; Deng, Y.; Yang, D.; Chen, L. Residual behavior and risk assessment of butralin in peanut fields. Environ. Monit. Assess. 2019, 192, 62. [Google Scholar] [CrossRef] [PubMed]
- Hussien, A.A. Utilization chemigation to reduce peas contamination with pesticide in new reclaimed lands. Egypt. J. Agric. Res. 2018, 96, 1543–1553. [Google Scholar]
- Sondhia, S. Fluazifop-p-Butyl Residues in Soybean Crop and Soil. Pestic. Res. J. 2007, 19, 248–250. [Google Scholar]


| Common Name | Trade Name | Recommended Rate (a.i ha−1) | Target Weed | Manufacturer |
|---|---|---|---|---|
| Pendimethalin | Stomp Extra 45.5%SC | 1.8 kg | Broad leaf and grass | BASF |
| Butralin | Amex 48% EC | 2.8 kg | Broad leaf and grass | Nufarm |
| Bentazon | Basagran 48% AS | 570 g | Broad leaf | BASF |
| Fluazifop-P butyl | Fuzilade 12.5% EC | 300 g | Grass | Syngenta |
| The Name of Weed | Relative Frequency of Weeds (%) |
|---|---|
| Cenchrus ciliaris | 79.2 |
| Malva parviflora | 12.8 |
| Chenopodium album | 6.5 |
| Rumex dentatus | 0.79 |
| Cichorium endivia | 0.56 |
| Total | 100 |
| Weed Dry Weight | Weed Efficacy % | N. of Active Nodules | Plant Length | Root Length | Plant Fresh Weight | Number of Leaves | Number of Branches | Dry Matter | Protein | Total Yield/Plant | Ch | Ca | T. Sugar | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weed Efficacy % | −0.97** | |||||||||||||
| N. of Active Nodule | −0.398 | 0.392 | ||||||||||||
| Plant length | −0.75 ** | 0.710 ** | 0.736 ** | |||||||||||
| Root length | 0.129 | 0.135 | 0.752 ** | 0.368 | ||||||||||
| Plant Fresh weight | −0.79 ** | 0.798 ** | 0.606 ** | 0.615 ** | 0.125 | |||||||||
| Number of leaves | 0.186 | −0.167 | 0.721 ** | 0.200 | 0.787 ** | 0.242 | ||||||||
| Number of branches | −0.043 | 0.015 | 0.627 ** | 0.378 | 0.672 ** | 0.156 | 0.492 ** | |||||||
| Dry matter | 0.298 | −0.343 | 0.151 | 0.027 | 0.445 | −0.478 * | 0.101 | 0.482 * | ||||||
| Protein | −0.138 | 0.128 | 0.831 ** | 0.556 * | 0.671 ** | 0.350 | 0.779 ** | 0.491 * | 0.049 | |||||
| Total yield/plant | −0.553 * | 0.556 * | 0.847 ** | 0.828 ** | 0.506 * | 0.748 ** | 0.533 * | 0.374 | -0.233 | 0.710 ** | ||||
| Chlorophyll | −0.517 * | 0.509 * | 0.535 * | 0.663 ** | 0.215 | 0.321 | 0.151 | 0.113 | 0.003 | 0.601 ** | 0.488 * | |||
| carotenoid | 0.006 | −0.068 | 0.047 | −0.066 | −0.186 | 0.333 | 0.135 | −0.037 | −0.474 * | 0.202 | 0.219 | 0.104 | ||
| T. sugar | −0.338 | 0.375 | 0.570 * | 0.614 ** | 0.306 | 0.383 | 0.465 | 0.130 | −0.262 | 0.696 ** | 0.728 ** | 0.583 * | 0.081 | |
| Vit. C | −0.359 | 0.310 | 0.719 ** | 0.854 ** | 0.584 * | 0.312 | 0.366 | 0.442 | 0.231 | 0.557 * | 0.780 ** | 0.449 | −0.129 | 0.629 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, I.S.; Abdelgawad, K.F.; El-Mogy, M.M.; El-Sawy, M.B.I.; Mahmoud, H.A.; Fahmy, M.A.M. Weed Control, Growth, Nodulation, Quality and Storability of Peas as Affected by Pre- and Postemergence Herbicides. Horticulturae 2021, 7, 307. https://doi.org/10.3390/horticulturae7090307
Abdallah IS, Abdelgawad KF, El-Mogy MM, El-Sawy MBI, Mahmoud HA, Fahmy MAM. Weed Control, Growth, Nodulation, Quality and Storability of Peas as Affected by Pre- and Postemergence Herbicides. Horticulturae. 2021; 7(9):307. https://doi.org/10.3390/horticulturae7090307
Chicago/Turabian StyleAbdallah, Ibrahim S., Karima F. Abdelgawad, Mohamed M. El-Mogy, Mohamed B. I. El-Sawy, Hend A. Mahmoud, and Mahmoud A. M. Fahmy. 2021. "Weed Control, Growth, Nodulation, Quality and Storability of Peas as Affected by Pre- and Postemergence Herbicides" Horticulturae 7, no. 9: 307. https://doi.org/10.3390/horticulturae7090307
APA StyleAbdallah, I. S., Abdelgawad, K. F., El-Mogy, M. M., El-Sawy, M. B. I., Mahmoud, H. A., & Fahmy, M. A. M. (2021). Weed Control, Growth, Nodulation, Quality and Storability of Peas as Affected by Pre- and Postemergence Herbicides. Horticulturae, 7(9), 307. https://doi.org/10.3390/horticulturae7090307







