Vineyard Fertilization Management for Iron Deficiency and Chlorosis Prevention on Carbonate Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Site and Experimental Design
2.2. Climate Conditions
2.3. Soil Analysis
2.4. Plant Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Soil Pedomorphological Characteristics
3.2. The Effects of Nitrogen Fertilization on Soil Properties
3.3. Influence of Nitrogen Fertilization and Foliar Fertilization on the Nutritional Status of Grapevine Leaves
3.4. Influence of Nitrogen Fertilization and Foliar Fertilization on Some Quality Variables of Grapes and Musts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bojnec, Š. Wine markets in central Europe. J. Cent. Eur. Agric. 2006, 7, 465–743. [Google Scholar]
- Maletić, E.; Pejić, I.; Kontić, J.K.; Zdunić, D.; Preiner, D.; Šimon, S.; Andabaka, Ž.; Žulj Mihaljević, M.; Bubola, M.; Marković, Z.; et al. Ampelographic and genetic characterization of Croatian grapevine varieties. VITIS-J. Grapevine Res. 2015, 54, 93–98. [Google Scholar] [CrossRef]
- Brataševec, K.; Sivilotti, P.; Vodopivec, B.M. Soil and foliar fertilization affects mineral contents in Vitis vinifera L. cv. Rebula leaves. J. Soil Sci. Plant Nutr. 2013, 13, 650–663. [Google Scholar]
- Mori, S. Iron acquisition by plants. Curr. Opin. Plant Biol. 1999, 2, 250–253. [Google Scholar] [CrossRef]
- Li, H.; Lian, C.; Zhang, Z. Agro-biofortification of iron and zinc in edible portion of crops for the global south. Adv. Plants Agric. Res. 2017, 6, 52–54. [Google Scholar] [CrossRef][Green Version]
- Drenjančević, M. Grapevine Fe-chlorosis on Podunavlje vinegrowing area. Poljoprivreda 2012, 18, 67–68. Available online: https://hrcak.srce.hr/83382 (accessed on 20 June 2021).
- Vukadinović, V.; Vukadinović, V. Land Resources (Zemljišni resursi); Faculty of Agriculture: Osijek, Croatia, 2018. [Google Scholar]
- Cataldo, E.; Salvi, L.; Sbraci, S.; Storchi, P.; Mattii, G.B. Sustainable Viticulture: Effects of Soil Management in Vitis vinifera. Agronomy 2020, 10, 1949. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition; International Potash Institute: Basel, Switzerland, 1987. [Google Scholar]
- Vukadinović, V.; Loncarić, Z. Plant Nutrition; Strossmayer University, Faculty of Agriculture: Osijek, Croatia, 1998; pp. 103–147. [Google Scholar]
- Rengel, Z. Availability of Mn, Zn and Fe in the rhizosphere. J. Soil Sci. Plant Nutr. 2015, 15, 397–409. [Google Scholar] [CrossRef]
- Ivezić, V.; Lončarić, Z.; Engler, M.; Kerovec, D.; Singh, B.R. Comparison of different extraction methods representing available and total concentrations of Cd, Cu, Fe, Mn and Zn in soil. Poljoprivreda 2013, 19, 53–58. [Google Scholar]
- Tagliavini, M.; Rombolà, A.D. Iron deficiency and chlorosis in orchard and vineyard ecosystems. Eur. J. Agron. 2001, 15, 71–92. [Google Scholar] [CrossRef]
- Zebec, V.; Rastija, D.; Lončarić, Z.; Bensa, A.; Popović, B.; Ivezić, V. Comparison of chemical extraction methods for determination of soil potassium in different soil types. Eurasian Soil Sci. 2017, 50, 1420–1427. [Google Scholar] [CrossRef]
- Karoglan, M.; Mihaljević, M.; Maslov, L.; Osrečak, M.; Jeromel, A.; Kozina, B.; Petrić, R. Impact of nitrogen fertilization on the chemical composition of chardonnay, italian riesling and white riesling grape cultivars. Poljoprivreda 2010, 16, 8–12. [Google Scholar]
- ISO, H. 10390:2005. Soil Quality—Determination of pH. In International Standard; Croatian Standards Institute: Zagreb, Croatia, 15 July 2005. [Google Scholar]
- ISO, H. 14235:1998. Soil Quality—Determination of Organic Carbon by Sulfochromic Oxidation. In International Standard; Croatian Standards Institute: Zagreb, Croatia, 1998. [Google Scholar]
- Egnér, H.; Riehm, H.; Domingo, W.R. Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor-und Kaliumbes timmung. K. Lantbr. Ann. 1960, 26, 199–215. [Google Scholar]
- ISO, H. 10693:1995. Soil Quality—Determination of Carbonate Content—Volumetric Method (ISO). In International Standard; Croatian Standards Institute: Zagreb, Croatia, 1995. [Google Scholar]
- Škorić, A. Handbook of Pedological Research; Faculty of Agricultural Sciences: Zagreb, Croatia, 1982. [Google Scholar]
- ISO, H. 11277:2009. Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation (ISO). In International Standard; Croatian Standards Institute: Zagreb, Croatia, 2009. [Google Scholar]
- Trierweiler, F.J.; Lindsay, W.L. EDTA-Ammonium carbonate soil test for Zn. Soil Sci. Soc. Am. Proc. 1969, 33, 49–54. [Google Scholar] [CrossRef]
- Begović, L.; Mlinarić, S.; Dunić, J.A.; Katanić, Z.; Lončarić, Z.; Lepeduš, H.; Cesar, V. Response of Lemna minor L. to short-term cobalt exposure: The effect on photosynthetic electron transport chain and induction of oxidtive damage. Aquat. Toxicol. 2016, 175, 117–126. [Google Scholar] [CrossRef]
- Škorić, A.; Filipovski, G.; Ćirić, M.; Vuković, T. Soil Classification of Yugoslavia; Academy of Sciences and Arts of Bosnia and Herzegovina: Sarajevo, Bosnia and Herzegovina, 1985. [Google Scholar]
- Husnjak, S. Systematics of Croatian Soils; University Edition of Croatia: Zagreb, Croatia, 2014. [Google Scholar]
- Zebec, V. Potassium Dynamics and Comparison of Methods for Determination of Available Potassium in Soils of Eastern Croatia. Ph.D. Thesis, University of Osijek, Osijek, Croatia, 2015. [Google Scholar]
- Đurđević, B.; Jug, I.; Jug, D.; Bogunovic, I.; Vukadinović, V.; Stipešević, B.; Brozović, B. Spatial variability of soil organic matter content in Eastern Croatia assessed using different interpolation methods. Int. Agrophysics 2019, 33, 31–39. [Google Scholar] [CrossRef]
- Popović, B. Comparison of Soil Phosphorus Test Methods. Ph.D. Thesis, University of Osijek, Osijek, Croatia, 2009. [Google Scholar]
- Lončarić, Z.; Karalić, K. Mineral Fertilizer and Crops Fertilization; University of Osijek, Faculty of Agriculture: Osijek, Croatia, 2015. [Google Scholar]
- Martinović, J. Management of Forest Soils in Croatia; Croatian Forest Research Institute: Zagreb, Croatia, 2003. [Google Scholar]
- Jones, J.B. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Fageria, N.K.; Dos Santos, A.B.; Moraes, M.F. Influence of urea and ammonium sulfate on soil acidity indices in lowland rice production. Commun. Soil Sci. Plant Anal. 2010, 41, 1565–1575. [Google Scholar] [CrossRef]
- Fisher, G.E. Micronutrients and animal nutrition and the link between the application of micronutrients to crops and animal health. Turk. J. Agric. For. 2008, 32, 221–233. [Google Scholar]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. Principles of Plant Nutrition; Springer: Dordrecht, Netherlands, 2001. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, F. Soil and crop management strategies to prevent iron deficiency in crops. Plant Soil 2011, 339, 83–95. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; He, J.Z.; Pinton, R.; Cesco, S. Review on iron availability in soil: Interaction of Fe minerals, plants, and microbes. J. Soils Sediments 2014, 14, 538–548. [Google Scholar] [CrossRef]
- Palčić, I.; Herak Ćustić, M.; Jeromel, A.; Karoglan, M.; Staver, M.; Damijanić, K.; Šegon, P.; Pasković, I. Status of Fe, Zn and Mn in cv. Istrian Malvasia (Vitis vinifera L.) leaf from four locations in north-western Istria. Glas. Zaštite Bilja 2015, 38, 12. [Google Scholar]
- Mielki, G.F.; Novais, R.F.; Ker, J.C.; Vergütz, L.; Castro, G.F.D. Iron availability in tropical soils and iron uptake by plants. Rev. Bras. Cienc. Solo 2016, 40, e0150174. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Naresh, R.K.; Mandal, A.; Singh, R.; Dhaliwal, M.K. Dynamics and transformations of micronutrients in agricultural soils as influenced by organic matter build-up: A review. Environ. Sustain. Indic. 2019, 1, 100007. [Google Scholar] [CrossRef]
- Bergmann, W.; Neubert, P. Plant Diagnosis and Plant Analysis as a Guide for Determination of Nutrition Disorders and for Determination of Nutrition Conditions of Cultivated Plants; FAO: Rome, Italy, 1976. [Google Scholar]
- Fregoni, M. Viticoltura di qualita. In La Nutrizione Minerale Della Vite; Edizioni Grafiche Lama: Piacenza, Italy, 1998; pp. 493–579. [Google Scholar]
- Čoga, L.; Slunjski, S.; Herak Ćustić, M.; Maslać, J.; Petek, M.; Ćosić, T.; Pavlović, I. Influence of soil reaction on phosphorus, potassium, calcium and magnesium dynamics in grapevine (Vitis vinifera L.). Agric. Conspec. Sci. 2009, 74, 39–43. [Google Scholar]
- Lacroux, F.; Trégoat, O.; Van Leeuwen, C.; Pons, A.; Tominaga, T.; Lavigne-Cruege, V.; Dubourdieu, D. Effect of foliar nitrogen and sulphur application on aromatic expression of Vitis vinifera L. cv. Sauvignon blanc. OEno One 2008, 42, 125–132. [Google Scholar] [CrossRef]
- Ancín-Azpilicueta, C.; Nieto-Rojo, R.; Gómez-Cordón, J. Influence of fertilisation with foliar urea on the content of amines in wine. Food Addit. Contam. 2011, 28, 877–884. [Google Scholar] [CrossRef]
- Hannam, K.D.; Neilsen, G.H.; Neilsen, D.; Midwood, A.J.; Millard, P.; Zhang, Z.; Thornton, B.; Steinke, D. Amino acid composition of grape (Vitis vinifera L.) juice in response to applications of urea to the soil or foliage. Am. J. Enol. Vitic. 2016, 67, 47–55. [Google Scholar] [CrossRef]
- Karoglan, M.; Maslov Bandic, L.; Osrecak, M.; Mihaljevic Zulj, M.; Kozina, B.; Jeromel, A. Amino Acid Composition of White Grape Juices as Affected by Soil Urea Fertilization. J. Agric. Sci. Technol. 2019, 21, 1507–1520. [Google Scholar]
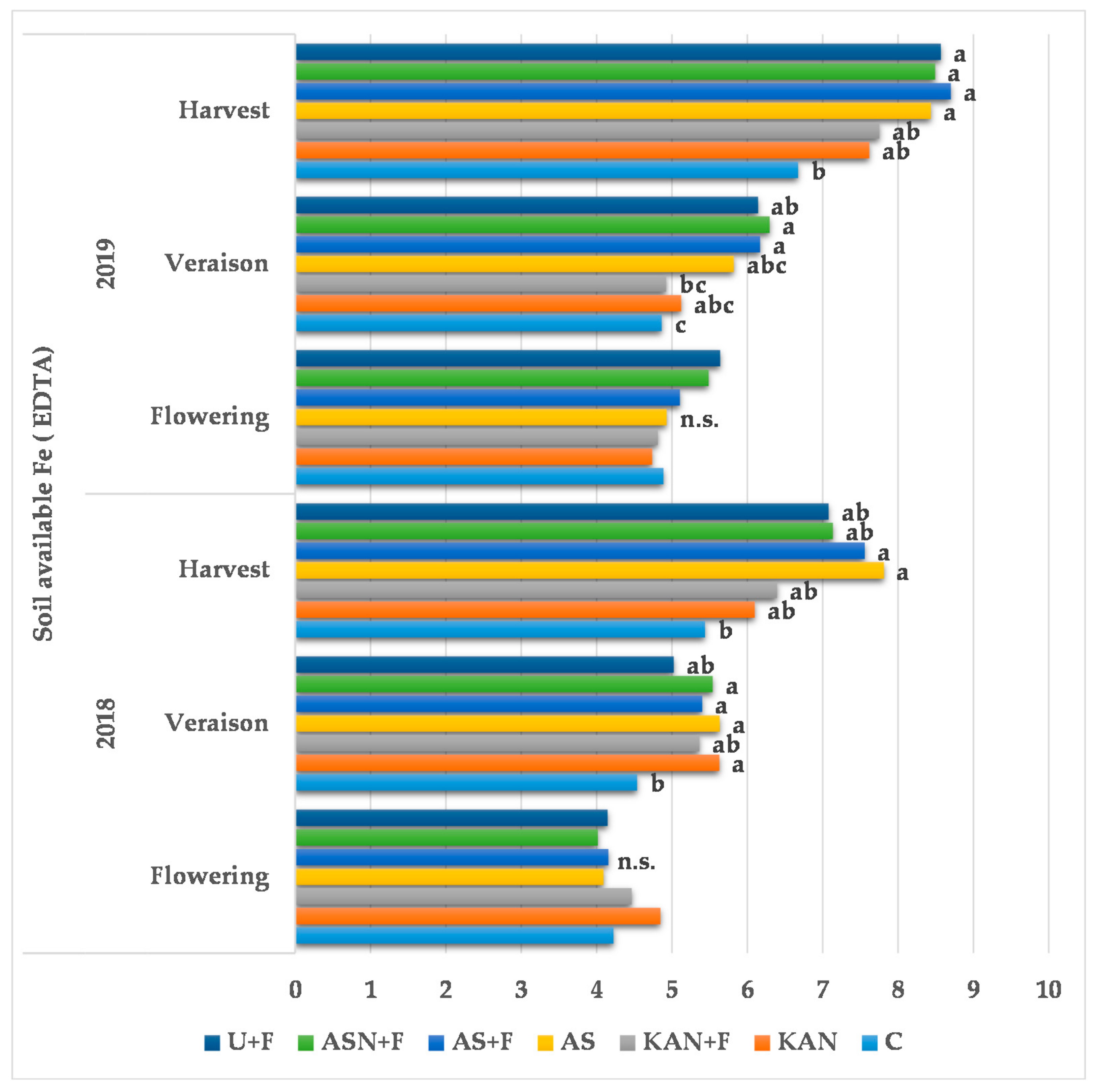
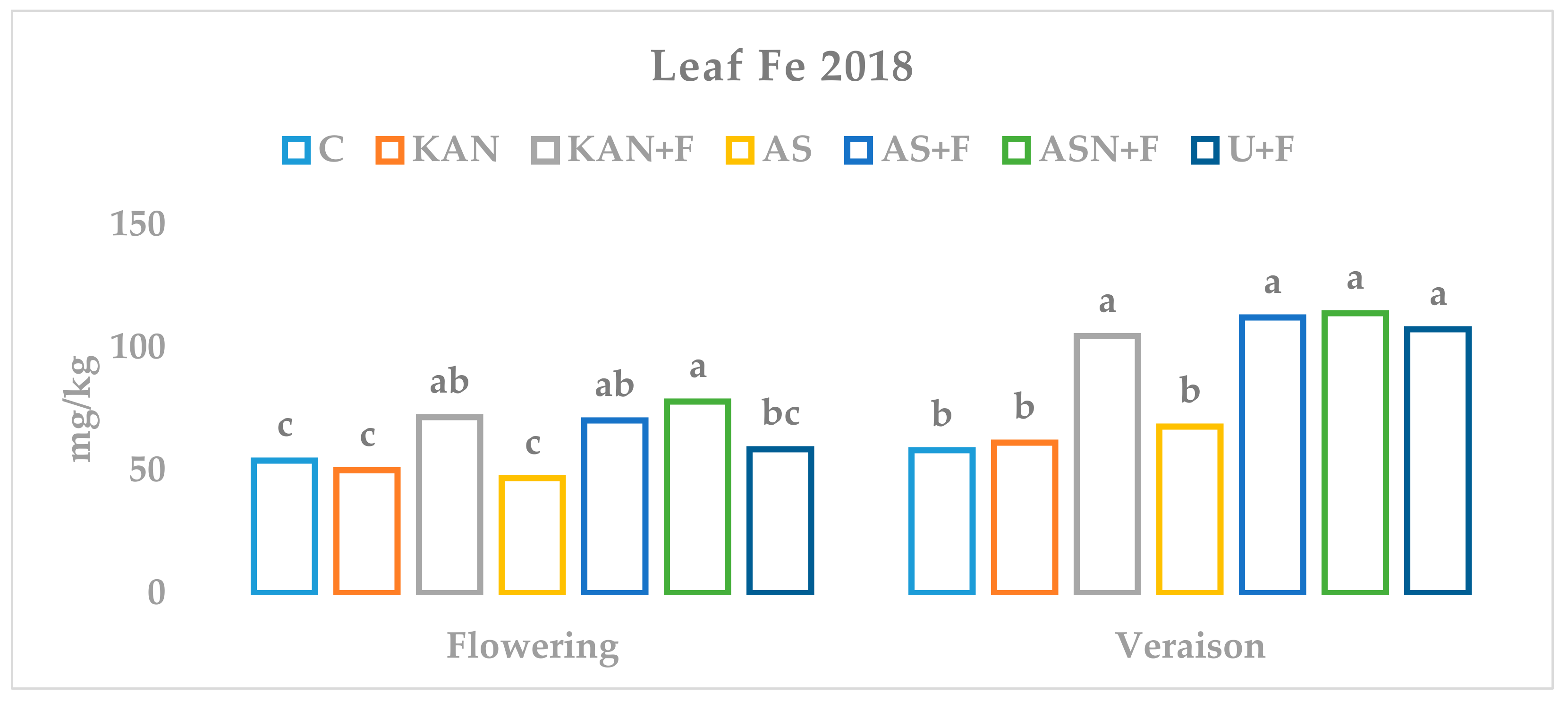
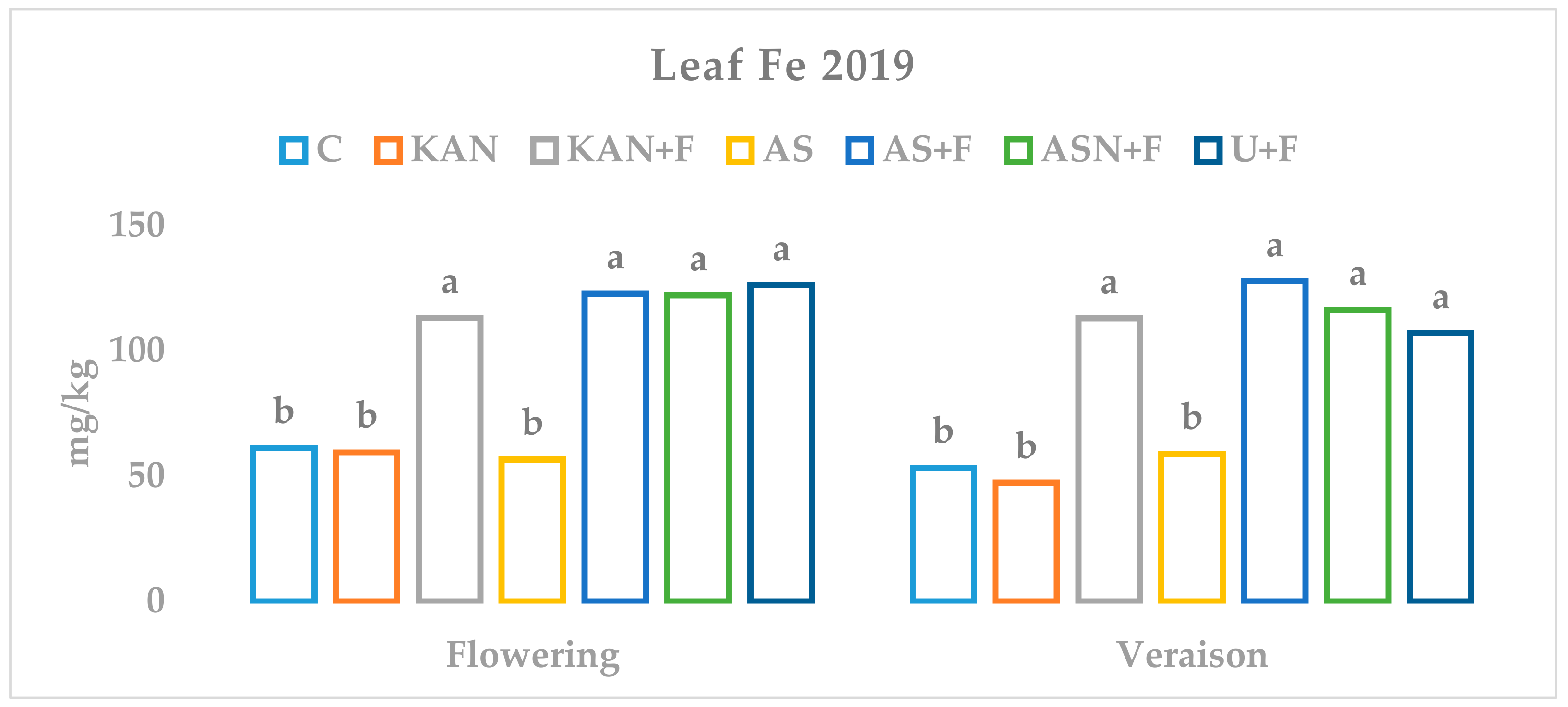
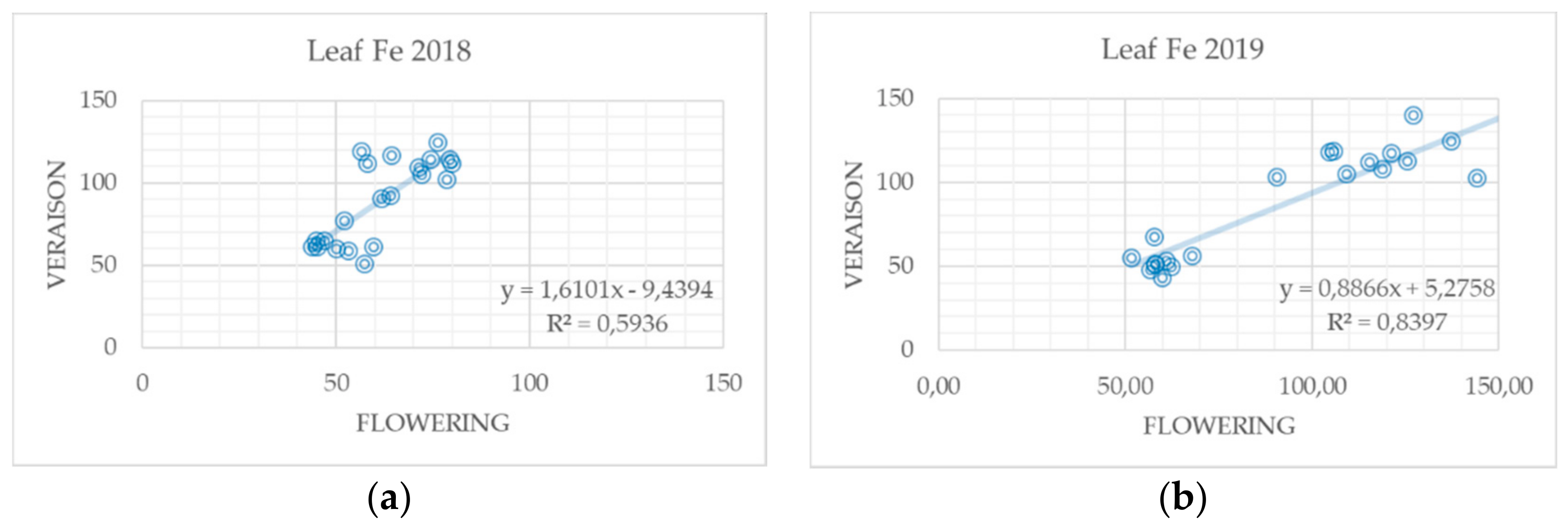
| Treatment (Fertilizers Type) | Required Amounts of Nutrients kg/ha | Applied Amounts of Fertilizers kg/ha | |||||
|---|---|---|---|---|---|---|---|
| N | P2O5 | K2O | Basic Fertilization (NPK 7-20-30) | 1st Top Dressing | 2nd Top Dressing | Foliar Fe Application | |
| C | 14 | 40 | 60 | 200 | 0 | 0 | NO |
| KAN | 70 | 40 | 60 | 200 | 142 | 65.5 | NO |
| KAN+F | 70 | 40 | 60 | 200 | 142 | 65.5 | YES |
| AS | 70 | 40 | 60 | 200 | 183 | 83.5 | NO |
| AS+F | 70 | 40 | 60 | 200 | 183 | 83.5 | YES |
| ASN+F | 70 | 40 | 60 | 200 | 148 | 67.5 | YES |
| U+F | 70 | 40 | 60 | 200 | 84 | 38 | YES |
| Year | Vegetation Period (IV–IX) | ||||
|---|---|---|---|---|---|
| Precipitation (mm) | Temperature (°C) | Precipitation (mm) | Temperature (°C) | ||
| 1981–2010 | absolute | 684.3 | 11.3 | 390.6 | 18.2 |
| 2018 | 664.8 | 12.6 | 347.7 | 20.2 | |
| 2019 | 650.5 | 10.8 | 522 | 18.9 | |
| 1981–2010 | relative | 100 | 100 | 100 | 100 |
| 2018 | 97.2 | 111.5 | 89.0 | 111.0 | |
| 2019 | 95.1 | 95.6 | 133.6 | 103.8 | |
| Soil Profile | Depth (cm) | Horizon Designation | Morphology Characteristics |
|---|---|---|---|
 | 0–25 | Ap | Soil color: 10YR 4/4 (dark yellowish-brown) Texture: Silt loam Structure: Granular-Medium (GR-ME) Carbonate reaction: Moderately calcareous, visible effervescence |
| 25–45 | B | Soil color: 7YR 4/2 (dark brown) Texture: Silt loam Structure: Granular-Fine/thin (GR-FI) Carbonate reaction: Strongly calcareous, strong visible effervescence. Bubbles form a low foam. | |
| 45–100 | C | Soil color: 2.5 Y 5/6 (light olive-brown) Texture: Silt loam to loam Structure: Granular-Very fine and fine (GR-FF) Carbonate reaction: Strongly calcareous, strong visible effervescence. Bubbles form a low foam. |
| Depth (cm) | ||||
|---|---|---|---|---|
| 0–25 | 25–45 | 45–100 | ||
| Chemical properties | ||||
| Actual soil acidity | pH H2O | 8.64 | 8.66 | 8.71 |
| Exchangeable soil acidity | pH KCl | 7.78 | 7.71 | 7.85 |
| Available phosphorus (P2O5) | mg/100 g | 31.53 | 7.46 | 1.62 |
| Available potassium (K2O) | mg/100 g | 27.1 | 8.64 | 6.74 |
| Organic matter content | % | 2.03 | 0.72 | 0.45 |
| Total carbonates (CaCO3) | % | 20.97 | 31.67 | 32.53 |
| Physical properties | ||||
| Coarse sand | 2.0–0.2 mm | 3.80 | 3.53 | 3.50 |
| Fine sand | 0.2–0.063 mm | 4.24 | 3.81 | 4.49 |
| Coarse silt | 0.063–0.02 mm | 44.92 | 41.21 | 46.95 |
| Fine silt | 0.02–0.002 mm | 26.26 | 33.36 | 29.55 |
| Clay | <0.002 mm | 20.78 | 18.09 | 15.51 |
| Texture | Silt loam | Silt loam | Silt loam | |
| Soil aggregate stability | % | 88.20 | - | - |
| Total porosity | % vol. | 40.55 | 47.24 | - |
| Packing density | g/cm3 | 2.22 | 1.86 | - |
| Hydrological properties | ||||
| Field capacity | % vol. | 38.46 | 39.98 | - |
| Air capacity | % vol. | 2.09 | 7.26 | - |
| Treatment | pH H2O | |||||
|---|---|---|---|---|---|---|
| 2018 | 2019 | |||||
| Flowering | Veraison | Harvest | Flowering | Veraison | Harvest | |
| C | 8.64 a | 8.59 a | 8.57 a | 8.56 a | 8.54 a | 8.59 a |
| KAN | 8.51 ab | 8.32 bc | 8.55 ab | 8.27 bc | 8.30 ab | 8.44 ab |
| KAN+F | 8.56 ab | 8.31 bc | 8.45 bc | 8.39 ab | 8.30 ab | 8.42 ab |
| AS | 8.44 b | 8.21 c | 8.34 de | 8.29 bc | 8.17 b | 8.36 b |
| AS+F | 8.47 ab | 8.23 bc | 8.36 cd | 8.16 c | 8.27 b | 8.36 b |
| ASN+F | 8.51 ab | 8.32 bc | 8.24 e | 8.19 bc | 8.26 b | 8.38 b |
| U+F | 8.62 ab | 8.37 b | 8.46 bc | 8.27 bc | 8.30 ab | 8.40 ab |
| Treatment | pH KCl | |||||
|---|---|---|---|---|---|---|
| 2018 | 2019 | |||||
| Flowering | Veraison | Harvest | Flowering | Veraison | Harvest | |
| C | 7.78 n.s. | 7.78 a | 7.88 n.s. | 7.91 a | 7.82 a | 7.81 a |
| KAN | 7.77 | 7.65 ab | 7.83 | 7.82 ab | 7.69 ab | 7.72 abc |
| KAN+F | 7.73 | 7.61 b | 7.83 | 7.79 ab | 7.61 ab | 7.77 a |
| AS | 7.65 | 7.65 ab | 7.80 | 7.83 ab | 7.59 b | 7.55 c |
| AS+F | 7.63 | 7.63 ab | 7.84 | 7.78 ab | 7.62 ab | 7.58 bc |
| ASN+F | 7.61 | 7.69 ab | 7.84 | 7.64 b | 7.66 ab | 7.56 c |
| U+F | 7.78 | 7.69 ab | 7.85 | 7.87 a | 7.69 ab | 7.75 ab |
| Treatment | Cluster Weight (kg) | Weight of 100 Berries (kg) | Must Density (°Oe) | Cluster Weight (kg) | Weight of 100 Berries (kg) | Must Density (°Oe) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | |||||||||||
| C | 0.109 | c | 0.178 | n.s. | 83.67 | b | 0.087 | c | 0.142 | b | 81.99 | b |
| KAN | 0.117 | bc | 0.19 | 89.67 | ab | 0.096 | bc | 0.152 | ab | 87.87 | a | |
| KAN+F | 0.136 | bc | 0.187 | 88.67 | ab | 0.113 | ab | 0.155 | ab | 87.78 | a | |
| AS | 0.138 | bc | 0.201 | 90.67 | a | 0.116 | a | 0.167 | a | 89.76 | a | |
| AS+F | 0.143 | a | 0.188 | 87.33 | ab | 0.122 | a | 0.158 | ab | 86.46 | ab | |
| ASN+F | 0.143 | ab | 0.188 | 87.33 | ab | 0.117 | a | 0.169 | a | 87.87 | a | |
| U+F | 0.132 | abc | 0.184 | 87.67 | ab | 0.117 | a | 0.157 | ab | 88.46 | a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zebec, V.; Lisjak, M.; Jović, J.; Kujundžić, T.; Rastija, D.; Lončarić, Z. Vineyard Fertilization Management for Iron Deficiency and Chlorosis Prevention on Carbonate Soil. Horticulturae 2021, 7, 285. https://doi.org/10.3390/horticulturae7090285
Zebec V, Lisjak M, Jović J, Kujundžić T, Rastija D, Lončarić Z. Vineyard Fertilization Management for Iron Deficiency and Chlorosis Prevention on Carbonate Soil. Horticulturae. 2021; 7(9):285. https://doi.org/10.3390/horticulturae7090285
Chicago/Turabian StyleZebec, Vladimir, Miroslav Lisjak, Jurica Jović, Toni Kujundžić, Domagoj Rastija, and Zdenko Lončarić. 2021. "Vineyard Fertilization Management for Iron Deficiency and Chlorosis Prevention on Carbonate Soil" Horticulturae 7, no. 9: 285. https://doi.org/10.3390/horticulturae7090285
APA StyleZebec, V., Lisjak, M., Jović, J., Kujundžić, T., Rastija, D., & Lončarić, Z. (2021). Vineyard Fertilization Management for Iron Deficiency and Chlorosis Prevention on Carbonate Soil. Horticulturae, 7(9), 285. https://doi.org/10.3390/horticulturae7090285






