Development of Growth Estimation Algorithms for Hydroponic Bell Peppers Using Recurrent Neural Networks
Abstract
1. Introduction
2. Materials and Methods
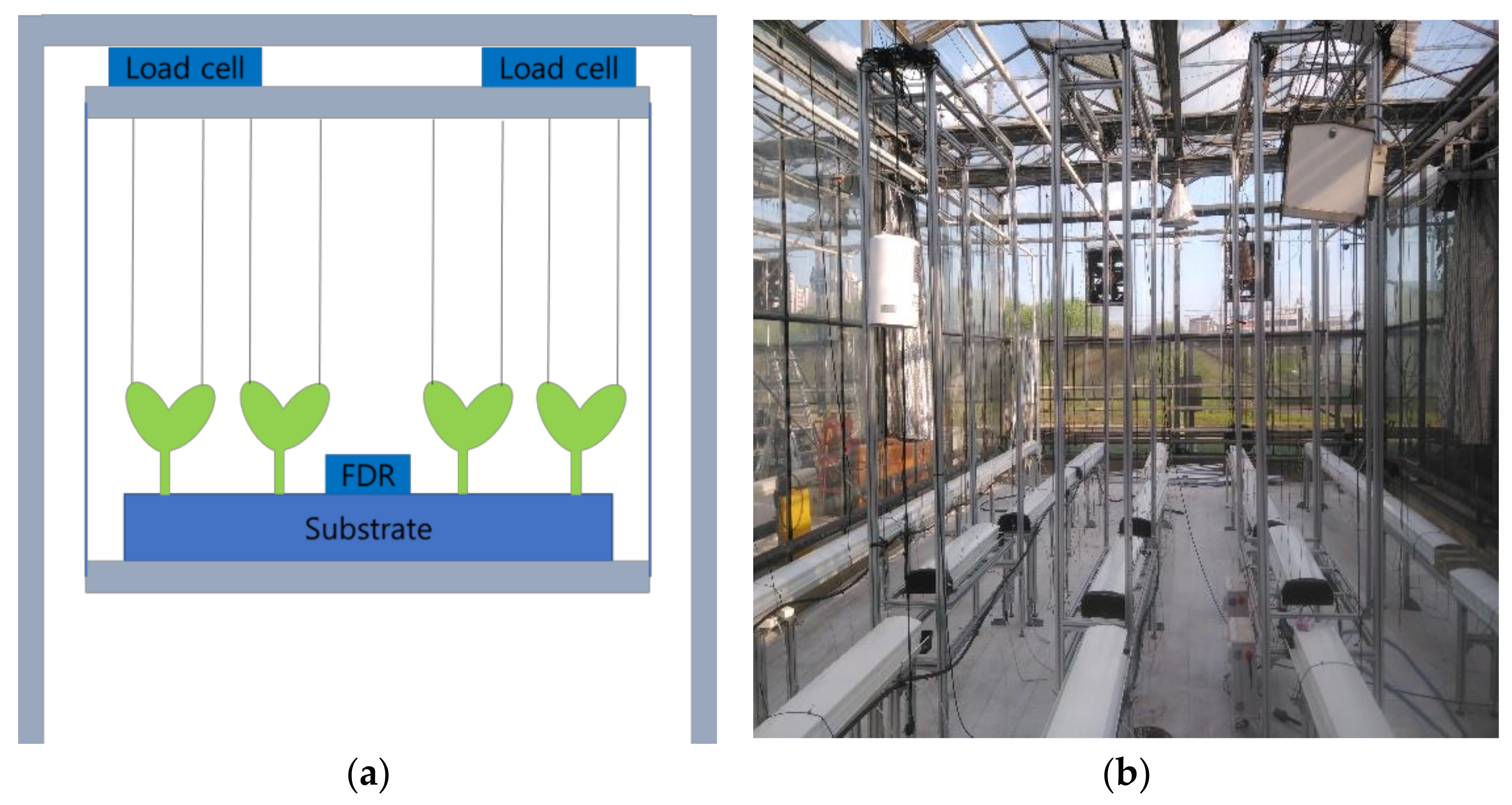
2.1. Crop Growth Conditions for Algorithm Training
2.2. Data Collection and Preprocessing
2.3. Recurrent Neural Network Application
2.4. Crop Growth Conditions for Validation
2.5. Evaluation of the Growth Rate Algorithm
2.6. Statistical Analysis
3. Results and Discussion
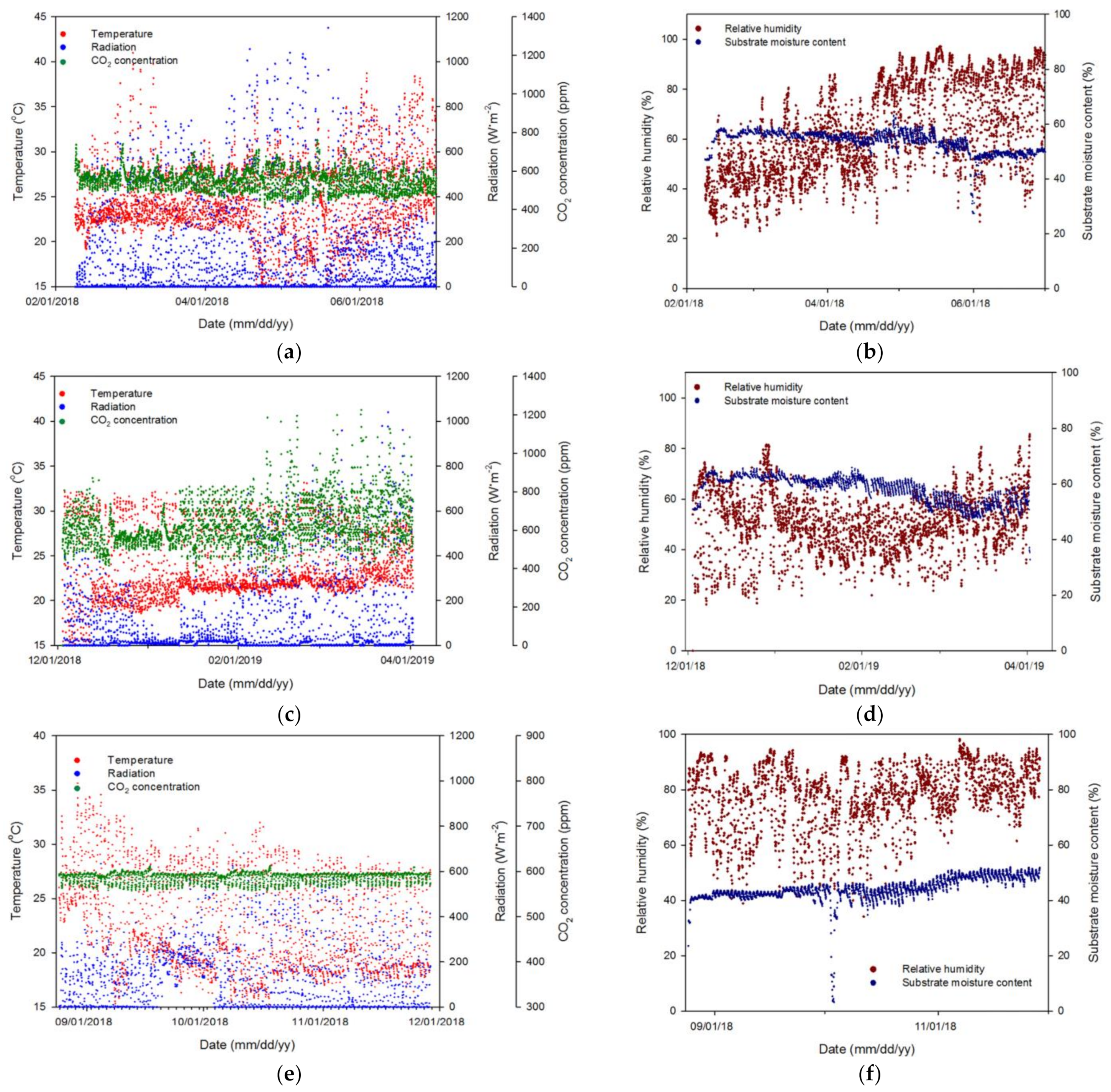
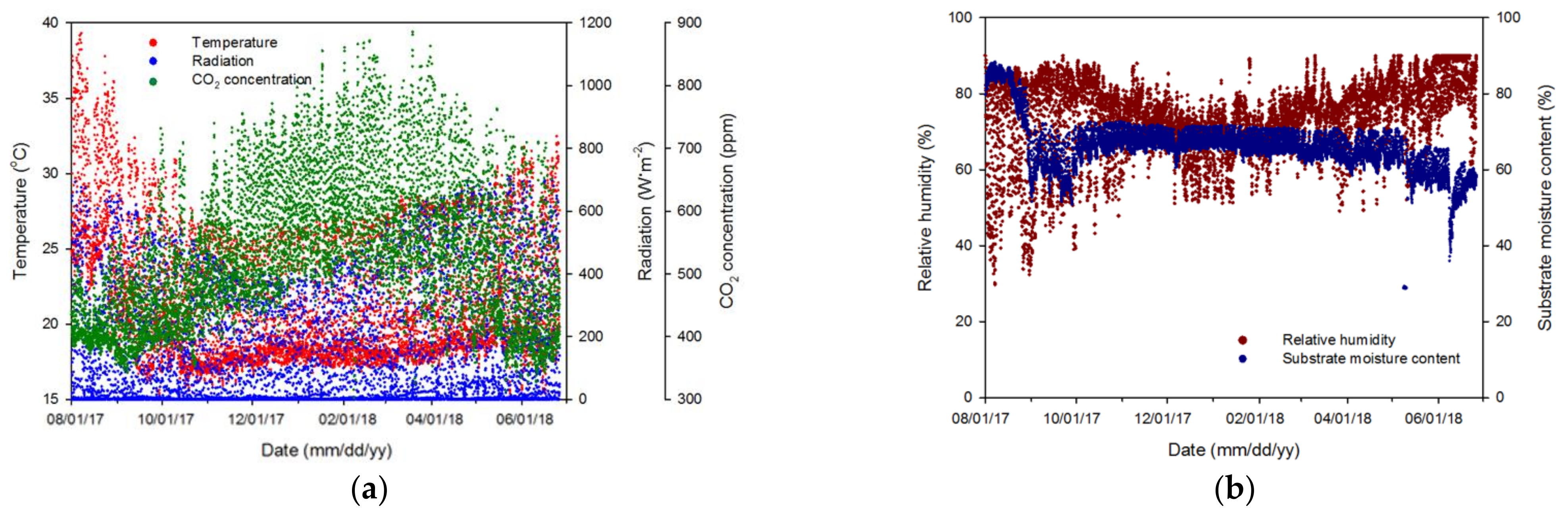
3.1. Variable Collection for Algorithm Training
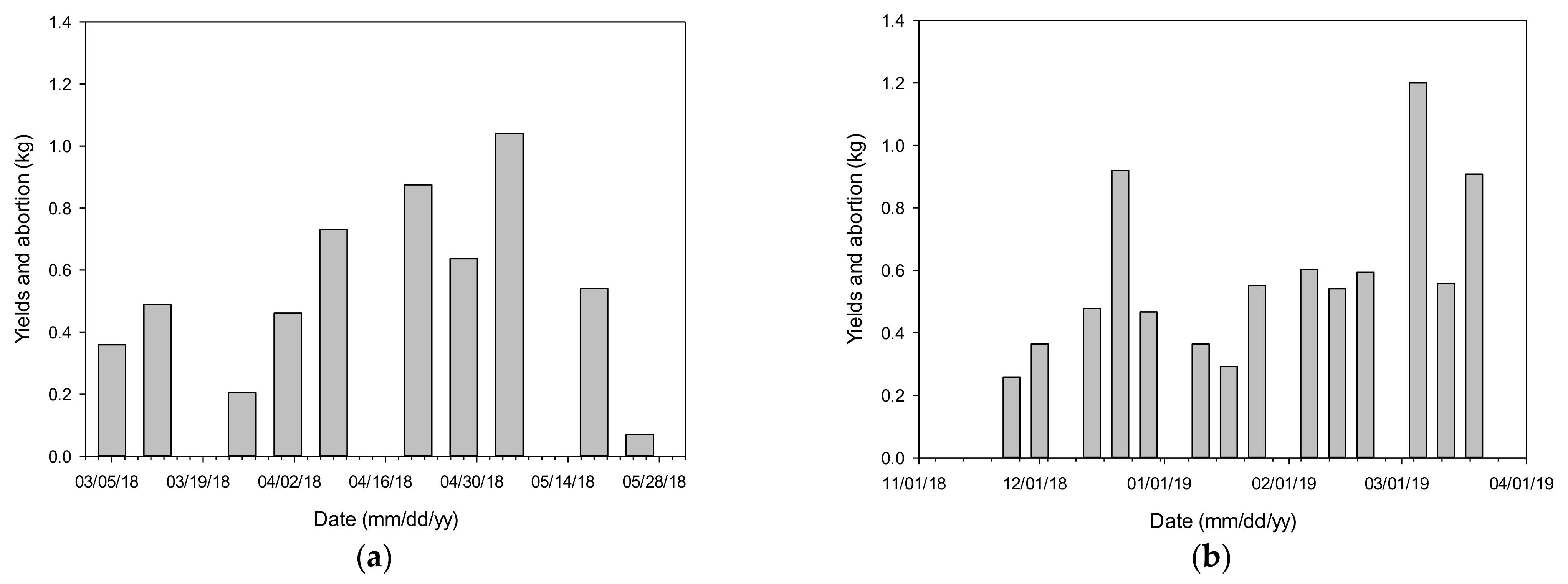
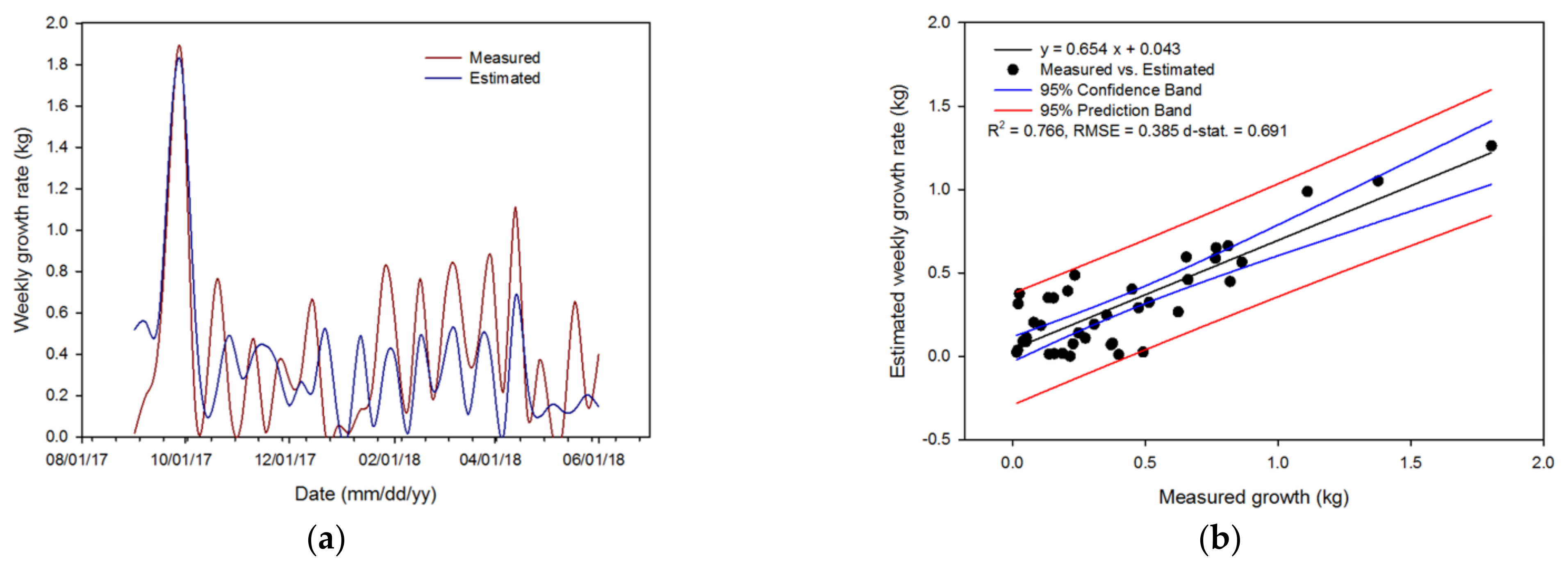
3.2. Crop Growth Rate Estimation of the Algorithm
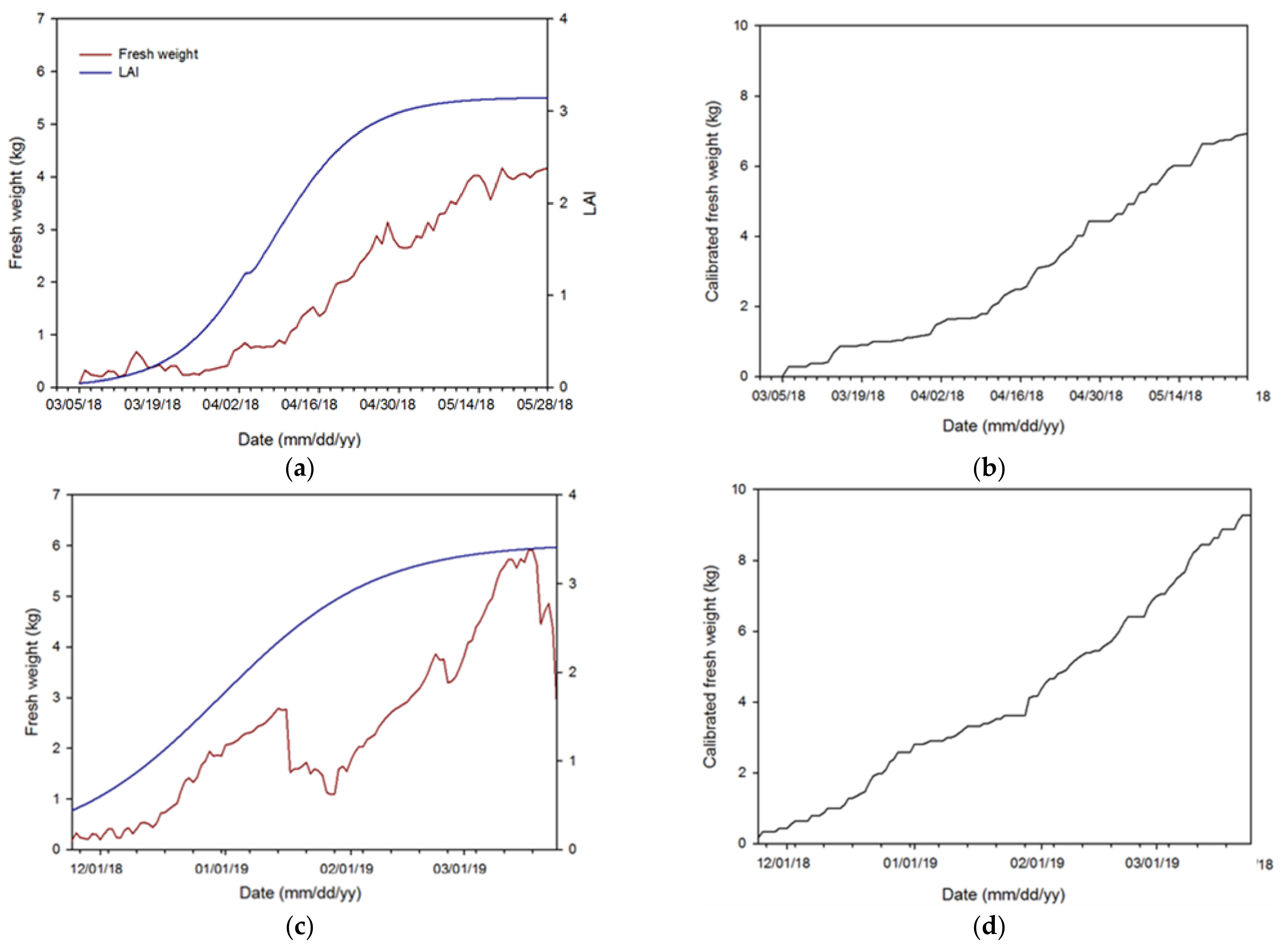
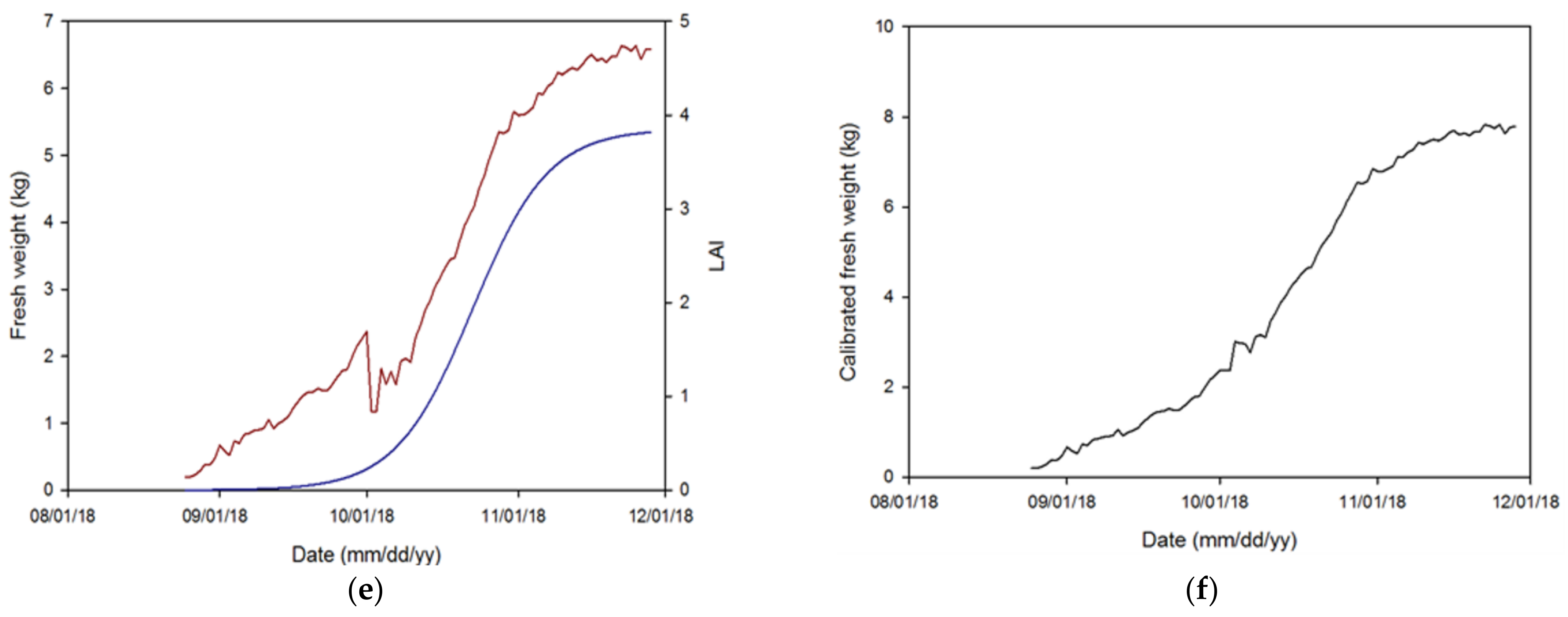
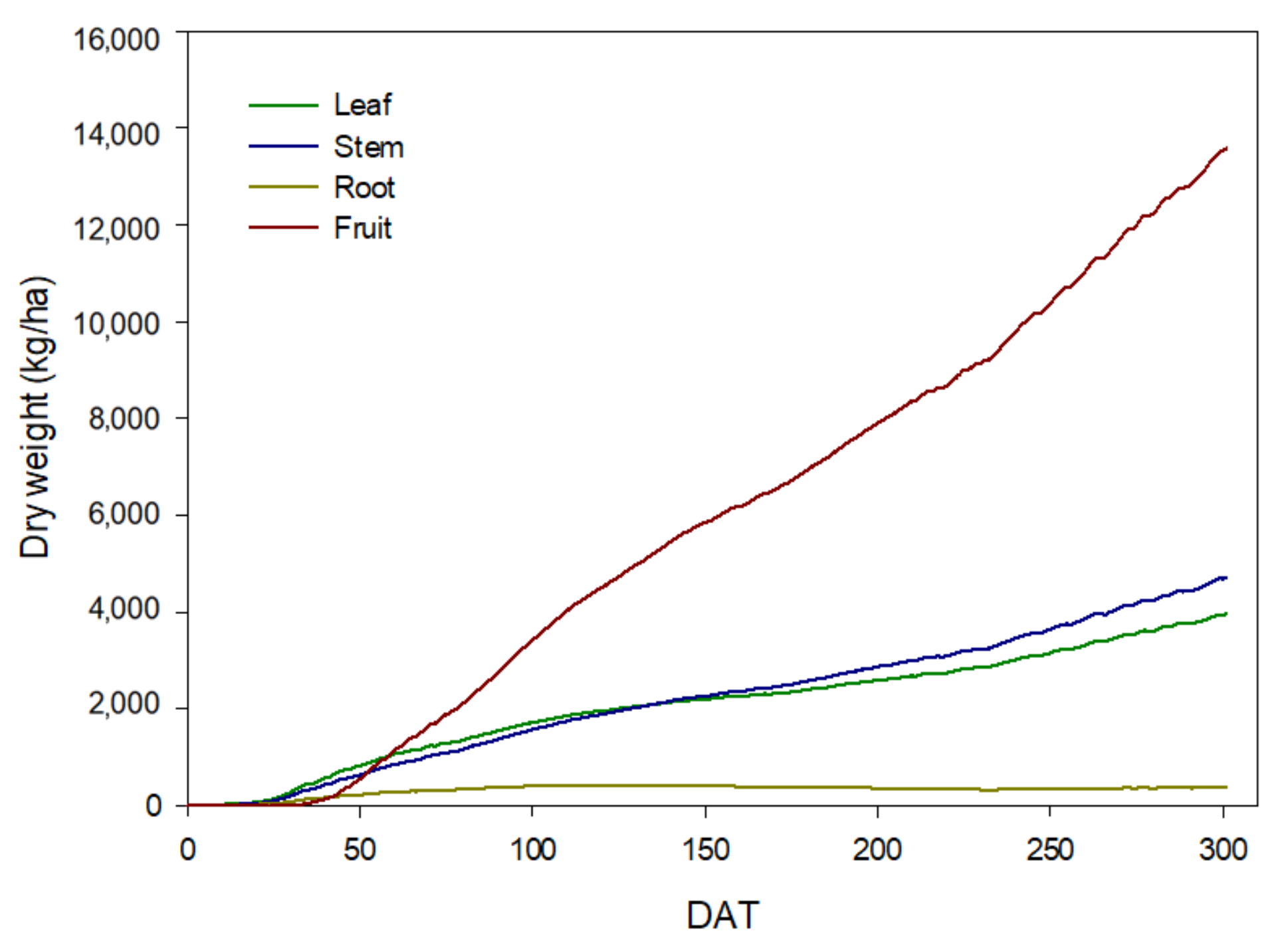
3.3. Calibration and Simulation of the PBM
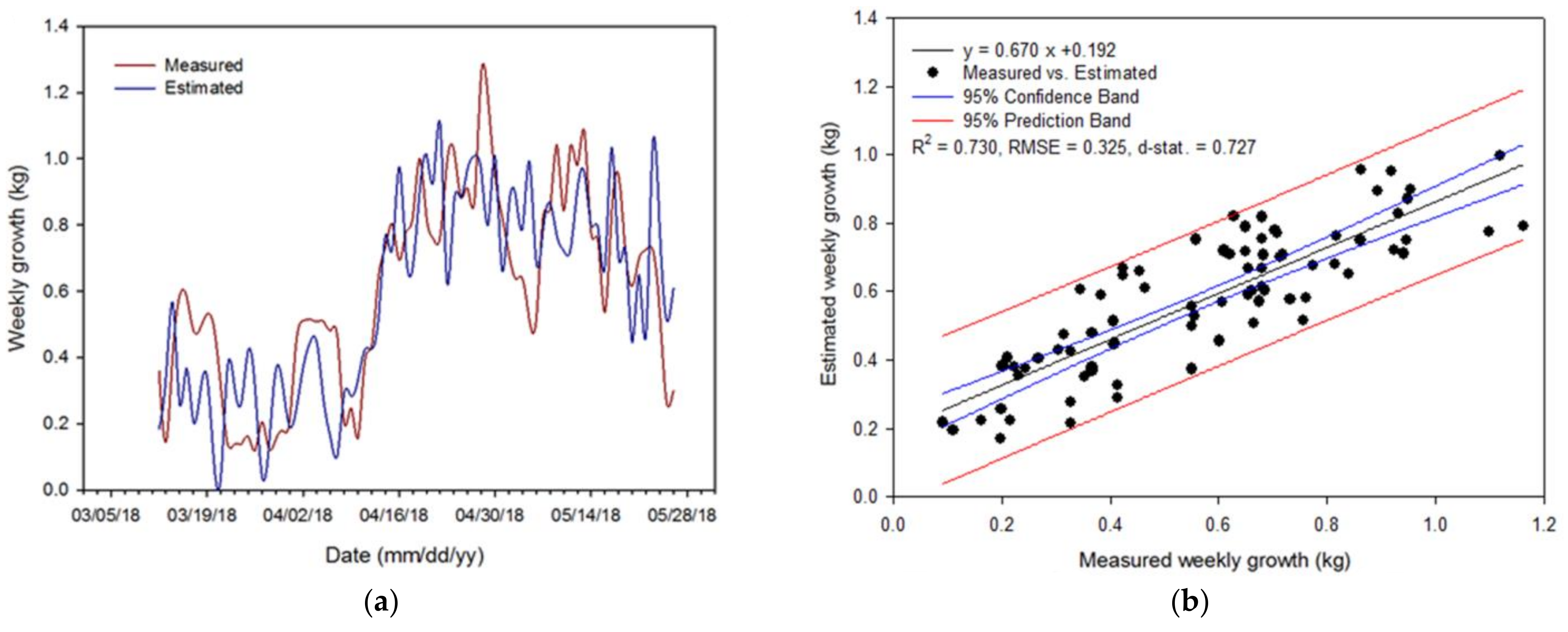
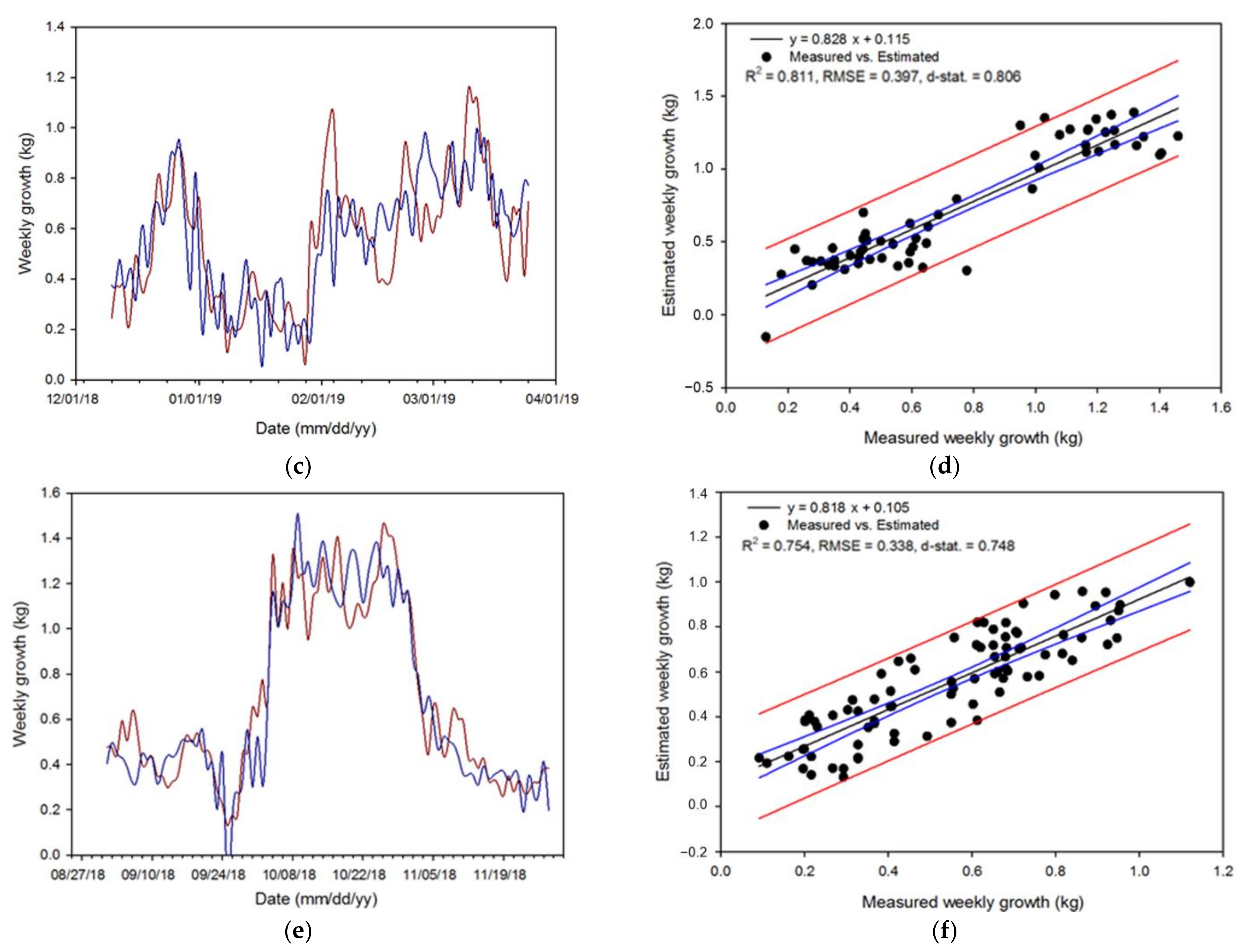
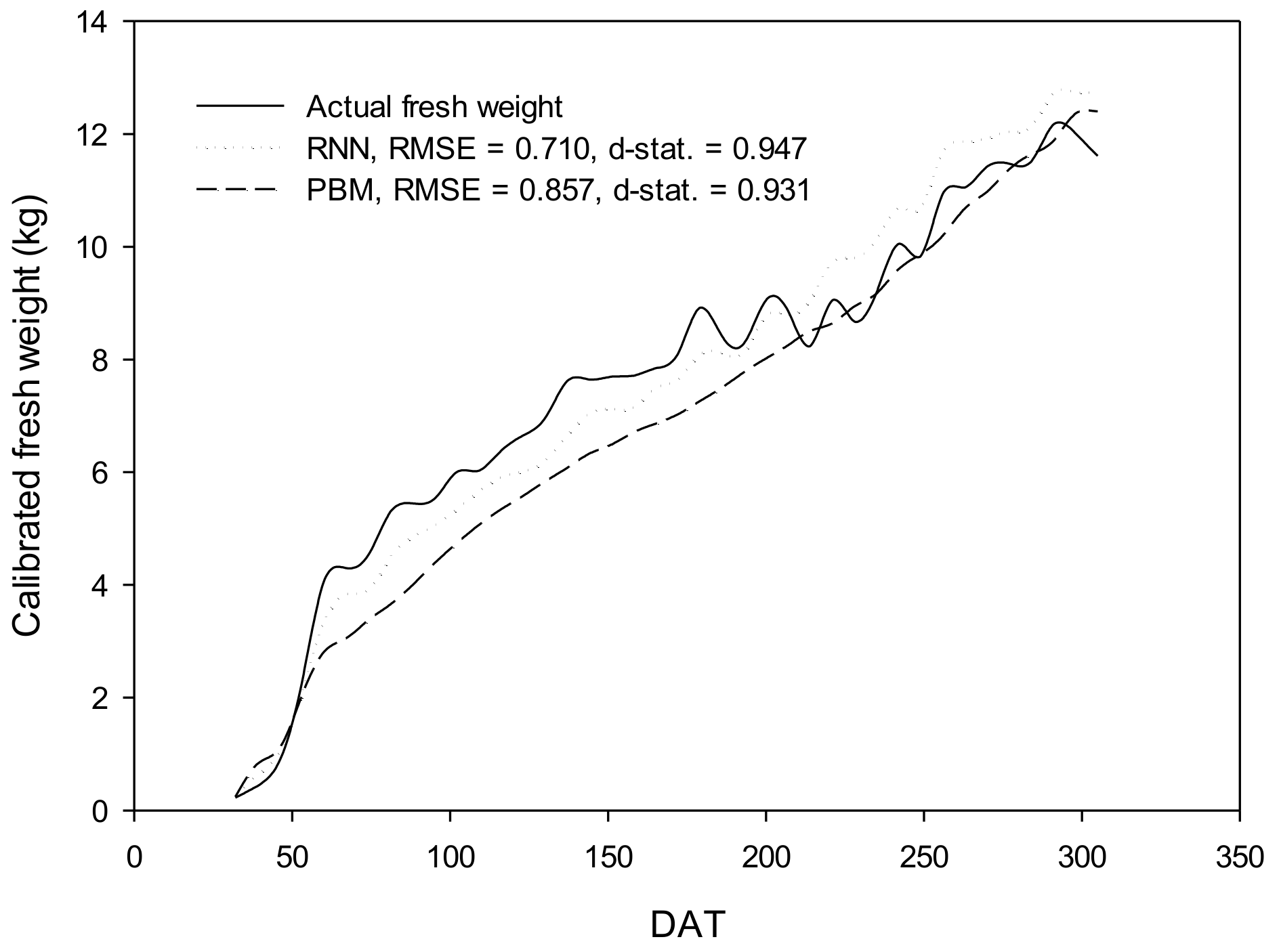
3.4. Validation and Evaluation of the Algorithm
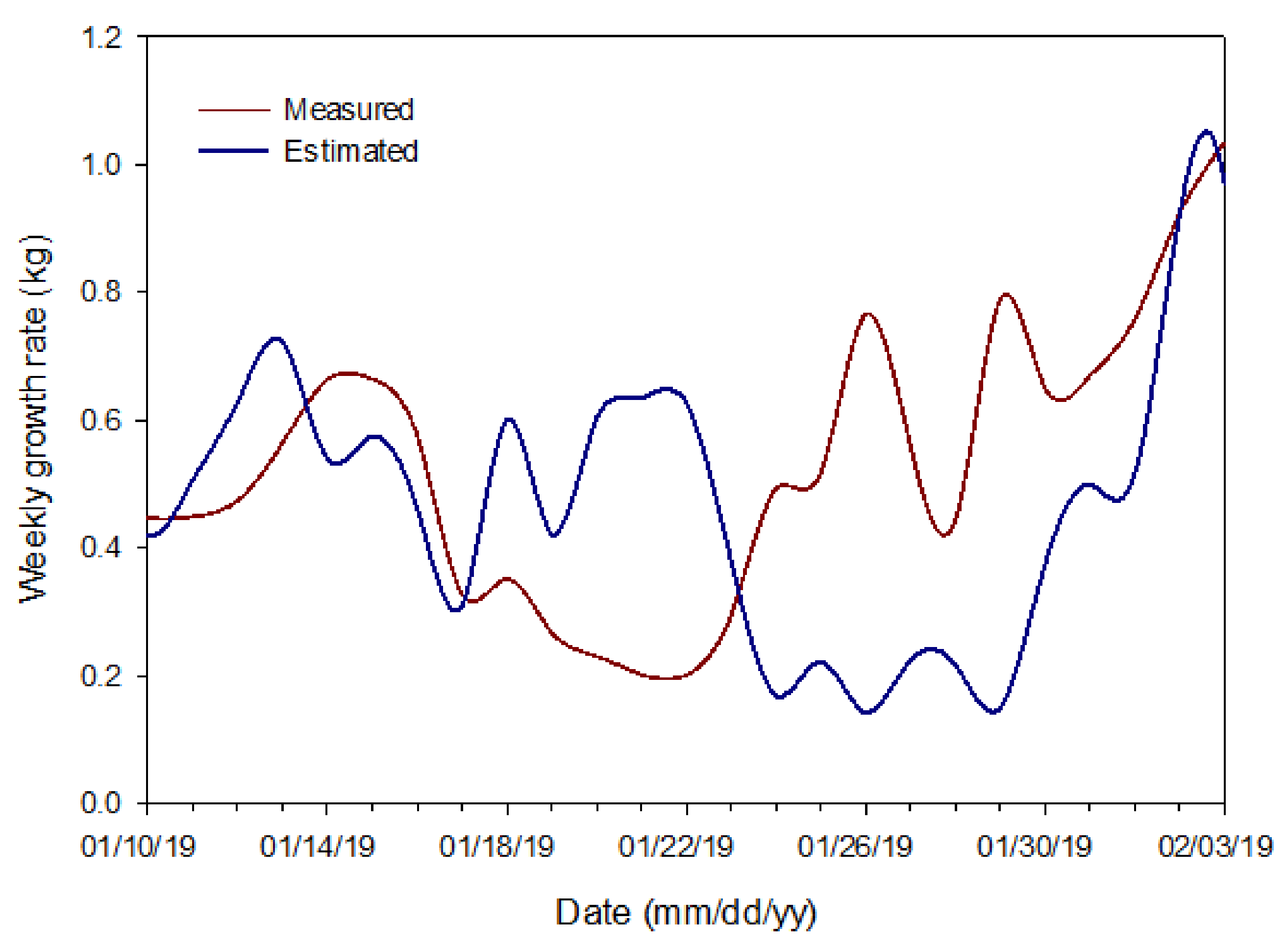
3.5. Advantages and Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atzberger, C. Advances in remote sensing of agriculture: Context description, existing operational monitoring systems and major information needs. Remote Sens. 2013, 5, 949–981. [Google Scholar] [CrossRef]
- Zamora-Izquierdo, M.A.; Santa, J.; Martínez, J.A.; Martínez, V.; Skarmeta, A.F. Smart farming IoT platform based on edge and cloud computing. Biosyst. Eng. 2019, 177, 4–17. [Google Scholar] [CrossRef]
- Baoyun, W. Review on internet of things. J. Electron. Meas. Instrum. 2009, 12, 1–7. [Google Scholar]
- Chi, M.; Plaza, A.; Benediktsson, J.A.; Sun, Z.; Shen, J.; Zhu, Y. Big data for remote sensing: Challenges and opportunities. Proc. IEEE 2016, 104, 2207–2219. [Google Scholar] [CrossRef]
- Sonka, S. Big data: Fueling the next evolution of agricultural innovation. J. Innov. Manag. 2016, 4, 114–136. [Google Scholar] [CrossRef]
- Wolfert, S.; Ge, L.; Verdouw, C.; Bogaardt, M.J. Big data in smart farming—A review. Agric. Syst. 2017, 153, 69–80. [Google Scholar] [CrossRef]
- Kamilaris, A.; Kartakoullis, A.; Prenafeta-Boldú, F.X. A review on the practice of big data analysis in agriculture. Comput. Electron. Agric. 2017, 143, 23–37. [Google Scholar] [CrossRef]
- Jones, J.W.; Antle, J.M.; Basso, B.; Boote, K.J.; Conant, R.T.; Foster, I.; Godfray, H.C.J.; Herrerog, M.; Howitth, R.E.; Janssen, S.; et al. Brief history of agricultural systems modeling. Agric. Syst. 2017, 155, 240–254. [Google Scholar] [CrossRef]
- Dayan, E.; Van Keulen, H.; Jones, J.W.; Zipori, I.; Shmuel, D.; Challa, H. Development, calibration and validation of a greenhouse tomato growth model: I. Description of the model. Agric. Syst. 1993, 43, 145–163. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Heuvelink, E.; Goudriaan, J. Modelling biomass production and yield of horticultural crops: A Review. Sci. Hortic. 1998, 74, 83–111. [Google Scholar] [CrossRef]
- Heuvelink, E. Evaluation of a dynamic simulation model for tomato crop growth and development. Ann. Bot. 1999, 83, 413–422. [Google Scholar] [CrossRef]
- Martínez-Ruiz, A.; López-Cruz, I.L.; Ruiz-García, A.; Pineda-Pineda, J.; Prado-Hernández, J.V. HortSyst: A dynamic model to predict growth, nitrogen uptake, and transpiration of greenhouse tomatoes. Chil. J. Agric. Res. 2019, 79, 89–102. [Google Scholar] [CrossRef]
- Wang, L. A hybrid genetic algorithm-neural network strategy for simulation optimization. Appl. Math. Comput. 2005, 170, 1329–1343. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Arab, M.M.; Yadollahi, A.; Shojaeiyan, A.; Ahmadi, H. Artificial neural network genetic algorithm as powerful tool to predict and optimize in vitro proliferation mineral medium for g × n15 rootstock. Front. Plant Sci. 2016, 7, 1526. [Google Scholar] [CrossRef]
- Ehret, D.L.; Hill, B.D.; Helmer, T.; Edwards, D.R. Neural network modeling of greenhouse tomato yield, growth and water use from automated crop monitoring data. Comput. Electron. Agric. 2011, 79, 82–89. [Google Scholar] [CrossRef]
- Küçükönder, H.; Boyaci, S.; Akyüz, A. A modeling study with an artificial neural network: Developing estimation models for the tomato plant leaf area. Turk. J. Agric For. 2016, 40, 203–212. [Google Scholar] [CrossRef]
- Adavanne, S.; Parascandolo, G.; Pertilä, P.; Heittola, T.; Virtanen, T. Sound event detection in multichannel audio using spatial and harmonic features. arXiv 2017, arXiv:1706.02293. [Google Scholar]
- Ororbia, A.G., II; Mikolov, T.; Reitter, D. Learning simpler language models with the differential state framework. Neural Comput. 2017, 29, 3327–3352. [Google Scholar] [CrossRef]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Jung, D.H.; Kim, H.S.; Jhin, C.; Kim, H.J.; Park, S.H. Time-serial analysis of deep neural network models for prediction of climatic conditions inside a greenhouse. Comput. Electron. Agric. 2020, 173, 105402. [Google Scholar] [CrossRef]
- Moon, T.; Ahn, T.I.; Son, J.E. Long short-term memory for a model-free estimation of macronutrient ion concentrations of root-zone in closed-loop soilless cultures. Plant Methods 2019, 15, 1–12. [Google Scholar] [CrossRef]
- Mouatadid, S.; Adamowski, J.F.; Tiwari, M.K.; Quilty, J.M. Coupling the maximum overlap discrete wavelet transform and long short-term memory networks for irrigation flow forecasting. Agric. Water Manag. 2019, 219, 72–85. [Google Scholar] [CrossRef]
- Jovicich, E.; Cantliffe, D.J.; Stoffella, P.J.; Haman, D.Z. Bell pepper fruit yield and quality as influenced by solar radiation-based irrigation and container media in a passively ventilated greenhouse. HortScience 2007, 42, 642–652. [Google Scholar] [CrossRef]
- Lee, J.; Moon, T.; Nam, D.S.; Park, K.S.; Son, J.E. Estimation of leaf area in paprika based on leaf length, leaf width, and node number using regression models and an artificial neural network. Hortic. Sci. Technol. 2018, 36, 183–192. [Google Scholar]
- Lee, J.W.; Son, J.E. Nondestructive and continuous fresh weight measurements of bell peppers grown in soilless culture systems. Agronomy 2019, 9, 652. [Google Scholar] [CrossRef]
- Bock, S.; Goppold, J.; Weiß, M. An improvement of the convergence proof of the ADAM-optimizer. arXiv 2018, arXiv:1804.10587. [Google Scholar]
- Kohavi, R.; George, H.J. Automatic parameter selection by minimizing estimated error. In Machine Learning Proceedings 1995; Morgan Kaufmann: Burlington, MA, USA, 1995; pp. 304–312. [Google Scholar]
- Jones, J.W.; Dayan, E.; Allen, L.H.; Van Keulen, H.; Challa, H. A dynamic tomato growth and yield model (TOMGRO). Trans. ASABE 1991, 34, 0663–0672. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.N.; Zhang, C.; Bai, M.J.; Wang, Y.K. Development and application of decision support system for agro-technology transfer DSSAT under water resources management. Adv. Mater. Res. 2015, 1073, 1596–1603. [Google Scholar] [CrossRef]
- Ratto, M.; Tarantola, S.; Saltelli, A. Sensitivity analysis in model calibration: GSA-GLUE approach. Comput. Phys. Commun. 2001, 136, 212–224. [Google Scholar] [CrossRef]
- Jones, J.W.; Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Batchelor, W.D.; Hunt, L.A.; Wilkens, P.W.; Singh, U.; Gijsman, A.J.; Ritchie, J.T. The DSSAT cropping system model. Eur. J. Agron. 2003, 18, 235–265. [Google Scholar] [CrossRef]
- Hoogenboom, G.; Jones, J.W.; Traore, P.C.; Boote, K.J. Experiments and data for model evaluation and application. In Improving Soil Fertility Recommendations in Africa Using the Decision Support System for Agrotechnology Transfer (DSSAT); Springer: Dordrecht, The Netherlands, 2012; pp. 9–18. [Google Scholar]
- Willmott, C.J.; Robeson, S.M.; Matsuura, K. A refined index of model performance. Int. J. Climatol. 2012, 32, 2088–2094. [Google Scholar] [CrossRef]
- Ćosić, M.; Stričević, R.; Djurović, N.; Moravčević, D.; Pavlović, M.; Todorović, M. Predicting biomass and yield of sweet pepper grown with and without plastic film mulching under different water supply and weather conditions. Agric. Water Manag. 2017, 188, 91–100. [Google Scholar] [CrossRef]
- Kaul, M.; Hill, R.L.; Walthall, C. Artificial neural networks for corn and soybean yield prediction. Agric. Syst. 2005, 85, 1–18. [Google Scholar] [CrossRef]
- Pantazi, X.E.; Moshou, D.; Alexandridis, T.; Whetton, R.L.; Mouazen, A.M. Wheat yield prediction using machine learning and advanced sensing techniques. Comput. Electron. Agric. 2016, 121, 57–65. [Google Scholar] [CrossRef]
- Teimouri, N.; Dyrmann, M.; Nielsen, P.; Mathiassen, S.; Somerville, G.; Jørgensen, R. Weed growth stage estimator using deep convolutional neural networks. Sensors 2018, 18, 1580. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Jiaqi, W.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 2018, 361, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.C.; Oliveira, F.A.; Brecht, J.K. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: A review. J. Food Eng. 2002, 52, 99–119. [Google Scholar] [CrossRef]
- Jozefowicz, R.; Zaremba, W.; Sutskever, I. An empirical exploration of recurrent network architectures. In Proceedings of the International Conference on Machine Learning, Lille, France, 6–11 July 2015; pp. 2342–2350. [Google Scholar]
- Csizinszky, A.A. Yield and nutrient uptake of Capistrano bell peppers in compost-amended sandy soil. Proc. Fla. State Hortic. Soc. 1999, 112, 333–336. [Google Scholar]
- Boote, K.J.; Jones, J.W.; Hoogenboom, G.; Pickering, N.B. Simulation of crop growth: CROPGRO model. Agric. Syst. 1998, 18, 651–692. [Google Scholar]
- Boote, K.J.; Rybak, M.R.; Scholberg, J.M.; Jones, J.W. Improving the CROPGRO-tomato model for predicting growth and yield response to temperature. HortScience 2012, 47, 1038–1049. [Google Scholar] [CrossRef]
- Vieira, M.I.; de Melo-Abreu, J.P.; Ferreira, M.E.; Monteiro, A.A. Dry matter and area partitioning, radiation interception and radiation-use efficiency in open-field bell pepper. Sci. Hortic. 2009, 121, 404–409. [Google Scholar] [CrossRef][Green Version]
- Hashem, I.A.T.; Yaqoob, I.; Anuar, N.B.; Mokhtar, S.; Gani, A.; Khan, S.U. The rise of “big data” on cloud computing: Review and open research issues. Inf. Syst. 2015, 47, 98–115. [Google Scholar] [CrossRef]
- Gupta, D.K.; Kumar, P.; Mishra, V.N.; Prasad, R.; Dikshit, P.K.S.; Dwivedi, S.B.; Ohri, A.; Singh, R.S.; Srivastava, V. Bistatic measurements for the estimation of rice crop variables using artificial neural network. Adv. Space Res. 2015, 55, 1613–1623. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, S.; Sankar, V.; Sakthivel, T.; Karunakaran, G.; Tripathi, P.C. Non-destructive estimation of leaf area of durian (Durio zibethinus)–An artificial neural network approach. Sci. Hortic. 2017, 219, 319–325. [Google Scholar]
| Parameter | Value | Description |
|---|---|---|
| Learning rate | 0.001 | Learning rate used by the AdamOptimizer |
| β1 | 0.9 | Exponential mass decay rate for the momentum estimates |
| β2 | 0.999 | Exponential velocity decay rate for the momentum estimates |
| E | 1 × 10−0.8 | A constant for numerical stability |
| Forget bias | 1.0 | Probability of forgetting information in the previous dataset |
| Time step | 2–24 | Number of datasets that the LSTM sees at one time |
| Index | Description (Unit) |
|---|---|
| PPSEN | Slope of the relative response of development to photoperiod with time (positive for short-day plants) (1/h) |
| EM-FL | Time between plant emergence and flower appearance (R1) (photothermal days) |
| FL-SH | Time between first flower and first pod (R3) (photothermal days) |
| FL-SD | Time between first flower and first seed (R5) (photothermal days) |
| SD-PM | Time between first seed (R5) and physiological maturity (R7) (photothermal days) |
| FL-LF | Time between first flower (R1) and end of leaf expansion (photothermal days) |
| LFMAX | Maximum leaf photosynthesis rate at 30 °C, 350 vpm CO2, and high light (mg CO2/m2/s) |
| SLAVR | Specific leaf area of cultivar under standard growth conditions (cm2/g) |
| SIZLF | Maximum size of full leaf (three leaflets) (cm2) |
| CSDL | Critical short day length below which reproductive development progresses with no daylength effect (for short-day plants) (hour) |
| XFRT | Maximum fraction of daily growth that is partitioned to seed + shell |
| WTPSD | Maximum weight per seed (g) |
| SFDUR | Seed filling duration for pod cohort at standard growth conditions (photothermal days) |
| SDPDV | Average seed per pod under standard growing conditions (#/pod) |
| PODUR | Time required for cultivar to reach final pod load under optimal conditions (photothermal days) |
| THRSH | Threshing percentage: the maximum ratio of (seed/(seed + shell)) at maturity |
| SDPRO | Fraction protein in seeds (g(protein)/g(seed)) |
| SDLIP | Fraction oil in seeds (g(oil)/g(seed)) |
| Index | Description (Unit) |
|---|---|
| MG | Maturity group number for this ecotype, such as maturity group |
| TM | Indicator of temperature adaptation |
| THVAR | Minimum rate of reproductive development under short days |
| PL-EM | Time between planting and emergence (V0), thermal days |
| EM-V1 | Time required from emergence to first true leaf (V1), thermal days |
| V1-JU | Time required from first true leaf to end of juvenile phase, thermal days |
| JU-R0 | Time required for floral induction, equal to the minimum number of days for floral induction under optimal temperature and daylengths, photothermal days |
| PM06 | Proportion of time between first flower and first pod for first peg |
| PM09 | Proportion of time between first seed and physiological maturity in which the last seed may be formed |
| LNGSH | Time required for growth of individual shells (photothermal days) |
| R7-R8 | Time between physiological (R7) and harvest maturity (R8) (days) |
| FL-VS | Time from first flower to last leaf on main stem (photothermal days) |
| TRIFOL | Rate of appearance of leaves on the mainstem (leaves per thermal day) |
| RWIDTH | Relative width of this ecotype in comparison to the standard width per node |
| RHGHT | Relative height of this ecotype in comparison to the standard height per node |
| R1PPO | Increase in daylength sensitivity after R1 (h) |
| OPTBI | Minimum daily temperature above which there is no effect on slowing normal development towards flowering (°C) |
| SLOBI | Slope of relationship reducing progress towards flowering if TMIN for the day is less than OPTBI |
| Index | Value | Index | Value |
|---|---|---|---|
| PPSEN | 0 | CSDL | 12.33 |
| EM-FL | 40 | XFRT | 0.6 |
| FL-SH | 10 | WTPSD | 0.007 |
| FL-SD | 15 | SFDUR | 40 |
| SD-PM | 330 | SDPDV | 150 |
| FL-LF | 200 | PODUR | 42 |
| LFMAX | 0.98 | THRSH | 6.5 |
| SLAVR | 275 | SDPRO | 0.3 |
| SIZLF | 350 | SDLIP | 0.05 |
| Index | Value | Index | Value |
|---|---|---|---|
| MG | 1 | LNGSH | 35 |
| TM | 1 | R7-R8 | 0 |
| THVAR | 0 | FL-VS | 330 |
| PL-EM | 5 | TRIFOL | 0.35 |
| EM-V1 | 10 | RWIDTH | 1 |
| V1-JU | 24 | RHGHT | 1 |
| JU-R0 | 5 | R1PPO | 0 |
| PM06 | 0 | OPTBI | 0 |
| PM09 | 0 | SLOBI | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-W.; Moon, T.; Son, J.-E. Development of Growth Estimation Algorithms for Hydroponic Bell Peppers Using Recurrent Neural Networks. Horticulturae 2021, 7, 284. https://doi.org/10.3390/horticulturae7090284
Lee J-W, Moon T, Son J-E. Development of Growth Estimation Algorithms for Hydroponic Bell Peppers Using Recurrent Neural Networks. Horticulturae. 2021; 7(9):284. https://doi.org/10.3390/horticulturae7090284
Chicago/Turabian StyleLee, Joon-Woo, Taewon Moon, and Jung-Eek Son. 2021. "Development of Growth Estimation Algorithms for Hydroponic Bell Peppers Using Recurrent Neural Networks" Horticulturae 7, no. 9: 284. https://doi.org/10.3390/horticulturae7090284
APA StyleLee, J.-W., Moon, T., & Son, J.-E. (2021). Development of Growth Estimation Algorithms for Hydroponic Bell Peppers Using Recurrent Neural Networks. Horticulturae, 7(9), 284. https://doi.org/10.3390/horticulturae7090284







