Research Advances in Allelopathy of Volatile Organic Compounds (VOCs) of Plants
Abstract
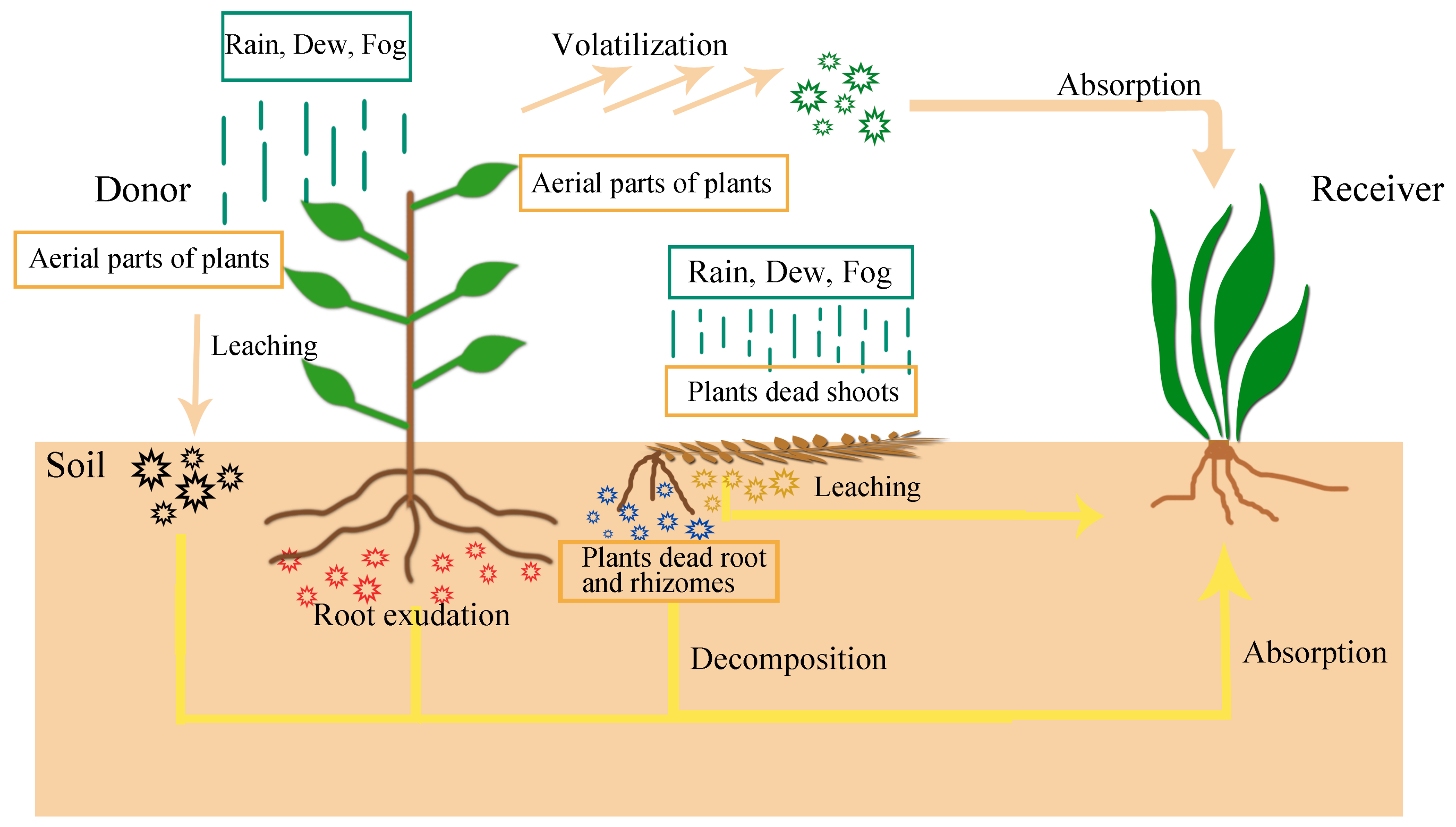
1. Introduction
2. Allelopathy of VOCs of Plants
2.1. VOCs and Plants Growth
2.2. VOCs and Weed Control
2.3. VOCs and Plants Dormancy
2.4. VOCs and the Inhibition of Plants Diseases and Insect Pests
2.4.1. Inhibition of Plants Disease
2.4.2. Inhibition of Plants Insect Pests
2.5. VOCs and Plants Respiration and Photosynthesis
2.6. VOCs and Plants ROS Content and Enzymatic Activity
2.7. VOCs and Plants Signal Transduction
2.7.1. Chemical Communications
2.7.2. Plant Kin Recognition
3. Method of VOCs Collection and Identification
3.1. Collection of Plants VOCs
3.2. Identification of Plants VOCs
4. Conclusions
5. Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Farooq, M.; Jabran, K.; Cheema, Z.A.; Wahid, A.; Siddique, K.H.M. The role of allelopathy in agricultural pest management. Pest Manag. Sci. 2011, 67, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, X.-Z.; Yan, Z.-Q.; Guo, H.-R.; Qin, B. Phytotoxicity of umbelliferone and its analogs: Structure-activity relationships and action mechanisms. Plant Physiol. Biochem. 2015, 97, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Boydston, R.A.; Morra, M.J.; Borek, V.; Clayton, L.; Vaughn, S.F. Onion and weed response to mustard (Sinapis alba) seed meal. Weed Sci. 2011, 59, 546–552. [Google Scholar] [CrossRef]
- Awan, F.K.; Rasheed, M.; Ashraf, M.; Khurshid, M.Y. Efficacy of brassica sorghum and sunflower aqueous extracts to control wheat weeds under rainfed conditions of pothwar. Pakistan J. Anim. Plant Sci. 2012, 22, 715–721. [Google Scholar]
- Bajwa, A.A.; Mahajan, G.; Chauhan, B.S. Nonconventional weed management strategies for modern agriculture. Weed Sci. 2015, 63, 723–747. [Google Scholar] [CrossRef]
- Tavella, L.B.; Lima e Silva, P.S.; Monteiro, A.L.; de Oliveira, V.R.; de Oliveira Fernandes, P.L. Gliricidia sepium intercropping for weed management in immature corn ear production. Rev. Cienc. Agron. 2017, 48, 650–656. [Google Scholar] [CrossRef]
- Avato, P.; D’Addabbo, T.; Leonetti, P.; Argentieri, M.P. Nematicidal potential of brassicaceae. Phytochem. Rev. 2013, 12, 791–802. [Google Scholar] [CrossRef]
- Liu, T.; Cheng, Z.; Meng, H.; Ahmad, I.; Zhao, H. Growth, yield and quality of spring tomato and physicochemical properties of medium in a tomato/garlic intercropping system under plastic tunnel organic medium cultivation. Sci. Hortic. 2014, 170, 159–168. [Google Scholar] [CrossRef]
- Glinwood, R.; Ninkovic, V.; Pettersson, J. Chemical interaction between undamaged plants—Effects on herbivores and natural enemies. Phytochemistry 2011, 72, 1683–1689. [Google Scholar] [CrossRef]
- Singh, A.; Weisser, W.W.; Hanna, R.; Houmgny, R.; Zytynska, S.E. Reduce pests, enhance production: Benefits of intercropping at high densities for okra farmers in Cameroon. Pest Manag. Sci. 2017, 73, 2017–2027. [Google Scholar] [CrossRef]
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Abdel-Monaim, M.F.; Abo-Elyousr, K.A.M. Effect of preceding and intercropping crops on suppression of lentil damping-off and root rot disease in New Valley—Egypt. Crop Prot. 2012, 32, 41–46. [Google Scholar] [CrossRef]
- Ma, Y.-h.; Fu, S.-l.; Zhang, X.-p.; Zhao, K.; Chen, H.Y.H. Intercropping improves soil nutrient availability, soil enzyme activity and tea quantity and quality. Appl. Soil Ecol. 2017, 119, 171–178. [Google Scholar] [CrossRef]
- Ndungu-Magiroi, K.W.; Wortmann, C.S.; Kibunja, C.; Senkoro, C.; Mwangi, T.J.K.; Wamae, D.; Kifuko-Koech, M.; Msakyi, J. Maize-bean intercrop response to nutrient application relative to maize sole crop response. Nutr. Cycl. Agroecosyst. 2017, 109, 17–27. [Google Scholar] [CrossRef]
- Bressan, M.; Roncato, M.-A.; Bellvert, F.; Comte, G.; Haichar, F.e.Z.; Achouak, W.; Berge, O. Exogenous glucosinolate produced by Arabidopsis thaliana has an impact on microbes in the rhizosphere and plant roots. Isme J. 2009, 3, 1243–1257. [Google Scholar] [CrossRef]
- Zhao, M.; Jones, C.M.; Meijer, J.; Lundquist, P.-O.; Fransson, P.; Carlsson, G.; Hallin, S. Intercropping affects genetic potential for inorganic nitrogen cycling by root-associated microorganisms in Medicago sativa and Dactylis glomerata. Appl. Soil Ecol. 2017, 119, 260–266. [Google Scholar] [CrossRef]
- Farooq, M.; Bajwa, A.A.; Cheema, S.A.; Cheema, Z.A. Application of allelopathy in crop production. Int. J. Agric. Biol. 2013, 15, 1367–1378. [Google Scholar]
- Alemayehu, A.; Tamado, T.; Nigussie, D.; Yigzaw, D.; Kinde, T.; Wortmann, C.S. Maize-common bean intercropping to optimize maize-based crop production. J. Agric. Sci. 2017, 155, 1124–1136. [Google Scholar] [CrossRef]
- Vivaldo, G.; Masi, E.; Taiti, C.; Caldarelli, G.; Mancuso, S. The network of plants volatile organic compounds. Sci. Rep. 2017, 7, 11050. [Google Scholar] [CrossRef] [PubMed]
- Kigathi, R.N.; Weisser, W.W.; Reichelt, M.; Gershenzon, J.; Unsicker, S.B. Plant volatile emission depends on the species composition of the neighboring plant community. BMC Plant Biol. 2019, 19, 58. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The role of volatiles in plant communication. Plant J. 2019, 100, 892–907. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Adebesin, F.; Widhalm, J.R.; Boachon, B.; Lefevre, F.; Pierman, B.; Lynch, J.H.; Alam, I.; Junqueira, B.; Benke, R.; Ray, S.; et al. Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter. Science 2017, 356, 1386–1388. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Hu, F.; Xu, X.; Zhang, M.; Liang, W. Volatile allelochemicals in the Ageratum conyzoides intercropped citrus orchard and their effects on mites Amblyseius newsami and Panonychus citri. J. Chem. Ecol. 2005, 31, 2193–2203. [Google Scholar] [CrossRef]
- McNickle, G.G.; St Clair, C.C.; Cahill, J.F., Jr. Focusing the metaphor: Plant root foraging behaviour. Trends Ecol. Evol. 2009, 24, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the “cry for help”. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef]
- Erb, M.; Veyrat, N.; Robert, C.A.M.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C.J. Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Gfeller, V.; Huber, M.; Foerster, C.; Huang, W.; Koellner, T.G.; Erb, M. Root volatiles in plant-plant interactions I: High root sesquiterpene release is associated with increased germination and growth of plant neighbours. Plant Cell Environ. 2019, 42, 1950–1963. [Google Scholar] [CrossRef]
- Molish, H. Der Einfluss Einer Pflanze auf die Andere-Allelopathie; Gustav Fischer Verlag: Jena, Germany, 1937. [Google Scholar]
- Simms, E.L.; Rausher, M.D. Costs and benefits of plant resistance to herbivory. Am. Nat. 1987, 130, 570–581. [Google Scholar] [CrossRef]
- Kim, J.; Felton, G.W. Priming of antiherbivore defensive responses in plants. Insect Sci. 2013, 20, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Langenheim, J.H. Higher plant terpenoids: A phytocentric overview of their ecological roles. J. Chem. Ecol. 1994, 20, 1223–1280. [Google Scholar] [CrossRef] [PubMed]
- Ameye, M.; Audenaert, K.; De Zutter, N.; Steppe, K.; Van Meulebroek, L.; Vanhaecke, L.; De Vleesschauwer, D.; Haesaert, G.; Smagghe, G. Priming of wheat with the green leaf volatile Z-3-hexenyl acetate enhances defense against fusarium graminearum but boosts deoxynivalenol production. Plant Physiol. 2015, 167, 1671–1684. [Google Scholar] [CrossRef]
- Siri-Udom, S.; Suwannarach, N.; Lumyong, S. Applications of volatile compounds acquired from Muscodor heveae against white root rot disease in rubber trees (Hevea brasiliensis Mull. Arg.) and relevant allelopathy effects. Fungal Biol. 2017, 121, 573–581. [Google Scholar] [CrossRef]
- Lerdau, M.; Gray, D. Ecology and evolution of light-dependent and light-independent phytogenic volatile organic carbon. New Phytol. 2003, 157, 199–211. [Google Scholar] [CrossRef]
- Cofer, T.M.; Engelberth, M.; Engelberth, J. Green leaf volatiles protect maize (Zea mays) seedlings against damage from cold stress. Plant Cell Environ. 2018, 41, 1673–1682. [Google Scholar] [CrossRef]
- Muller, C.H.; Muller, W.H.; Haines, B.L. Volatile growth Inhibitors produced by aromatic shrubs. Science 1964, 143, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.H. Inhibitory terpenes volatilized from salvia shrubs. Bull. Torrey Bot. Club 1965, 92, 38–45. [Google Scholar] [CrossRef]
- Abrahim, D.; Braguini, W.L.; Kelmer-Bracht, A.M.; Ishii-Iwamoto, E.L. Effects of four monoterpenes on germination, primary root growth, and mitochondrial respiration of maize. J. Chem. Ecol. 2000, 26, 611–624. [Google Scholar] [CrossRef]
- Norton, J.M.; Harman, G.E. Responses of soil microorganisms to volatile exudates from germinating pea seeds. Can. J. Bot. 1985, 63, 1040–1045. [Google Scholar] [CrossRef]
- Won Yun, K.; Kil, B.S.; Han, D.M. Phytotoxic and antimicrobial activity of volatile constituents of Artemisia princeps var. orientalis. J. Chem. Ecol. 1993, 19, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Jassbi, A.R.; Zamanizadehnajari, S.; Baldwin, I.T. Phytotoxic volatiles in the roots and shoots of Artemisia tridentata as detected by headspace solid-phase microextraction and gas chromatographic-mass spectrometry analysis. J. Chem. Ecol. 2010, 36, 1398–1407. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Augustin, J.; Merbach, W. Plant rhizodeposition—An important source for carbon turnover in soils. J. Plant Nutr. Soil Sci. 2002, 165, 397–407. [Google Scholar] [CrossRef]
- Lin, C.; Owen, S.M.; Peñuelas, J. Volatile organic compounds in the roots and rhizosphere of Pinus spp. Soil Biol. Biochem. 2007, 39, 951–960. [Google Scholar] [CrossRef]
- Delory, B.M.; Delaplace, P.; Fauconnier, M.-L.; du Jardin, P. Root-emitted volatile organic compounds: Can they mediate belowground plant-plant interactions? Plant Soil 2016, 402, 1–26. [Google Scholar] [CrossRef]
- Fitter, A. Making allelopathy respectable. Science 2003, 301, 1337–1338. [Google Scholar] [CrossRef] [PubMed]
- Brilli, F.; Loreto, F.; Baccelli, I. Exploiting plant volatile organic compounds (VOCs) in agriculture to Improve sustainable defense strategies and productivity of crops. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Oleszek, W. Allelopathic effects of volatiles from some Cruciferae species on lettuce, barnyard grass and wheat growth. Plant Soil 1987, 102, 271–273. [Google Scholar] [CrossRef]
- Romagni, J.G.; Allen, S.N.; Dayan, F.E. Allelopathic effects of volatile cineoles on two weedy plant species. J. Chem. Ecol. 2000, 26, 303–313. [Google Scholar] [CrossRef]
- Schmidt-Silva, V.; Pawlowski, Â.; Kaltchuk-Santos, E.; Zini, C.; Soares, G. Cytotoxicity of essential oils from two species of Heterothalamus (Asteraceae). Aust. J. Bot. 2011, 59, 682–691. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z.; Meng, H.; Tang, X. The garlic allelochemical diallyl disulfide affects tomato root growth by influencing cell division, phytohormone balance and expansin gene expression. Front. Plant Sci. 2016, 7, 1199. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z.-H.; Meng, H.-W. Transcriptomic insights into the allelopathic effects of the garlic allelochemical diallyl disulfide on tomato roots. Sci. Rep. 2016, 6, 38902. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kil, B.-S. Allelopathic effects of some volatile substances from the tomato plant. J. Crop Prod. 2001, 4, 313–321. [Google Scholar] [CrossRef]
- Alves, M.d.C.S.; Medeiros Filho, S.; Innecco, R.; Torres, S.B. Alelopatia de extratos voláteis na germinação de sementes e no comprimento da raiz de alface. Pesqui. Agropecuária Bras. 2004, 39, 1083–1086. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Meehan, J.T. Use of isothiocyanates for suppression of Palmer amaranth (Amaranthus palmeri), pitted morningglory (Ipomoea lacunosa), and yellow nutsedge (Cyperus esculentus). Weed Sci. 2017, 53, 884–890. [Google Scholar] [CrossRef]
- Horiuchi, J.-i.; Badri, D.V.; Kimball, B.A.; Negre, F.; Dudareva, N.; Paschke, M.W.; Vivanco, J.M. The floral volatile, methyl benzoate, from snapdragon (Antirrhinum majus) triggers phytotoxic effects in Arabidopsis thaliana. Planta 2007, 226, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.R.; Overbeck, G.E.; Soares, G.L.G. Phytotoxicity of volatiles from fresh and dry leaves of two Asteraceae shrubs: Evaluation of seasonal effects. S. Afr. J. Bot. 2014, 93, 14–18. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; Novoa, A.; González, L. Soil biochemical alterations and microbial community responses under Acacia dealbata Link invasion. Soil Biol. Biochem. 2014, 79, 100–108. [Google Scholar] [CrossRef]
- Haramoto, E.R.; Gallandt, E.R. Brassica cover cropping for weed management: A review. Renew. Agric. Food Syst. 2007, 19, 187–198. [Google Scholar] [CrossRef]
- Morra, M.J.; Kirkegaard, J.A. Isothiocyanate release from soil-incorporated Brassica tissues. Soil Biol. Biochem. 2002, 34, 1683–1690. [Google Scholar] [CrossRef]
- Santonja, M.; Bousquet-Mélou, A.; Greff, S.; Ormeño, E.; Fernandez, C. Allelopathic effects of volatile organic compounds released from Pinus halepensis needles and roots. Ecol. Evol. 2019, 9, 8201–8213. [Google Scholar] [CrossRef]
- Ren, K.; Hayat, S.; Qi, X.; Liu, T.; Cheng, Z. The garlic allelochemical DADS influences cucumber root growth involved in regulating hormone levels and modulating cell cycling. J. Plant Physiol. 2018, 230, 51–60. [Google Scholar] [CrossRef]
- Wei, C.; Zhou, S.; Li, W.; Jiang, C.; Yang, W.; Han, C.; Zhang, C.; Shao, H. Chemical composition and allelopathic, phytotoxic and pesticidal activities of Atriplex cana Ledeb. (Amaranthaceae) essential oil. Chem. Biodivers. 2019, 16, e1800595. [Google Scholar] [CrossRef]
- Effah, E.; Holopainen, J.K.; McCormick, A.C. Potential roles of volatile organic compounds in plant competition. Perspect. Plant Ecol. Evol. Syst. 2019, 38, 58–63. [Google Scholar] [CrossRef]
- Macias, F.A.; Molinillo, J.M.; Varela, R.M.; Galindo, J.C. Allelopathy—A natural alternative for weed control. Pest Manag Sci 2007, 63, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Luiza Ishii-Iwamoto, E.; Marusa Pergo Coelho, E.; Reis, B.; Sebastiao Moscheta, I.; Moacir Bonato, C. Effects of monoterpenes on physiological processes during seed germination and seedling growth. Curr. Bioact. Compd. 2012, 8, 50–64. [Google Scholar] [CrossRef]
- Arroyo, A.I.; Pueyo, Y.; Pellissier, F.; Ramos, J.; Espinosa-Ruiz, A.; Millery, A.; Alados, C.L. Phytotoxic effects of volatile and water soluble chemicals of Artemisia herba-alba. J. Arid Environ. 2018, 151, 1–8. [Google Scholar] [CrossRef]
- Runyon, J.B.; Mescher, M.C.; De Moraes, C.M. Volatile chemical cues guide host location and host selection by parasitic plants. Science 2006, 313, 1964–1967. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.D.; Hill, J.D.; Liebman, M. Reducing freshwater toxicity while maintaining weed control, profits, and productivity: Effects of increased crop rotation diversity and reduced herbicide usage. Environ. Sci. Technol. 2017, 51, 1707–1717. [Google Scholar] [CrossRef]
- Arimura, G.-i.; Shiojiri, K.; Karban, R. Acquired immunity to herbivory and allelopathy caused by airborne plant emissions. Phytochemistry 2010, 71, 1642–1649. [Google Scholar] [CrossRef]
- Verdeguer, M.; Blázquez, M.; Boira, H. Phytotoxic effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus essential oils in weeds of Mediterranean summer crops. Biochem. Syst. Ecol. 2009, 37, 362–369. [Google Scholar] [CrossRef]
- Benvenuti, S.; Cioni, P.L.; Flamini, G.; Pardossi, A. Weeds for weed control: Asteraceae essential oils as natural herbicides. Weed Res. 2017, 57, 342–353. [Google Scholar] [CrossRef]
- Mushtaq, W.; Ain, Q.; Siddiqui, M.B.; Alharby, H.; Hakeem, K.R. Allelochemicals change macromolecular content of some selected weeds. S. Afr. J. Bot. 2020, 130, 177–184. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; Belz, R.; Walker, F.; Hurle, K. Weed suppression by release of Isothiocyanates from turnip-rape mulch. Agron. J. 2001, 93, 37–43. [Google Scholar] [CrossRef]
- Pardo-Muras, M.; Puig, C.G.; López-Nogueira, A.; Cavaleiro, C.; Pedrol, N. On the bioherbicide potential of Ulex europaeus and Cytisus scoparius: Profiles of volatile organic compounds and their phytotoxic effects. PLoS ONE 2018, 13, e0205997. [Google Scholar] [CrossRef]
- Pardo-Muras, M.; Puig, C.G.; Pedrol, N. Cytisus scoparius and Ulex europaeus produce volatile organic compounds with powerful synergistic herbicidal effects. Molecules 2019, 24, 4539. [Google Scholar] [CrossRef] [PubMed]
- Vokou, D.; Douvli, P.; Blionis, G.J.; Halley, J.M. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003, 29, 2281–2301. [Google Scholar] [CrossRef]
- Barney, J.N.; Sparks, J.P.; Greenberg, J.; Whitlow, T.H.; Guenther, A. Biogenic volatile organic compounds from an invasive species: Impacts on plant–plant interactions. Plant Ecol. 2009, 203, 195–205. [Google Scholar] [CrossRef][Green Version]
- Campbell, M.; Segear, E.; Beers, L.; Knauber, D.; Suttle, J. Dormancy in potato tuber meristems: Chemically induced cessation in dormancy matches the natural process based on transcript profiles. Funct. Integr. Genom. 2008, 8, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, T.; Hiura, H.; Hamada, M. Breaking bud dormancy in corms, tubers, and trees with sulfur-containing compounds. HortScience 1985, 20, 290–291. [Google Scholar]
- Hosoki, T. Breaking bud dormancy in corms and trees with sulfide compounds in garlic and horseradish. HortScience 1986, 21, 114–116. [Google Scholar]
- Kubota, N.; Yamane, Y.; Toriu, K.; Kawazu, K.; Higuchi, T.; Nishimura, S. Identification of active substances in garlic responsible for breaking bud dormancy in grapevines. J. Jpn. Soc. Hortic. Sci. 1999, 68(6), 1111–1117. [Google Scholar] [CrossRef][Green Version]
- Shukla, S.; Pandey, S.S.; Chandra, M.; Pandey, A.; Bharti, N.; Barnawal, D.; Chanotiya, C.S.; Tandon, S.; Darokar, M.P.; Kalra, A. Application of essential oils as a natural and alternate method for inhibiting and inducing the sprouting of potato tubers. Food Chem. 2019, 284, 171–179. [Google Scholar] [CrossRef]
- Hartmans, K.J.; Diepenhorst, P.; Bakker, W.; Gorris, L.G.M. The use of carvone in agriculture: Sprout suppression of potatoes and antifungal activity against potato tuber and other plant diseases. Ind. Crop. Prod. 1995, 4, 3–13. [Google Scholar] [CrossRef]
- Song, X.; Bandara, M.S.; Tanino, K.K. Potato dormancy regulation: Use of essential oils for sprout suppression in potato storage. Fruit Veg. Cereal Sci. Biotechnol 2009, 2, 110–117. [Google Scholar]
- Finger, F.L.; Santos, M.M.d.S.; Araujo, F.F.; Lima, P.C.C.; Costa, L.C.d.; França, C.d.F.M.; Queiroz, M.d.C. Action of essential oils on sprouting of non-dormant potato tubers. Braz. Arch. Biol. Technol. 2018, 61. [Google Scholar] [CrossRef]
- Owolabi, M.S.; Olowu, R.A.; Lajide, L.; Oladimeji, M.O.; Padilla-Camberos, E.; Flores-Fernández, J.M. Inhibition of potato tuber sprouting during storage by the controlled release of essential oil using a wick application method. Ind. Crop. Prod. 2013, 45, 83–87. [Google Scholar] [CrossRef]
- Komai, K.; Tang, C.-S. A chemotype of Cyperus rotundus in Hawaii. Phytochemistry 1989, 28, 1883–1886. [Google Scholar] [CrossRef]
- Neri, F.; Mari, M.; Brigati, S.; Bertolini, P. Fungicidal activity of plant volatile compounds for controlling Monilinia laxa in stone fruit. Plant Dis. 2007, 91, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.C.; de Souza, P.E.; Amaral, D.C.; Zeviani, W.M.; Brasil Pereira Pinto, J.E. Essential oils from Hyptis marrubioides, Aloysia gratissima and Cordia verbenacea reduce the progress of Asian soybean rust. Acta Sci. Agron. 2014, 36, 159–166. [Google Scholar] [CrossRef][Green Version]
- Rienth, M.; Crovadore, J.; Ghaffari, S.; Lefort, F. Oregano essential oil vapour prevents Plasmopara viticola infection in grapevine (Vitis Vinifera) and primes plant immunity mechanisms. PLoS ONE 2019, 14, e0222854. [Google Scholar] [CrossRef] [PubMed]
- Asthana, A.; Tripathi, N.N.; Dixit, S.N. Fungitoxic and phytotoxic studies with essential oil of Ocimum adscendens. J. Phytopathol. 1986, 117, 152–159. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Dikshit, A.; Dixit, S.N. Adenocalymma allicea, a new source of a natural fungitoxicant. Trop. Agric. 1987, 64, 318–322. [Google Scholar]
- Dube, S.; Upadhyay, P.D.; Tripathi, S.C. Antifungal, physicochemical, and insect-repelling activity of the essential oil of Ocimum basilicum. Can. J. Bot. 1989, 67, 2085–2087. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Chen, H.; Fan, Y.; Shi, Z. Antifungal activity of eugenol against Botrytis cinerea. Trop. Plant Pathol. 2010, 35, 137–143. [Google Scholar] [CrossRef]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid Based Complement Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef]
- Quintana-Rodriguez, E.; Rivera-Macias, L.E.; Adame-Alvarez, R.M.; Torres, J.M.; Heil, M. Shared weapons in fungus-fungus and fungus-plant interactions? Volatile organic compounds of plant or fungal origin exert direct antifungal activity in vitro. Fungal Ecol. 2018, 33, 115–121. [Google Scholar] [CrossRef]
- Mohammad, S.F.; Woodward, S.C. Characterization of a potent inhibitor of platelet aggregation and release reaction isolated from Allium sativum (Garlic). Thromb. Res. 1986, 44, 793–806. [Google Scholar] [CrossRef]
- Yang, F.; Liu, X.; Wang, H.; Deng, R.; Yu, H.; Cheng, Z. Identification and allelopathy of green garlic (Allium sativum L.) volatiles on scavenging of cucumber (Cucumis sativus L.) reactive oxygen species. Molecules 2019, 24, 3263. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Sugano, M.; Majid, A.; Fujii, Y. Antifungal effects of volatile compounds from black zira (Bunium persicum) and other spices and herbs. J. Chem. Ecol. 2007, 33, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mandal, M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pac. J. Trop. Biomed. 2015, 5, 421–428. [Google Scholar] [CrossRef]
- Friberg, M.; Schwind, C.; Roark, L.C.; Raguso, R.A.; Thompson, J.N. Floral scent contributes to interaction specificity in coevolving plants and their insect pollinators. J. Chem. Ecol. 2014, 40, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K.; Arimura, G.-i.; Ohnishi, T. ‘Hidden’ terpenoids in plants: Their biosynthesis, localization and ecological roles. Plant Cell Physiol. 2017, 58, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Alborn, H.; Turlings, T.; Jones, T.H.; Stenhagen, G.; Loughrin, J.; Tumlinson, J. An elicitor of plant volatiles from beet armyworm oral secretion. Science 1997, 276, 945–949. [Google Scholar] [CrossRef]
- Degenhardt, J.; Hiltpold, I.; Köllner, T.G.; Frey, M.; Gierl, A.; Gershenzon, J.; Hibbard, B.E.; Ellersieck, M.R.; Turlings, T.C.J. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc. Natl. Acad. Sci. USA 2009, 106, 13213–13218. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-H.; Nishimura, H.; Hasegawa, K.; Mizutani, J. Allelopathy of Sasa cernua. J. Chem. Ecol. 1992, 18, 1785–1796. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Weaver, T.; Klarich, D. Allelopathic effects of volatile substances from Artemisia tridentata Nutt. Am. Midl. Nat. 1977, 92, 508–512. [Google Scholar] [CrossRef]
- Einhellig, F.A. Mechanism of Action of Allelochemicals in Allelopathy; ACS Publications: Washington, DC, USA, 1995. [Google Scholar]
- Kohli, R.K.; Batish, D.R.; Singh, H.P. Eucalypt oils for the control of Parthenium (Parthenium hysterophorus L.). Crop Prot. 1998, 17, 119–122. [Google Scholar] [CrossRef]
- Einhellig, F.A.; Rasmussen, J.A. Effects of three phenolic acids on chlorophyll content and growth of soybean and grain sorghum seedlings. J. Chem. Ecol. 1979, 5, 815–824. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Yeh, S. Isoprene emission from plants. Annu. Rev. Plant Biol. 2001, 52, 407–436. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Chemical characterization and allelopathic potential of volatile oil of Eucalyptus tereticornis against Amaranthus viridis. J. Plant Interact. 2011, 6, 297–302. [Google Scholar] [CrossRef]
- Tsubo, M.; Nishihara, E.; Nakamatsu, K.; Cheng, Y.; Shinoda, M. Plant volatiles inhibit restoration of plant species communities in dry grassland. Basic Appl. Ecol. 2012, 13, 76–84. [Google Scholar] [CrossRef]
- Yang, X.; Deng, S.; De Philippis, R.; Chen, L.; Hu, C.; Zhang, W. Chemical composition of volatile oil from Artemisia ordosica and its allelopathic effects on desert soil microalgae, Palmellococcus miniatus. Plant Physiol. Biochem. 2012, 51, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, L.; Zhou, L.; Bai, Y.; Wang, B.; Hou, P.; Xu, Q.; Yang, W.; Zuo, Z. Inhibitory effects of eucalyptol and limonene on the photosynthetic abilities in Chlorella vulgaris (Chlorophyceae). Phycologia 2016, 55, 696–702. [Google Scholar] [CrossRef]
- Tsai, C.W.; Yang, J.J.; Chen, H.W.; Sheen, L.Y.; Lii, C.K. Garlic organosulfur compounds upregulate the expression of the pi class of glutathione S-transferase in rat primary hepatocytes. J. Nutr. 2005, 135, 2560–2565. [Google Scholar] [CrossRef]
- Hsiung, Y.-C.; Chen, Y.-A.; Chen, S.-Y.; Chi, W.-C.; Lee, R.-H.; Chiang, T.-Y.; Huang, H.-J. Volatilized myrcene inhibits growth and activates defense responses in rice roots. Acta Physiol. Plant. 2013, 35, 2475–2482. [Google Scholar] [CrossRef]
- Mutlu, S.; Atici, Ö.; Esim, N.; Mete, E. Essential oils of catmint (Nepeta meyeri Benth.) induce oxidative stress in early seedlings of various weed species. Acta Physiol. Plant. 2011, 33, 943–951. [Google Scholar] [CrossRef]
- Jin, P.; Wu, X.; Xu, F.; Wang, X.; Wang, J.; Zheng, Y. Enhancing antioxidant capacity and reducing decay of Chinese bayberries by essential oils. J. Agric. Food Chem. 2012, 60, 3769–3775. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; von Dahl, C.C.; Preston, C.A. Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef]
- Heil, M.; Silva Bueno, J.C. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl. Acad. Sci. USA 2007, 104, 5467–5472. [Google Scholar] [CrossRef]
- Kessler, A.; Halitschke, R.; Diezel, C.; Baldwin, I.T. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 2006, 148, 280–292. [Google Scholar] [CrossRef]
- Ton, J.; D’Alessandro, M.; Jourdie, V.; Jakab, G.; Karlen, D.; Held, M.; Mauch-Mani, B.; Turlings, T.C.J. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 2007, 49, 16–26. [Google Scholar] [CrossRef]
- Heil, M.; Ton, J. Long-distance signalling in plant defence. Trends Plant Sci. 2008, 13, 264–272. [Google Scholar] [CrossRef]
- Biedrzycki, M.L.; Bais, H.P. Kin recognition in plants: A mysterious behaviour unsolved. J. Exp. Bot. 2010, 61, 4123–4128. [Google Scholar] [CrossRef]
- Broz, A.K.; Broeckling, C.D.; De-la-Peña, C.; Lewis, M.R.; Greene, E.; Callaway, R.M.; Sumner, L.W.; Vivanco, J.M. Plant neighbor identity influences plant biochemistry and physiology related to defense. BMC Plant Biol. 2010, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.J.; During, H.J.; Anten, N.P. Detect thy neighbor: Identity recognition at the root level in plants. Plant Sci. Int. J. Exp. Plant Biol. 2012, 195, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Shiojiri, K. Self-recognition affects plant communication and defense. Ecol. Lett. 2009, 12, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Masclaux, F.; Hammond, R.L.; Meunier, J.; Gouhier-Darimont, C.; Keller, L.; Reymond, P. Competitive ability not kinship affects growth of Arabidopsis thaliana accessions. New Phytol. 2010, 185, 322–331. [Google Scholar] [CrossRef]
- Milla, R.; Forero, D.M.; Escudero, A.; Iriondo, J.M. Growing with siblings: A common ground for cooperation or for fiercer competition among plants? Proc. R. Soc. B Biol. Sci. 2009, 276, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-F.; Li, L.-L.; Xu, Y.; Kong, C.-H. Kin recognition in rice (Oryza sativa) lines. New Phytol. 2018, 220, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Torices, R.; Gómez, J.M.; Pannell, J.R. Kin discrimination allows plants to modify investment towards pollinator attraction. Nat. Commun. 2018, 9, 2018. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.T. Comprehensive insect physiology, biochemistry and pharmacology: Edited by G. A. Kerkut and L. I. Gilbert. Pergamon Press, Oxford. 1985. 13 Volumes. 8200 pp approx. £1700.00/$2750.00. ISBN 0 08 026850 1. Insect Biochem. 1985, 15, i–xiv. [Google Scholar] [CrossRef]
- Kimparis, A.; Siatis, N.; Daferera, D.; Tarantilis, P.; Pappas, C.; Polissiou, M. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason. Sonochem. 2006, 13, 54–60. [Google Scholar] [CrossRef]
- Lee, S.N.; Kim, N.S.; Lee, D.S. Comparative study of extraction techniques for determination of garlic flavor components by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2003, 377, 749–756. [Google Scholar] [CrossRef]
- Mutarutwa, D.; Navarini, L.; Lonzarich, V.; Compagnone, D.; Pittia, P. GC-MS aroma characterization of vegetable matrices: Focus on 3-alkyl-2-methoxypyrazines. J. Mass Spectrom. 2018, 53, 871–881. [Google Scholar] [CrossRef]
- Sgorbini, B.; Cagliero, C.; Liberto, E.; Rubiolo, P.; Bicchi, C.; Cordero, C. Strategies for accurate quantitation of volatiles from foods and plant-origin materials: A challenging task. J. Agric. Food Chem. 2019, 67, 1619–1630. [Google Scholar] [CrossRef]
- Verpoorte, R.; Choi, Y.H.; Kim, H.K. NMR-based metabolomics at work in phytochemistry. Phytochem. Rev. 2007, 6, 3–14. [Google Scholar] [CrossRef]
- Marshall, T.L.; Chaffin, C.T.; Makepeace, V.D.; Hoffman, R.M.; Hammaker, R.M.; Fateley, W.G.; Saarinen, P.; Kauppinen, J. Investigation of the effects of resolution on the performance of classical least-squares (CLS) spectral interpretation programs when applied to volatile organic compounds (VOCs) of interest in remote sensing using open-air long-path Fourier transform infrared (FT-IR) spectrometry. J. Mol. Struct. 1994, 324, 19–28. [Google Scholar] [CrossRef]
- Stierlin, É.; Nicolè, F.; Fernandez, X.; Michel, T. Development of a headspace solid-phase microextraction gas chromatography-mass spectrometry method to study volatile organic compounds (VOCs) emitted by lavender roots. Chem. Biodivers. 2019, 16, e1900280. [Google Scholar] [CrossRef] [PubMed]
- Danner, H.; Samudrala, D.; Cristescu, S.M.; Van Dam, N.M. Tracing hidden herbivores: Time-resolved non-invasive analysis of belowground volatiles by proton-transfer-reaction mass spectrometry (PTR-MS). J. Chem. Ecol. 2012, 38, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Lonzarich, V.; Khomenko, I.; Cappellin, L.; Navarini, L.; Biasioli, F. Unveiling the molecular basis of mascarpone cheese aroma: VOCs analysis by SPME-GC/MS and PTR-ToF-MS. Molecules 2020, 25, 1242. [Google Scholar] [CrossRef]
- Lebanov, L.; Ghiasvand, A.; Paull, B. Data handling and data analysis in metabolomic studies of essential oils using GC-MS. J. Chromatogr. A 2021, 1640, 461896. [Google Scholar] [CrossRef]
- Majchrzak, T.; Wojnowski, W.; Lubinska-Szczygeł, M.; Różańska, A.; Namieśnik, J.; Dymerski, T. PTR-MS and GC-MS as complementary techniques for analysis of volatiles: A tutorial review. Anal. Chim. Acta 2018, 1035, 1–13. [Google Scholar] [CrossRef]
- Hare, J.D. Ontogeny and season constrain the production of herbivore-inducible plant volatiles in the field. J. Chem. Ecol. 2010, 36, 1363–1374. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Tian, L.; Han, X.; Yang, Y. Research Advances in Allelopathy of Volatile Organic Compounds (VOCs) of Plants. Horticulturae 2021, 7, 278. https://doi.org/10.3390/horticulturae7090278
Xie Y, Tian L, Han X, Yang Y. Research Advances in Allelopathy of Volatile Organic Compounds (VOCs) of Plants. Horticulturae. 2021; 7(9):278. https://doi.org/10.3390/horticulturae7090278
Chicago/Turabian StyleXie, Yiqi, Libo Tian, Xu Han, and Yan Yang. 2021. "Research Advances in Allelopathy of Volatile Organic Compounds (VOCs) of Plants" Horticulturae 7, no. 9: 278. https://doi.org/10.3390/horticulturae7090278
APA StyleXie, Y., Tian, L., Han, X., & Yang, Y. (2021). Research Advances in Allelopathy of Volatile Organic Compounds (VOCs) of Plants. Horticulturae, 7(9), 278. https://doi.org/10.3390/horticulturae7090278





