Exogenous Treatments to Enhance Splice-Grafted Watermelon Survival
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location and Design
2.2. Plant Material
2.3. Exogenous Treatments
2.4. Grafting Methods and Healing
2.5. Environmental Conditions
2.6. Grafted Plant Survival
2.7. Data Analyses
3. Results
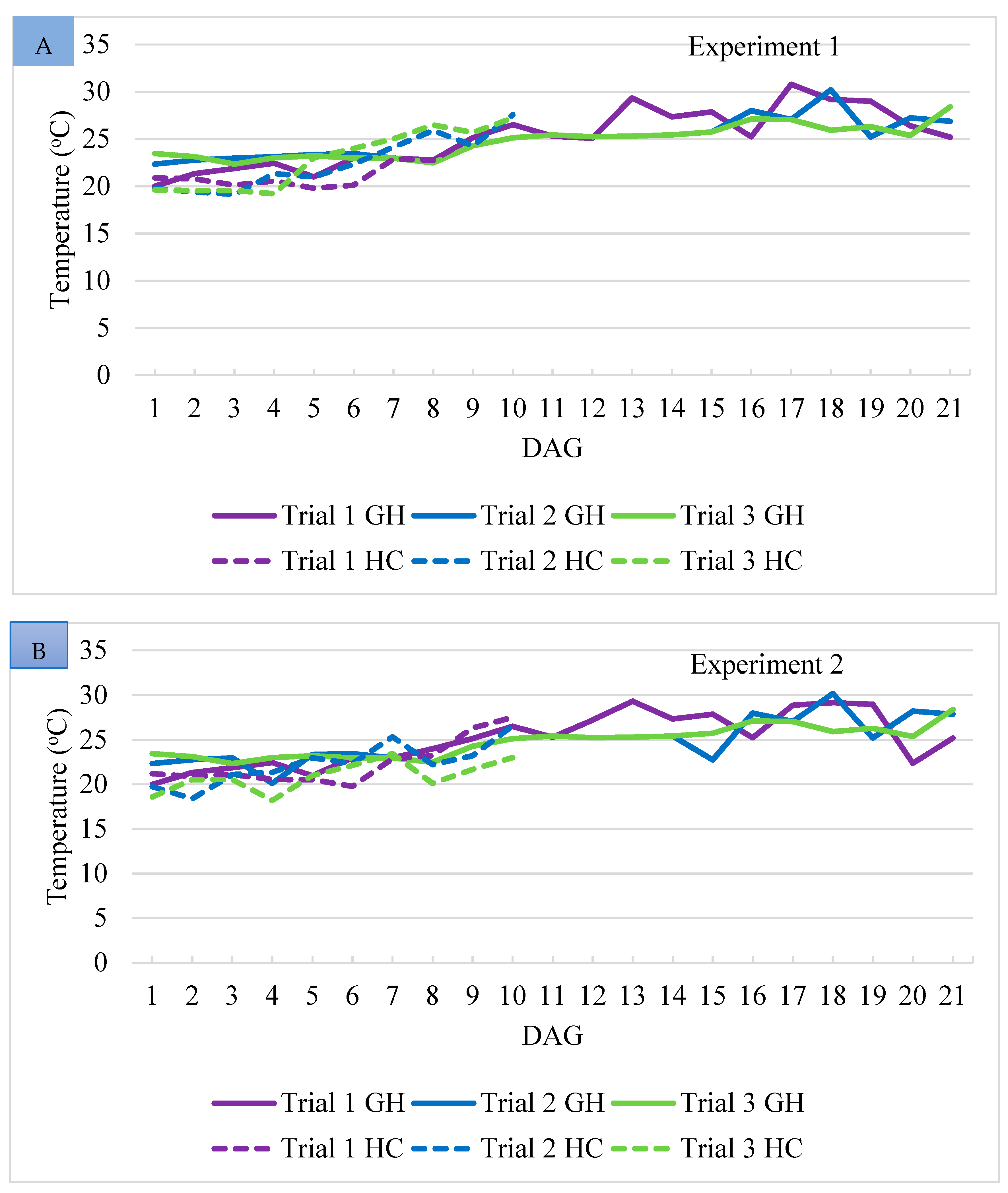
3.1. Environmental Conditions in the Greenhouse
3.2. Grafted Plant Survival
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, D.C.; Kwon, S.W.; Ko, B.R.; Choi, J.S. Using chemical controls to inhibit axillary buds of Lagenaria as rootstock for grafted watermelon (Citrullus lanatus). Acta Hort. 2002, 588, 43–48. [Google Scholar] [CrossRef]
- Davis, A.R.; Perkins-Veazie, P.; Sakata, Y.; López-Galarza, S.; Maroto, J.V.; Lee, S.G.; Huh, Y.C.; Miguel, A.; King, S.R.; Cohen, R.; et al. Cucurbit grafting. Crit. Rev. Plant Sci. 2008, 27, 50–74. [Google Scholar] [CrossRef]
- Memmott, F.D.; Hassell, R.L. Watermelon (Citrullus lanatus) grafting method to reduce labor cost by eliminating rootstock side shoots. Acta Hort. 2010, 871, 389–394. [Google Scholar] [CrossRef]
- Galinato, S.P.; Miles, C.A.; Wimer, J.A. Non-Grafted and Grafted Seedless Watermelon Transplants: Comparative Economic Feasibility Analysis. Available online: http://cru.cahe.wsu.edu/CEPublications/TB08E/TB08.pdf (accessed on 10 April 2019).
- Galinato, S.P.; Gallardo, R.K. Chapter 6.1 Cost analysis for vegetable grafting. In Grafting Manual: How to Produce Grafted Vegetable Plants; Kubota, C., Miles, C., Zhao, X., Eds.; 2017; Available online: http://www.vegetablegrafting.org/resources/grafting-manual/ (accessed on 18 September 2019).
- Lewis, M.; Kubota, C.; Tronstad, R.; Son, Y.J. Scenario-based cost analysis for vegetable grafting nurseries of different technologies and sizes. HortScience 2014, 49, 917–930. [Google Scholar] [CrossRef]
- Dabirian, S.; Miles, C.A. A Increasing survival of splice-grafted watermelon seedlings using a sucrose application. HortScience 2017, 52, 579–583. [Google Scholar] [CrossRef]
- Devi, P.; Lukas, S.; Miles, C. Fruit maturity and quality of splice-grafted and one-cotyledon grafted watermelon. HortScience 2020, 55, 1090–1098. [Google Scholar] [CrossRef]
- Kubota, C.; McClure, M.A.; Kokalis-Burelle, N.; Bausher, M.G.; Rosskopf, E.N. Vegetable grafting: History, use and current technology status in North America. HortScience 2008, 43, 1664–1669. [Google Scholar] [CrossRef]
- Aloni, B.; Cohen, R.; Karni, L.; Aktas, H.; Edelstein, M. Hormonal signaling in rootstock-scion interactions. Sci. Hortic. 2010, 127, 119–126. [Google Scholar] [CrossRef]
- Pina, A.; Errea, P. A review of new advances in mechanism of graft compatibility–incompatibility. Sci. Hortic. 2005, 106, 1–11. [Google Scholar] [CrossRef]
- Melnyk, C.W. Monitoring vascular regeneration and xylem connectivity in Arabidopsis thaliana. Methods Mol. Biol. 2017, 1544, 91–102. [Google Scholar] [CrossRef]
- Hunter, J.J.; Volschenk, C.G.; Le Roux, D.J.; Fouché, G.W.; Adams, L. Plant Material Quality: A Compilation of Research; ARC Infruitec-Nietvoorbij: Stellenbosch, South Africa, 2004; Available online: http://www.winetech.co.za/documents/plantmaterial/plantmaterialquality.pdf (accessed on 28 September 2019).
- Ogata, T.; Kabashima, Y.; Shiozaki, S.; Horiuchi, S. Regeneration of the vascular bundle at the graft interface in auto- and heterografts with juvenile nucellar seedlings of satsuma mandarin, yuzu and trifoliate orange. J. Jpn. Soc. Hort. Sci. 2005, 74, 214–220. [Google Scholar] [CrossRef][Green Version]
- Rapaka, V.K.; Faust, J.E.; Dole, J.M.; Runkle, E.S. Diurnal carbohydrate dynamics affect postharvest ethylene responsiveness in portulaca (Portulaca grandiflora ‘Yubi Deep Rose’) unrooted cuttings. Postharvest Biol. Technol. 2007, 44, 293–299. [Google Scholar] [CrossRef]
- Asahina, M.; Iwai, H.; Kikuchi, A.; Yamaguchi, S.; Kamiya, Y.; Kamada, H.; Satoh, S. Gibberellin produced in the cotyledon is required for cell division during tissue-reunion in the cortex of cut cucumber and tomato hypocotyls. Plant Physiol. 2002, 129, 201–210. [Google Scholar] [CrossRef]
- Sabatino, L. Advances in Vegetable Grafting and New Nursery Patterns for Grafted Plant Production. Ph.D. Thesis, Università degli Studi di Palermo, Palermo, Italy, 2013. [Google Scholar]
- Guan, W.; Zhao, X. Effects of grafting methods and root excision on growth characteristics of grafted muskmelon plants. HortTechnology 2015, 25, 706–713. [Google Scholar] [CrossRef]
- Memmott, F.D. Refinement of Innovative Watermelon Grafting Methods with Appropriate Choice of Developmental Stage, Rootstock Type, and Root Treatment to Increase Grafting Success. Master’s Thesis, Clemson University, Clemson, SC, USA, 2010. [Google Scholar]
- Lee, J.M. Cultivation of grafted vegetables I. Current status, grafting methods, and benefits. HortScience 1994, 29, 235–239. [Google Scholar] [CrossRef]
- Penny, M.G.; Moore, K.G.; Lovell, P.H. The effects of inhibition of cotyledon photosynthesis on seedling development in Cucumis sativus L. Ann. Bot. 1976, 40, 815–824. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G.; Islami, E. Root pruning effects on seedlings’ growth and plant stand establishment rate of watermelon grafted seedlings. Acta Hortic. 2014, 1033, 19–24. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Q.; Sanchez, M.T.; Dufault, N.S. Performance of grafted seedless watermelon plants with and without root excision under inoculation with Fusarium oxysporum f. sp. niveum race 2. HortScience 2018, 53, 1340–1346. [Google Scholar] [CrossRef]
- Grill, E.; Ziegler, H. A plants dilemma. Science 1998, 282, 252–253. [Google Scholar] [CrossRef]
- Hetherington, A. Plant physiology: Spreading a drought warning. Curr. Biol. 1998, 8, 911–913. [Google Scholar] [CrossRef]
- Nitzsche, P.; Berkowitz, G.A.; Rabin, J. Development of a seedling applied antitranspirant formulation to enhance water status, growth, and yield of transplanted bell pepper. J. Am. Soc. Hort. Sci. 1991, 116, 405–411. [Google Scholar] [CrossRef]
- Kümpers, B.M.; Bishopp, A. Plant grafting: Making the right connections. Curr. Biol. 2015, 25, R411–R413. [Google Scholar] [CrossRef] [PubMed]
- Preece, J.E.; Read, P.E. The Biology of Horticulture; Wiley & Sons: NewYork, NY, USA, 1993; p. 480. [Google Scholar]
- Salisbury, F.B.; Ross, C.W. Plant Physiology; Wadsworth Publishing Company: Belmont, CA, USA, 1992; p. 682. [Google Scholar]
- Bhalerao, R.P.; Eklöf, J.; Ljung, K.; Marchant, A.; Bennett, M.; Sandberg, G. Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 2002, 29, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.K.; Melnyk, C.W. The role of plant hormones during grafting. J. Plant Res. 2018, 131, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Procko, C.; Crenshaw, C.M.; Ljung, K.; Noel, J.P.; Chory, J. Cotyledon-generated auxin is required for shade-induced hypocotyl growth in Brassica rapa. Plant Physiol. 2014, 165, 1285–1301. [Google Scholar] [CrossRef]
- Friml, J.; Palme, K. Polar auxin transport–old questions and new concepts? Plant Mol. Biol. 2002, 49, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, T.; Fujihara, K. Prevention of auxin-induced vascular differentiation in wound callus by surface-to-surface adhesion between calluses of stock and scion in cactus grafts. Plant Cell Physiol. 1978, 19, 877–886. [Google Scholar] [CrossRef]
- Yin, H.; Yan, B.; Sun, J.; Jia, P.; Zhang, Z.; Yan, X.; Liu, H. Graft-union development: A delicate process that involves cell–cell communication between scion and stock for local auxin accumulation. J. Exp. Bot. 2012, 63, 4219–4232. [Google Scholar] [CrossRef]
- Matsuoka, K.; Sugawara, E.; Aoki, R.; Takuma, K.; Terao-Morita, M.; Satoh, S.; Asahina, M. Differential cellular control by cotyledon-derived phytohormones involved in graft reunion of Arabidopsis hypocotyls. Plant Cell Physiol. 2016, 57, 2620–2631. [Google Scholar] [CrossRef]
- Sachs, T. On the determination of the pattern of vascular tissue in peas. Ann. Bot. 1968, 32, 781–790. [Google Scholar] [CrossRef]
- El-Eslamboly, A.S.A. Seedless Watermelon Propagation by Cuttings: Effect of Planting Containers, Cutting Types and IBA on Transplants Production from Cuttings. Bulletin of Faculty of Agriculture. 2014. Available online: https://www.researchgate.net/publication/269331198_SEEDLESS_WATERMELON_PROPAGATION_BY_CUTTINGS_A_Effect_of_planting_containers_cutting_types_and_IBA_on_transplants_production_from_cuttings (accessed on 10 March 2018).
- Katsumi, M.; Chiba, Y.; Fukuvama, M. The roles of the cotyledons and auxin in the adventitious root formation of hypocotyl cuttings of light-grown cucumber seedlings. Physiol. Plant. 1969, 22, 993–1000. [Google Scholar] [CrossRef]
- Dunn, B.L.; Cole, J.C.; Payton, M.E. Use of antitranspirants to reduce water stress on herbaceous and woody ornamentals. J. Environ. Hortic. 2012, 30, 137–145. [Google Scholar] [CrossRef]
- Johnson, G. Can Antitranspirants and Antidesiccants Improve Vegetable Transplant Survival? Weekly Crop Update, University of Delaware Cooperative Extension. Available online: https://agdev.anr.udel.edu/weeklycropupdate/?p=3942 (accessed on 15 March 2019).
- Miles, C.; Hesnault, L.; Johnson, S.; Kreider, P.; Dabirian, S. Vegetable Grafting: Watermelon. Wash. State Univ. Ext. Pub. FS100E. Available online: https://s3.wp.wsu.edu/uploads/sites/2071/2014/04/Grafting-Watermelon-FS100E.pdf (accessed on 20 March 2018).
- Dabirian, S.; Miles, C.A. Antitranspirant application increases grafting success of watermelon. HortTechnology 2017, 52, 579–583. [Google Scholar] [CrossRef]
- Borel, C.; Frey, A.; Marion-Poll, A.; Tardieu, F.; Simonneau, T. Does engineering abscisic acid biosynthesis in Nicotiana plumbaginifolia modify stomatal response to drought? Plant Cell Environ. 2001, 24, 477–489. [Google Scholar] [CrossRef]
- Niu, M.; Huang, Y.; Sun, S.; Sun, J.; Cao, H.; Shabala, S.; Bie, Z. Root respiratory burst oxidase homologue-dependent H2O2 production confers salt tolerance on a grafted cucumber by controlling Na+ exclusion and stomatal closure. J. Exp. Bot. 2018, 69, 3465–3476. [Google Scholar] [CrossRef] [PubMed]
- Aloni, B.; Karni, L.; Deventurero, G.; Cohen, R.; Katzir, N.; Edelstein, M.; Aktas, H. The use of plant grafting and plant growth regulators for enhancing abiotic stress tolerance in vegetable transplants. Acta. Hortic. 2011, 898, 255–264. [Google Scholar] [CrossRef]
- Růžička, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed]
- Van Breusegem, F.; Dat, J.F. Reactive oxygen species in plant cell death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef]


| Treatment Product x | Product and Manufacturer |
|---|---|
| Sucrose 2% (w/v) | IB37160 Sucrose; IBI Scientific, Peosta, IA, USA |
| Antitranspirant A 2% (v/v) | Root-Drench; Zorro Technology Inc., Clackamas, OR, USA |
| Antitranspirant B 2% (v/v) | Glycerin 99.7% USP/BP grade; Deepthi Organics LLC, Greensboro, NC, USA |
| Auxin | Indole-3-butyric acid (IBA), 98%, Alfa Aesar™; Fisher Scientific, Waltham, MA, USA |
| Tap water |
| Survival (%) | ||||
|---|---|---|---|---|
| Treatment z | 4 DAG | 9 DAG | 16 DAG | 21 DAG |
| Sucrose | 99 (1.02) y | 88 (2.18) | 63 b x (4.82) | 60 b (4.13) |
| Sucrose + antitranspirant A | 100 (0.00) | 98 (1.05) | 92 a (1.65) | 90 a (1.87) |
| Sucrose + antitranspirant B | 99 (1.02) | 92 (1.62) | 80 a (2.34) | 75 a (3.52) |
| Sucrose + 10 mg·L−1 auxin | 100 (0.00) | 93 (1.71) | 85 a (2.07) | 80 a (2.30) |
| Sucrose + 20 mg·L−1 auxin | 100 (0.00) | 89 (2.15) | 72 ab (3.22) | 70 ab (3.77) |
| Sucrose + antitranspirant A+ 10 mg·L−1 auxin | 99 (1.02) | 92 (1.62) | 90 a (1.87) | 88 a (2.08) |
| Sucrose + antitranspirant A+ 20 mg·L−1 auxin | 100 (0.00) | 90 (1.82) | 70 ab (3.47) | 61 b (4.01) |
| Sucrose + antitranspirant B+ 10 mg·L−1 auxin | 99 (1.02) | 85 (2.32) | 71 ab (3.57) | 58 b (5.30) |
| Sucrose + antitranspirant B+ 20 mg·L−1 auxin | 99 (1.02) | 88 (2.18) | 65 b (4.71) | 48 bc (5.92) |
| Tap water (control) | 98 (1.23) | 70 (3.67) | 40 c (5.12) | 18 c (8.17) |
| p-value | 0.68 | 0.07 | 0.003 | <0.0001 |
| Survival (%) | ||||
|---|---|---|---|---|
| Treatment z | 4 DAG | 9 DAG | 16 DAG | 21 DAG |
| Root-intact | 99 (1.07) y | 86 (1.88) | 73 (2.87) | 65 a x (3.75) |
| Root-excised | 99 (1.07) | 80 (1.71) | 68 (2.56) | 58 b (4.54) |
| p-value | 0.24 | 0.29 | 0.17 | 0.02 |
| Sucrose + antitranspirant A | 99 (1.02) | 91 (1.67) | 89 a (2.14) | 87 a (2.53) |
| Sucrose + auxin 10 mg·L−1 | 98 (1.22) | 85 (2.37) | 69 b (4.33) | 59 b (5.43) |
| Sucrose + antitranspirant A+ auxin 10 mg·L−1 | 98 (1.22) | 92 (1.59) | 89 a (2.14) | 86 a (2.71) |
| Tap water (control) | 98 (1.22) | 64 (3.07) | 37 c (6.89) | 14 c (8.32) |
| p-value | 0.09 | 0.06 | 0.002 | 0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devi, P.; DeVetter, L.W.; Lukas, S.; Miles, C. Exogenous Treatments to Enhance Splice-Grafted Watermelon Survival. Horticulturae 2021, 7, 197. https://doi.org/10.3390/horticulturae7070197
Devi P, DeVetter LW, Lukas S, Miles C. Exogenous Treatments to Enhance Splice-Grafted Watermelon Survival. Horticulturae. 2021; 7(7):197. https://doi.org/10.3390/horticulturae7070197
Chicago/Turabian StyleDevi, Pinki, Lisa Wasko DeVetter, Scott Lukas, and Carol Miles. 2021. "Exogenous Treatments to Enhance Splice-Grafted Watermelon Survival" Horticulturae 7, no. 7: 197. https://doi.org/10.3390/horticulturae7070197
APA StyleDevi, P., DeVetter, L. W., Lukas, S., & Miles, C. (2021). Exogenous Treatments to Enhance Splice-Grafted Watermelon Survival. Horticulturae, 7(7), 197. https://doi.org/10.3390/horticulturae7070197







