Effects of Soil Aeration and Fertilization Practices on Alleviating Iron Deficiency Chlorosis in “Huangguan” Pears Grafted onto Quince A in Calcareous Soils
Abstract
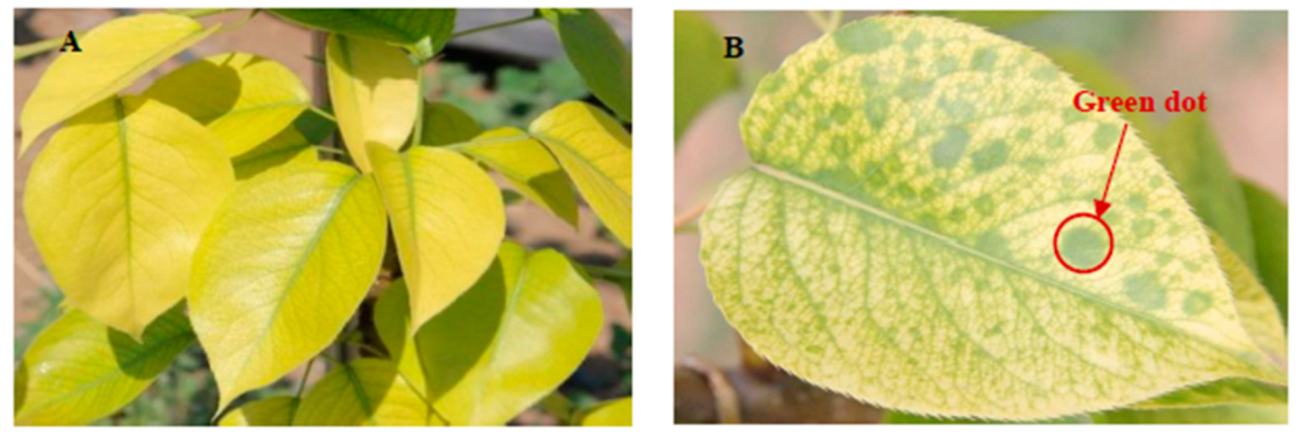
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Conditions
2.2. Experimental Implementation
2.3. Leaf Soil Plant Analysis Development (SPAD) Value
2.4. Rhizosphere pH, Soil Active Fe Concentration, and Soil Moisture
2.5. Root FCR Activity
2.6. Leaf Fe Concentration
2.7. Statistical Data Analysis
3. Results
3.1. Leaf SPAD Values
3.2. Soil Active Fe Concentrations and Soil Moisture
3.3. Root FCR Activities and Rhizosphere pH
3.4. Leaf Fe Concentrations and the Ratio of SPAD to the Leaf Fe Concentration (RSFe)
4. Discussion
4.1. Improving the Leaf Fe Concentration
4.2. Fe Activation in Leaves
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Fe | Iron |
| SPAD | Soil plant analysis development |
| FCR | Ferric-chelate reductase |
| FDR | False discovery rate |
| HG-QA | “Huangguan” grafted onto quince A with Hardy as an interstock |
| FM | Flattening with landscape fabric mulching |
| R | Ridging without landscape fabric mulching |
| F | Flattening without landscape fabric mulching |
| M | Manure application |
| SFe | Fe application in soil |
| FFe | Foliar Fe application |
| RSFe | The ratio of SPAD to the leaf Fe concentration |
References
- Briat, J.F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Thomine, S.; Vert, G. Iron transport in plants: Better be safe than sorry. Curr. Opin. Plant Biol. 2013, 16, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Bavaresco, L.; Giachino, E.; Pezzutto, S. Grapevine rootstock effects on lime-induced chlorosis, nutrient uptake, and source-sink relationships. J. Plant Nutr. 2003, 7, 1451–1465. [Google Scholar] [CrossRef]
- Mengel, K.; Planker, R.; Hoffmann, B. Relationship between leaf apoplast pH and iron chlorosis of sunflower (Helianthus annuus L.). J. Plant Nutr. 1994, 17, 1053–1065. [Google Scholar] [CrossRef]
- Zribi, K.; Gharsalli, M. Effect of bicarbonate on growth and iron nutrition of pea. J. Plant Nutr. 2002, 25, 2143–2149. [Google Scholar] [CrossRef]
- Kosegarten, H.; Englisch, G. Effect of various nitrogen forms on the pH in leaf apoplast and iron chlorosis of Glycine max L. Z. Planzenernaehr. Bodenk. 1994, 157, 401–405. [Google Scholar] [CrossRef]
- Mengel, K. Iron availability in plant tissues-iron chlorosis in calcareous soils. Plant Soil 1994, 165, 275–283. [Google Scholar] [CrossRef]
- Tagliavini, M.; Scudellari, D.; Marangoni, B.; Toselli, M. Acid-spray regreening of kiwifruit leaves affected by lime-induced iron chlorosis. In Iron Nutrition in Soils and Plants. Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 1995; Volume 59. [Google Scholar]
- Zhao, Y.; Sun, M.; Liang, Z.; Li, H.; Yu, F.; Liu, S. Analysis of contrast iron chlorosis tolerance in the pear cv. ‘Huangguan’ grafted onto pyrus betulifolia and quince A grown in calcareous soils. Sci. Hortic. 2020, 271, 109488. [Google Scholar] [CrossRef]
- FAOSTAT. 2020. Available online: http://www.fao.org/faostat/zh/#data (accessed on 1 January 2021).
- Jiang, S.; Ou, Q.; Wang, F.; Zhang, Y.; Ma, L.; Zhao, Y. Development and application of pear dwarf stock in China. North. Fruits 2018, 2, 1–3. (In Chinese) [Google Scholar]
- Prado, R.M.; Alcántara-Vara, E. Tolerance to iron chlorosis in non-grafted quince seedlings and in pear grafted onto quince plants. J. Soil Sci. Plant Nutr. 2011, 11, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, P.R.; Meza, B.I.C.; Requena, F.R.G.; Flores, G.M.; Barra, J.D.E. Evaluation of different iron compounds in chlorotic Italian lemon trees (Citrus lemon). Plant Physiol. Bioch. 2007, 45, 330–334. [Google Scholar] [CrossRef]
- Lucena, J.J. Fe chelates for remediation of Fe chlorosis in strategy I plants. J. Plant Nutr. 2003, 26, 1969–1984. [Google Scholar] [CrossRef]
- Fernández, V.; Río, V.D.; Pumariño, L.; Igartua, E.; Abadía, J.; Abadía, A. Foliar fertilization of peach (Prunus persica (L.) Batsch) with different iron formulations: Effects on re-greening, iron concentration and mineral composition in treated and untreated leaf surfaces. Sci. Hortic. 2008, 117, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Fernández, V.; Ebert, G. Foliar iron fertilization: A critical review. J. Plant Nutr. 2005, 28, 2113–2124. [Google Scholar] [CrossRef] [Green Version]
- Fernández, V.; Ebert, G.; Winkelmann, G. The use of microbial siderophores for foliar iron application studies. Plant Soil 2005, 272, 245–252. [Google Scholar] [CrossRef]
- García-Laviña, P.; Álvarez-Fernández, A.; Abadía, J.; Abadía, A. Foliar applications of acids with and without FeSO4 to control iron chlorosis in pear. Acta Hort. 2002, 594, 217–222. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, X.; Zhao, Z.; Cai, K.; Wang, Z. Releasing of N, P and K of organic fertilizers and their effects on the contents of available Cu, Zn, Fe and Mn in soil. Chin. J. Eco Agric. 2007, 15, 47–50. (In Chinese) [Google Scholar]
- Liu, Y.; Yang, S.; Li, S.; Chen, X.; Chen, F. Growth and development of maize (Zea mays L.) in response to different field water management practices: Resource capture and use efficiency. Agric. For. Meteorol. 2010, 150, 606–613. [Google Scholar]
- Shen, P.; Wang, C.; Yu, T.; Wu, Z.; Zheng, Y.; Sun, X.; Zheng, Y.; Sun, X.; Luo, S. Differences in iron nutrition characteristics under no tillage and conventional tillage systems in typical brown soils of main peanut producing areas. J. Nucl. Agric. Sci. 2017, 31, 1818–1826. (In Chinese) [Google Scholar]
- Raese, J.T.; Parish, C.L.; Staiff, D.C. Nutrition of apple and pear trees with foliar sprays, trunk injections or soil application of iron compounds. J. Plant Nutr. 1986, 9, 987–999. [Google Scholar] [CrossRef]
- Mo, F.; Li, X.; Niu, F.; Zhang, C.; Li, S.; Zhang, L.; Xiong, Y. Alternating small and large ridges with full film mulching increase linseed (Linum usitatissimum L.) productivity and economic benefit in a rainfed semiarid environment. Field Crop. Res. 2018, 219, 120–130. [Google Scholar]
- Zhang, P.; Wei, T.; Han, Q.; Ren, X.; Jia, Z. Effects of different film mulching methods on soil water productivity and maize yield in a semiarid area of China. Agric. Water Manag. 2020, 241, 106382. (In Chinese) [Google Scholar] [CrossRef]
- Liu, M.; Chai, Z.; Sheng, J.; Ding, K.; He, C. Effect of organic fertilizer on the elements of leaves and the yield, quality of Korla fragrant pear. North Hortic. 2014, 10, 159–163. (In Chinese) [Google Scholar] [CrossRef]
- Martínez-Cuenca, M.R.; Legaz, F.; Forner-Giner, M.Á.; Primo-Millo, E.; Iglesias, D.J. Bicarbonate blocks iron translocation from cotyledons inducing iron stress responses in Citrus roots. J. Plant Physiol. 2013, 170, 899–905. [Google Scholar] [CrossRef]
- Zheng, S.; Tang, C.; Arakawa, Y.; Masaoka, Y. The responses of red clover (Trifolium pratense L.) to iron deficiency: A root Fe(III) chelate reductase. Plant Sci. 2013, 164, 679–687. [Google Scholar] [CrossRef]
- Lucena, J.J.; Hernandez-Apaolaza, L. Iron nutrition in plants: An overview. Plant. Soil 2017, 418, 1–4. [Google Scholar] [CrossRef]
- Mortvedt, J.J.; Stevenson, F.J. Organic Matter-Micronutrient Reactions in Soil; SSSA Book Series; Soil Science Society of America Inc.: Madison, WI, USA, 1991. [Google Scholar]
- Tang, J.; Xu, J.; Wen, Y.; Tian, C.; Lin, Z.; Zhao, B. Effects of organic fertilizer and inorganic fertilizer on the wheat yields and soil nutrients under long-term fertilization. J. Plant Nutr. Fertil. 2019, 25, 1827–1834. (In Chinese) [Google Scholar]
- Manna, M.C.; Swarup, A.; Wanjari, R.H.; Mishra, B.; Shahi, D.K. Long-term fertilization, manure and liming effects on soil organic matter and crop yield. Soil Till. Res. 2007, 94, 397–409. [Google Scholar] [CrossRef]
- Masunaga, T.; Marques-Fong, J.D. Strategies for increasing micronutrient availability in soil for plant uptake. In Plant Micronutrient Use Efficiency; Elsevier/Academic Press: San Diego, CA, USA, 2018; pp. 195–208. [Google Scholar]
- Zhang, X.; Zhang, D.; Sun, W.; Wang, T. The adaptive mechanism of plants to iron deficiency via iron uptake, transport, and homeostasis. Int. J. Mol. Sci. 2019, 20, 2424. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Peng, Z.; Wu, X.; Wu, X.; Yu, J.; Liu, H.; Yang, L.; Zhang, Z. Effects of applying amino acids on root active of Chinese cabbage and soil biological activity. Chin. Agric. Sci. Bull. 2014, 30, 108–194. (In Chinese) [Google Scholar]
- Jolley, V.D.; Cook, K.A.; Hansen, N.C.; Stevens, W.B. Plant physiological responses for genotypic evaluation of iron efficiency in strategy I and strategy II plants-a review. J. Plant Nutr. 1996, 19, 1241–1255. [Google Scholar] [CrossRef]
- Garnica, M.; Bacaicoa, E.; Mora, V.; Francisco, S.S.; Baigorri, R.; Zamarreño, A.M.; Garcia-Mina, J.M. Shoot iron status and auxin are involved in iron deficiency-induced phytosiderophores release in wheat. BMC Plant Biol. 2018, 18, 1471–2229. [Google Scholar] [CrossRef]
- Yuan, Y. FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res. 2008, 18, 385–402. [Google Scholar] [CrossRef]
- En-Jung, H.; Waters, B.M. Alkaline stress and iron deficiency regulate iron uptake and riboflavin synthesis gene expression differently in root and leaf tissue: Implications for iron deficiency chlorosis. J. Exp. Bot. 2016, 67, 5671–5685. [Google Scholar]
- Romera, F.J.; Alcantara, E.; Guardia, M.D. Effects of bicarbonate, phosphate and high pH on the reducing capacity of Fe-deficient sunflower and cucumber plants. J. Plant Nutr. 1992, 15, 1519–1530. [Google Scholar] [CrossRef]
- White, P.F.; Robson, A.D. Response of lupins (Lupinus angustifolius L.) and peas (Pisum sativum L.) to Fe deficiency induced by low concentrations of Fe in solution or by addition of HCO3−. Plant Soil 1990, 125, 39–47. [Google Scholar] [CrossRef]
- Fleming, A.L.; Chane, R.L.; Coulombe, B.A. Bicarbonate inhibits Fe stress response and Fe uptake translocation of chlorosis susceptible soybeans cultivars. J. Plant Nutr. 1984, 7, 699–714. [Google Scholar] [CrossRef]
- Deal, G.M.; Alcantara, E. Bicarbonate and low iron level increase root to total plant weight ratio in olive and peach rootstock. J. Plant Nutr. 2002, 25, 1021–1032. [Google Scholar]
- Gharsalli, M.; Zribi, K.; Hajji, M. Physiological responses of pea to iron deficiency induced by bicarbonate. In Plant Nutrition-Food Security and Sustainability of Agro-Ecosystems; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 606–607. [Google Scholar]
- Zuo, Y.; Ren, L.; Zhang, F.; Jiang, R.F. Bicarbonate concentration as affected by soil water content controls iron nutrition of peanut plants in a calcareous soil. Plant Physiol. Bioch. 2007, 45, 357–364. [Google Scholar] [CrossRef]
- Lucena, J.J. Effects of bicarbonate, nitrate and other environmental factors on iron deficiency chlorosis: A review. J. Plant Nutr. 2000, 23, 1591–1606. [Google Scholar] [CrossRef]
- Morales, F.; Grasa, R.; Abadía, A.; Abadía, J. Iron chlorosis paradox in fruit trees. J. Plant Nutr. 1998, 21, 815–825. [Google Scholar] [CrossRef]
- Abadía, J.; Vázquez, S.; Rellán-Álvarez, R.; El-Jendoubi, H.; Abadía, A.; Álvarez-Fernández, A.; López-Millán, A.F. Towards a knowledge-based correction of iron chlorosis. Plant. Physiol. Bioch. 2011, 49, 471–482. [Google Scholar] [CrossRef]
- Martínez-Cuenca, M.R.; Primo-Capella, A.; Quiñones, A.; Bermejo, A.; Forner-Giner, M.A. Rootstock influence on iron uptake responses in citrus leaves and their regulation under the Fe paradox effect. PeerJ 2017, 5, 3553. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Clode, P.L.; Lambers, H. Effects of pH and bicarbonate on the nutrient status and growth of three Lupinus species. Plant Soil 2020, 447, 9–28. [Google Scholar] [CrossRef]
- Jassim, M.A.A.; Sukri, H.S.B. Effect of iron and sulfur on leaves nutrient concentrations of dixired peach trees. Int. J. Plant Res. 2020, 10, 27–32. [Google Scholar]
- Bityutskii, N.P.; Yakkonen, K.L.; Lukina, K.A.; Semenov, K.N. Fullerenol increases effectiveness of foliar iron fertilization in iron-deficient cucumber. PLoS ONE 2020, 15, e0232765. [Google Scholar] [CrossRef]
- Adhikari, R.; Bristow, K.L.; Casey, P.S.; Freischmidt, G.; Hornbuckle, J.W.; Adhikari, B. Preformed and sprayable polymeric mulch film to improve agricultural water use efficiency. Agric. Water Manag. 2016, 169, 1–13. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Li, H.; Sun, M.; Liang, Z.; Yu, F.; Li, F.; Liu, S. Effects of Soil Aeration and Fertilization Practices on Alleviating Iron Deficiency Chlorosis in “Huangguan” Pears Grafted onto Quince A in Calcareous Soils. Horticulturae 2021, 7, 172. https://doi.org/10.3390/horticulturae7070172
Zhao Y, Li H, Sun M, Liang Z, Yu F, Li F, Liu S. Effects of Soil Aeration and Fertilization Practices on Alleviating Iron Deficiency Chlorosis in “Huangguan” Pears Grafted onto Quince A in Calcareous Soils. Horticulturae. 2021; 7(7):172. https://doi.org/10.3390/horticulturae7070172
Chicago/Turabian StyleZhao, Yanyan, Haigang Li, Mingde Sun, Zhenxu Liang, Futong Yu, Fei Li, and Songzhong Liu. 2021. "Effects of Soil Aeration and Fertilization Practices on Alleviating Iron Deficiency Chlorosis in “Huangguan” Pears Grafted onto Quince A in Calcareous Soils" Horticulturae 7, no. 7: 172. https://doi.org/10.3390/horticulturae7070172
APA StyleZhao, Y., Li, H., Sun, M., Liang, Z., Yu, F., Li, F., & Liu, S. (2021). Effects of Soil Aeration and Fertilization Practices on Alleviating Iron Deficiency Chlorosis in “Huangguan” Pears Grafted onto Quince A in Calcareous Soils. Horticulturae, 7(7), 172. https://doi.org/10.3390/horticulturae7070172




