Development of Micropropagation in Bigleaf Maple (Acer macrophyllum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.1.1. Shoot Explants from Sprouts of Field-Grown Trees
2.1.2. Shoot Explants from 1-Year-Old Greenhouse Grown Plants
2.2. Surface Sterilization
2.3. Shoot Initiation Media Preparation and Culture Condition
2.4. Shoot Multiplication
2.5. In Vitro Rooting
2.6. Ex Vitro Acclimatization
2.7. Statistical Design and Analysis
3. Results
3.1. Surface Sterilization
3.2. Shoot Growth Induction
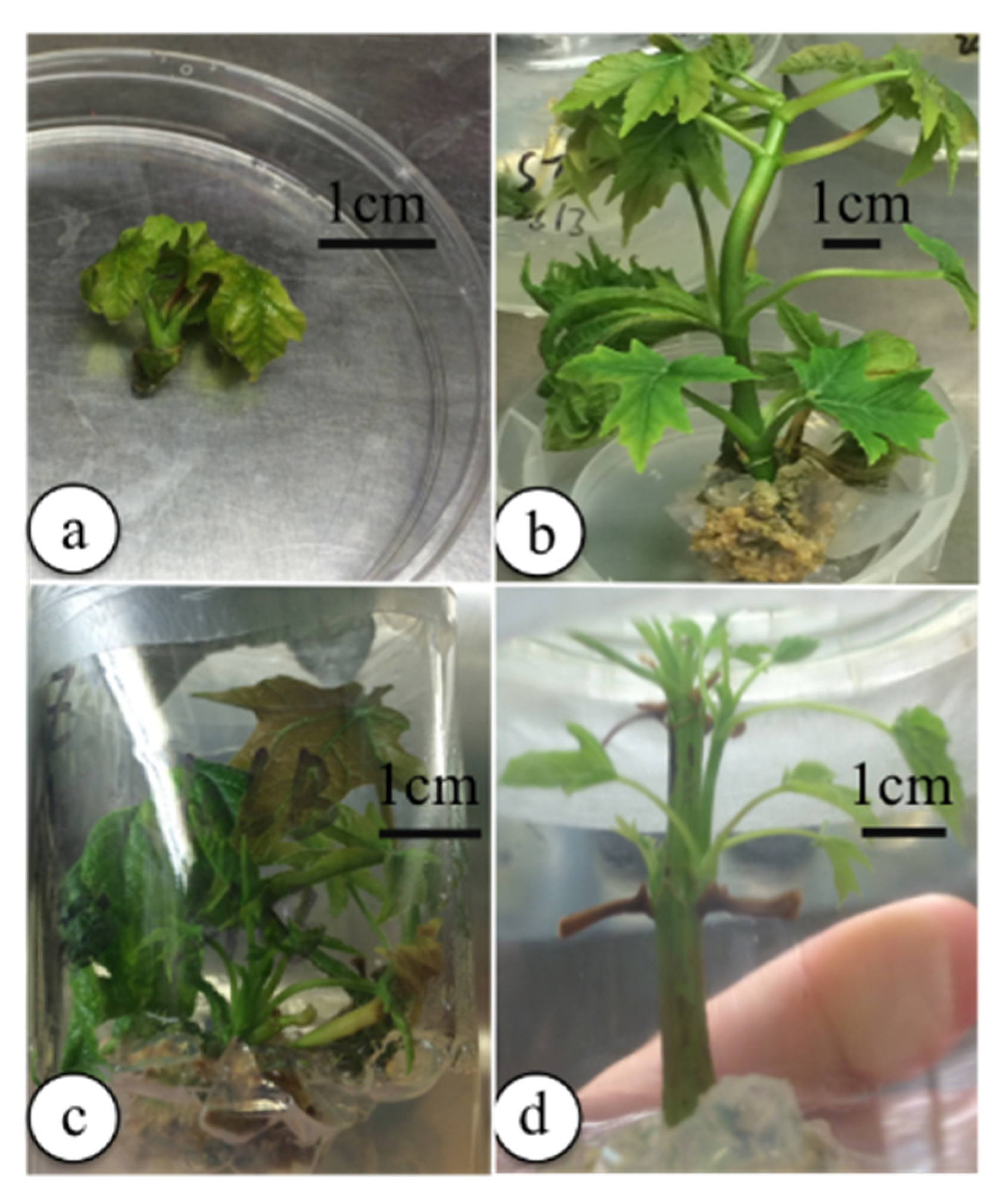
3.2.1. Preliminary Tests
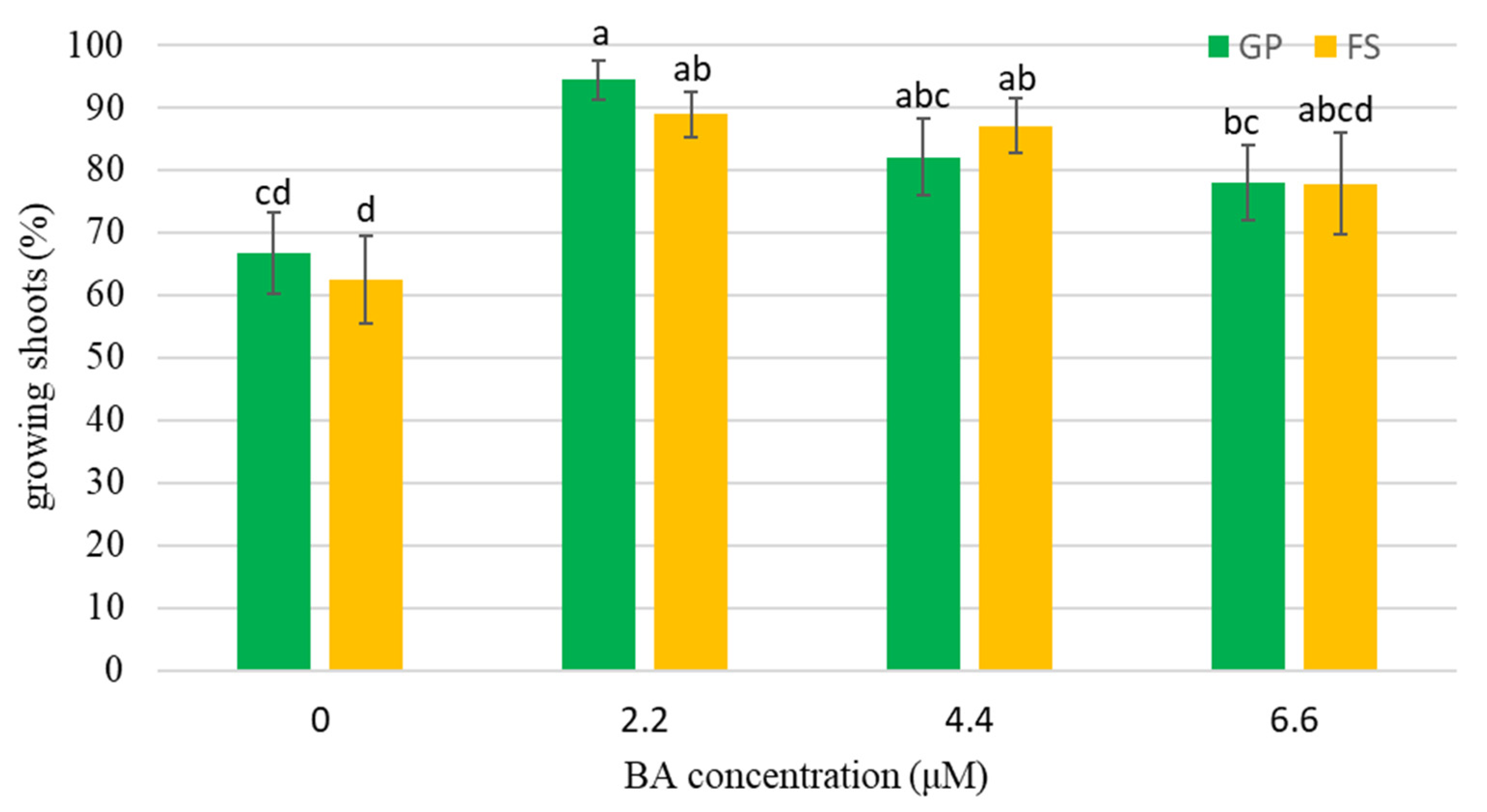
3.2.2. Basal Media and BA Concentrations Experiments
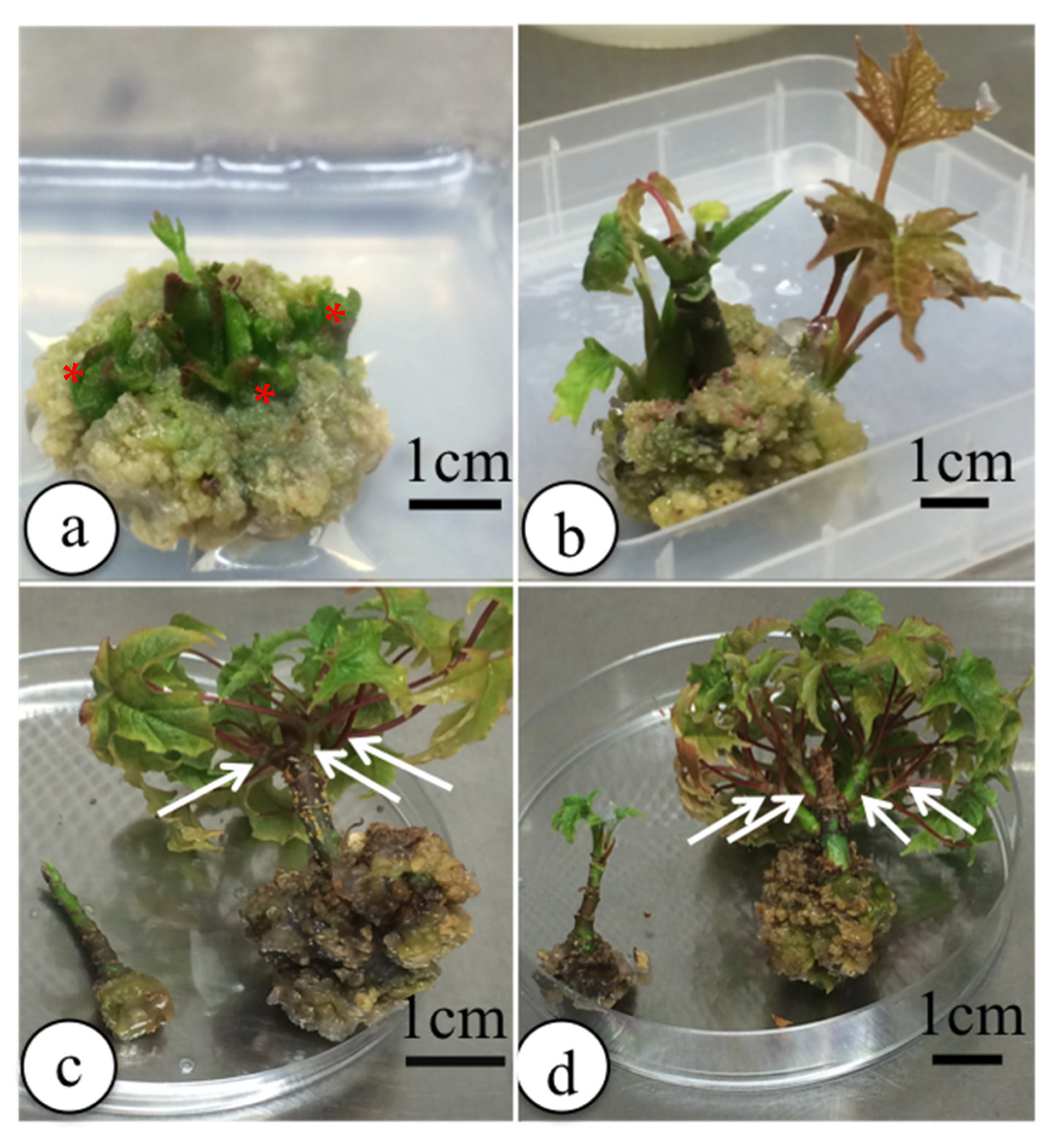
3.2.3. Shoot Proliferation
Initial Exploratory Experiments
Hormone Combination Screen on Shoots without Apices
- (A)
- Effect of BA, BA+NAA and TDZ+NAA on shoots from greenhouse plants after decapitation
- (B)
- Effect of TDZ on shoots from greenhouse plants after decapitation
- (C)
- Effect of TDZ on shoots from field sprouts after decapitation
3.2.4. Rooting of Shoots
3.2.5. Acclimatization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Iddrisu, M.N.; Ritland, K. Genetic variation, population structure, and mating system in bigleaf maple (Acer macrophyllum Pursh). Can. J. Bot. 2004, 82, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Peterson, E. Bigleaf Maple Managers’ Handbook for British Columbia; Technical Report; British Columbia, Ministry of Forests Research Program: Terrace, BC, Canada, 1999.
- Panshin, A.J.; De Zeeuw, C. Textbook of Wood Technology; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Beals, H.O.; Davis, T.C. Figure in Wood: An Illustrated Review; Auburn University: Auburn, AL, USA, 1977. [Google Scholar]
- Rieder, A. Ahorn-Wertholzproduktion in kurzen Umtrieben. AFZ Der Wald 1998, 15, 776–779. [Google Scholar]
- Krajnc, L.; Čufar, K.; Brus, R. Characteristics and Geographical Distribution of Fiddleback Figure in Wood of Acer pseudoplatanus L. in Slovenia. Drv. Ind. 2015, 66, 213–220. [Google Scholar] [CrossRef]
- Kobal, M.; Kristan, S.; Grudnik, P.; Vilhar, U. Supply and demand at auctions of value wood assortments in Slovenj Gradec. Gozdarski Vestn. 2013, 71, 462–470. [Google Scholar]
- Fan, Y.; Rupert, K.; Wiedenhoeft, A.C.; Woeste, K.; Lexer, C.; Meilan, R. Figured grain in aspen is heritable and not affected by graft-transmissible signals. Trees Struct. Funct. 2013, 27, 973–983. [Google Scholar] [CrossRef] [Green Version]
- Persson, A. Seed orchard production of figured birch. Skogen 1954, 41, 160–163. [Google Scholar]
- Václav, E. The quality of wood of the technical forms of birch. In Proceedings of the Second World Consultation on Forest Tree Breeding, Washington, DC, USA, 7–16 August 1969; FAO: Rome, Italy, 1970; Volume 1, pp. 437–446. [Google Scholar]
- Kärkkäinen, K.; Viherä-Aarnio, A.; Vakkari, P.; Hagqvist, R.; Nieminen, K. Simple inheritance of a complex trait: Figured wood in curly birch is caused by one semi-dominant and lethal Mendelian factor? Can. J. For. Res. 2017, 47, 991–995. [Google Scholar] [CrossRef] [Green Version]
- Ewald, D.; Naujoks, G. Vegetative propagation of wavy grain Acer pseudoplatanus and confirmation of wavy grain in wood of vegetatively propagated trees: A first evaluation. Dendrobiology 2015, 74, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Rohr, R.; Hanus, D. Vegetative propagation of wavy grain sycamore maple. Can. J. For. Res. 1987, 17, 418–420. [Google Scholar] [CrossRef]
- Harrington, C.A.; McGrath, J.M.; Kraft, J.M. Propagating Native Species: Experience at the Wind River Nursery. West. J. Appl. For. 1999, 14, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Ďurkovič, J.; Mišalová, A. Micropropagation of temperate noble hardwoods: An overview. Funct. Plant Sci. Biotechnol. 2008, 2, 1–9. [Google Scholar]
- Lattier, J.D.; Touchell, D.H.; Ranney, T.G. Micropropagation of Acer platanoides L. “Crimson Sentry.” SNA Res. Conf. 2012, 57, 296–300. [Google Scholar]
- Wilhelm, E. Micropropagation of juvenile sycamore maple via adventitious shoot formation by use of thidiazuron. Plant Cell Tissue Organ Cult. 1999, 57, 57–60. [Google Scholar] [CrossRef]
- Wann, S.R.; Gates, E.E. Micropropagation of mature red maple (Acer rubrum L.). In Proceedings of the Southern Forest Tree Improvement Conference, Atlanta, Georgia, 14–17 June 1993; pp. 99–105. [Google Scholar]
- Ďurkovič, J. In vitro regeneration of Norway maple (Acer platanoides L.). Biol. Plant. 1996, 38, 303–307. [Google Scholar] [CrossRef]
- Kerns, H.R.; Meyer, M.M., Jr. Diligence finds the chemical key to micropropagating a new maple. Am. Nurserym. 1987, 165, 104–105. [Google Scholar]
- Preece, J.E.; Huetteman, C.A.; Ashby, W.C.; Roth, P.L. Micro-and Cutting Propagation of Silver Maple. I. Results with Adult and Juvenile Propagules. J. Am. Soc. Hortic. Sci. 1991, 116, 142–148. [Google Scholar] [CrossRef]
- Driver, J.; Kuniyuki, A. In vitro propagation of Paradox walnut rootstock. HortScience 1984, 19, 507–509. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Int. Plant Propagators’ Soc. Proc. 1980, 30, 421–427. [Google Scholar]
- Dou, Y. The Tissue Culture of Two Maple and Preliminary Research on Prevention of Tissue Pollution. Master’s Thesis, Liaoning Normal University, Dalian, China, 2010. [Google Scholar]
- Bowen-O’Connor, C.A.; Hubstenberger, J.; Killough, C.; VanLeeuwen, D.M.; St. Hilaire, R. In vitro propagation of Acer grandidentatum Nutt. In Vitro Cell. Dev. Biol. Plant 2007, 43, 40–50. [Google Scholar] [CrossRef]
- Ďurkovič, J. Regeneration of Acer caudatifolium Hayata plantlets from juvenile explants. Plant Cell Rep. 2003, 21, 1060–1064. [Google Scholar] [CrossRef]
- Marks, T.R.; Simpson, S.E. Factors affecting shoot development in apically dominant Acer cultivars in vitro. J. Hortic. Sci. 1994, 69, 543–551. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. Plant growth regulators I: Introduction; auxins, their analogues and inhibitors. In Plant Propagation by Tissue Culture; Springer: Heidelberg/Berlin, Germany, 2008; pp. 175–204. [Google Scholar]
- Bhojwani, S.S.; Dantu, P.K. Plant Tissue Culture: An Introductory Text; Springer: New Delhi, India, 2013. [Google Scholar]
- Chaturvedi, R.; Razdan, M.K.; Bhojwani, S.S. An efficient protocol for the production of triploid plants from endosperm callus of neem, Azadirachta indica A. Juss. J. Plant Physiol. 2003, 160, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Dun, E.A.; Ferguson, B.J.; Beveridge, C.A. Apical dominance and shoot branching. Divergent opinions or divergent mechanisms? Plant Physiol. 2006, 142, 812–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cline, M.G. Concepts and terminology of apical dominance. Am. J. Bot. 1997, 84, 1064–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A.S. Plant Physiology and Development; Sinauer Associates: Sunderland, MA, USA, 2015. [Google Scholar]
- Savidge, R.A. Tree growth and wood quality. Wood Qual. Biol. Basis 2003, 240, 1–29. [Google Scholar]
- Kristiansen, K. Micropropagation of Ficus benjamina clones. Plant Cell Tissue Organ Cult. 1992, 28, 53–58. [Google Scholar] [CrossRef]
- Grant, N.J.; Hammatt, N. Increased root and shoot production during micropropagation of cherry and apple rootstocks: Effect of subculture frequency. Tree Physiol. 1999, 19, 899–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, R.A.; Mantell, S.H. Effects of Stage II subculture durations on the multiplication rate and rooting capacity of micropropagated shoots of tree paeony (Paeonia suffruticosa Andr.). J. Hortic. Sci. 1991, 66, 95–102. [Google Scholar] [CrossRef]
- Huetteman, C.A.; Preece, J.E. Thidiazuron: A potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult. 1993, 33, 105–119. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Nurmansyah; Naidoo, Y.; Teixeira da Silva, J.A. Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Rep. 2018, 37, 1451–1470. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.L.; Srinivasan, C.; Lomberk, D. Effect of nutrient media on axillary shoot proliferation and preconditioning for adventitious shoot regeneration of pears. In Vitro Cell. Dev. Biol. Plant 2009, 45, 708–714. [Google Scholar] [CrossRef]


| BA (μM) | NAA (μM) | TDZ (μM) | No. Expl. | Resp. (%) | No. Shoots | Axillary Shoot Length (cm) | Nodes per Axillary Shoot |
|---|---|---|---|---|---|---|---|
| 0.44 | - | - | 31 | 39 | 1.1 ± 0.2f | 0.67 ± 0.08g | 1.3 ± 0.1g |
| 1.33 | - | - | 31 | 52 | 1.7 ± 0.1cd | 1.11 ± 0.07cde | 2.1 ± 0.1cde |
| 2.22 | - | - | 28 | 61 | 2.3 ± 0.1ab | 1.38 ± 0.13ab | 2.5 ± 0.1ab |
| 4.44 | - | - | 32 | 41 | 1.3 ± 0.1ef | 0.80 ± 0.06fg | 1.7 ± 0.1fg |
| 1.33 | 0.054 | - | 34 | 59 | 1.8 ± 0.1c | 1.03 ± 0.06def | 2.2 ± 0.1cd |
| 1.33 | 0.27 | - | 36 | 64 | 2.2 ± 0.1ab | 1.29 ± 0.06abc | 2.6 ± 0.1a |
| 1.33 | 0.54 | - | 36 | 47 | 1.4 ± 0.1e | 0.81 ± 0.06fg | 1.8 ± 0.1efg |
| - | 0.27 | 0.01 | 32 | 47 | 1.5 ± 0.1de | 0.88 ± 0.09efg | 1.8 ± 0.1def |
| - | 0.27 | 0.05 | 33 | 58 | 2.1 ± 0.1bc | 1.19 ± 0.08bcd | 2.2 ± 0.2bc |
| - | 0.27 | 0.1 | 35 | 69 | 2.4 ± 0.1a | 1.40 ± 0.06a | 2.9 ± 0.1a |
| TDZ (µM) | No. Expl. | Resp. (%) | No. Axillary Shoots | Axillary Shoot Length (cm) | Nodes per Axillary Shoot |
|---|---|---|---|---|---|
| Greenhouse explants, round 1 | |||||
| 0.01 | 32 | 69 | 1.9 ± 0.2c | 1.06 ± 0.07c | 2.2 ± 0.2c |
| 0.05 | 36 | 67 | 2.6 ± 0.3b | 1.33 ± 0.06b | 2.7 ± 0.1b |
| 0.1 | 43 | 84 | 3.2 ± 0.2a | 1.81 ± 0.06a | 3.7 ± 0.1a |
| 1 | 26 | 39 | 1.4 ± 0.2d | 0.60 ± 0.05d | 1.7 ± 0.2d |
| Greenhouse explants, round 2 | |||||
| 0.01 | 40 | 63 | 2.4 ± 0.2c | 1.45 ± 0.08c | 3.1 ± 0.1c |
| 0.05 | 36 | 64 | 3.0 ± 0.2b | 1.66 ± 0.07b | 3.7 ± 0.1b |
| 0.1 | 43 | 79 | 3.5 ± 0.2a | 2.21 ± 0.06a | 4.8 ± 0.1a |
| Field sprout explants, round 1 | |||||
| 0.01 | 27 | 63 | 2.3 ± 0.3b | 0.92 ± 0.04b | 2.5 ± 0.2b |
| 0.05 | 30 | 67 | 2.4 ± 0.3b | 1.01 ± 0.06b | 2.8 ± 0.1b |
| 0.1 | 29 | 83 | 3.9 ± 0.3a | 1.31 ± 0.05a | 3.5 ± 0.2a |
| IBA (μM) | No. Expl. | Rooting (%) | No. Roots/Shoot | Lateral Roots | Root Length (cm) |
|---|---|---|---|---|---|
| Rooting after 1st round of multiplication (explants originally from field sprouts) | |||||
| 0 | 28 | 54 | 1.3 | 0 | 2.49 ± 0.17d |
| 0.25 | 25 | 52 | 1.9 | 1 | 3.26 ± 0.29c |
| 0.5 | 27 | 56 | 2.5 | 2 | 4.54 ± 0.09b |
| 1 | 28 | 64 | 2.7 | 6 | 7.15 ± 0.29a |
| 5 | 25 | 40 | 2.9 | 3 | 3.73 ± 0.14c |
| Rooting after 2nd round of multiplication (explants originally from greenhouse plants) | |||||
| 0 | 22 | 41 | 2 | 0 | 2.05 ± 0.10d |
| 0.25 | 42 | 52 | 2.1 | 2 | 4.33 ± 0.26c |
| 0.5 | 32 | 59 | 2.4 | 6 | 5.42 ± 0.28b |
| 1 | 37 | 68 | 2.8 | 4 | 6.67 ± 0.24a |
| 5 | 31 | 45 | 3.4 | 1 | 3.86 ± 0.56c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Mattsson, J. Development of Micropropagation in Bigleaf Maple (Acer macrophyllum). Horticulturae 2021, 7, 170. https://doi.org/10.3390/horticulturae7070170
Zhou C, Mattsson J. Development of Micropropagation in Bigleaf Maple (Acer macrophyllum). Horticulturae. 2021; 7(7):170. https://doi.org/10.3390/horticulturae7070170
Chicago/Turabian StyleZhou, Chen, and Jim Mattsson. 2021. "Development of Micropropagation in Bigleaf Maple (Acer macrophyllum)" Horticulturae 7, no. 7: 170. https://doi.org/10.3390/horticulturae7070170
APA StyleZhou, C., & Mattsson, J. (2021). Development of Micropropagation in Bigleaf Maple (Acer macrophyllum). Horticulturae, 7(7), 170. https://doi.org/10.3390/horticulturae7070170





