Genetic Engineering of Eggplant (Solanum melongena L.): Progress, Controversy and Potential
Abstract
1. Introduction
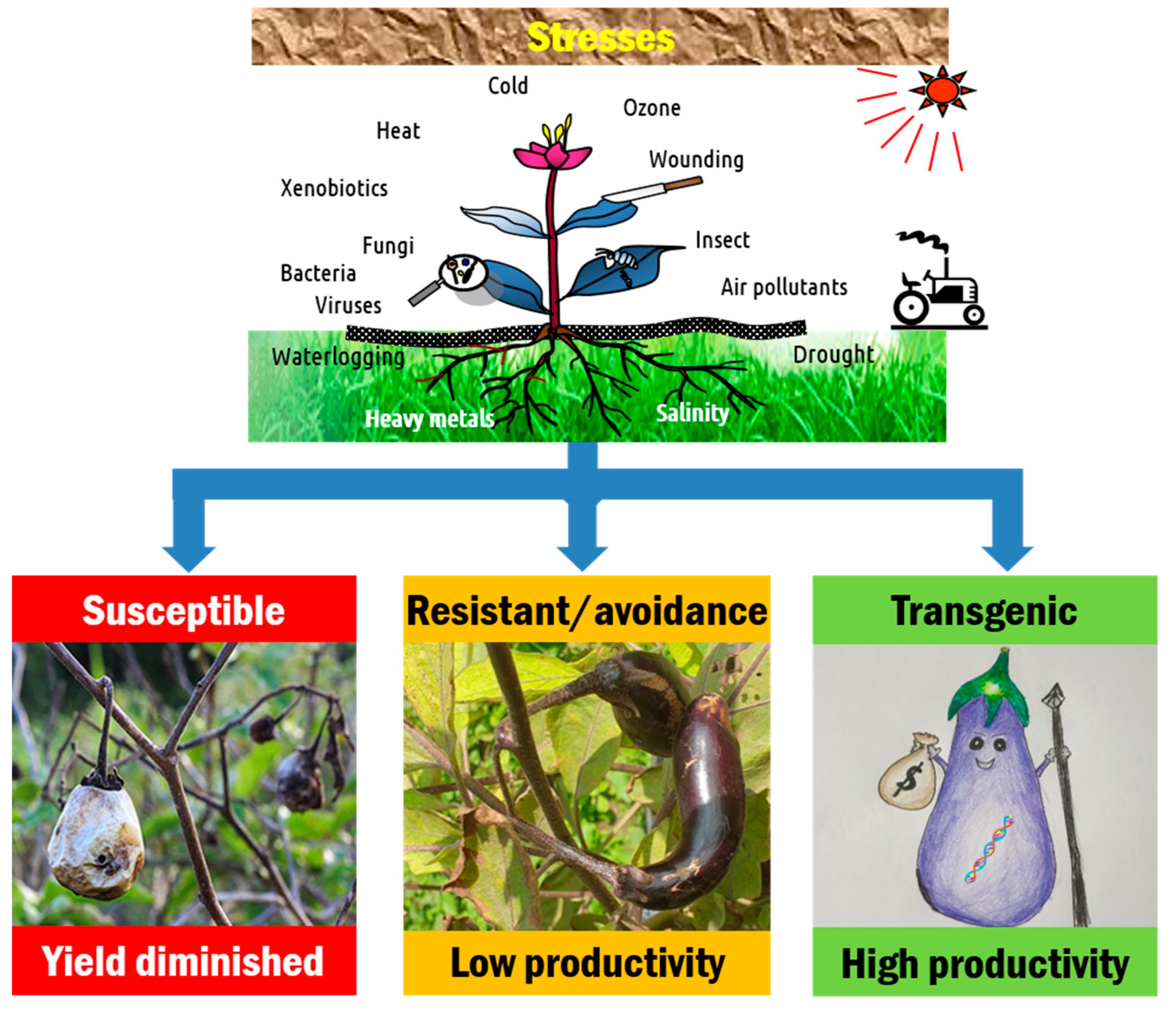
2. Eggplant Production Constraints
2.1. Biotic Stresses
2.2. Abiotic Stresses
3. Quality and Other Issues
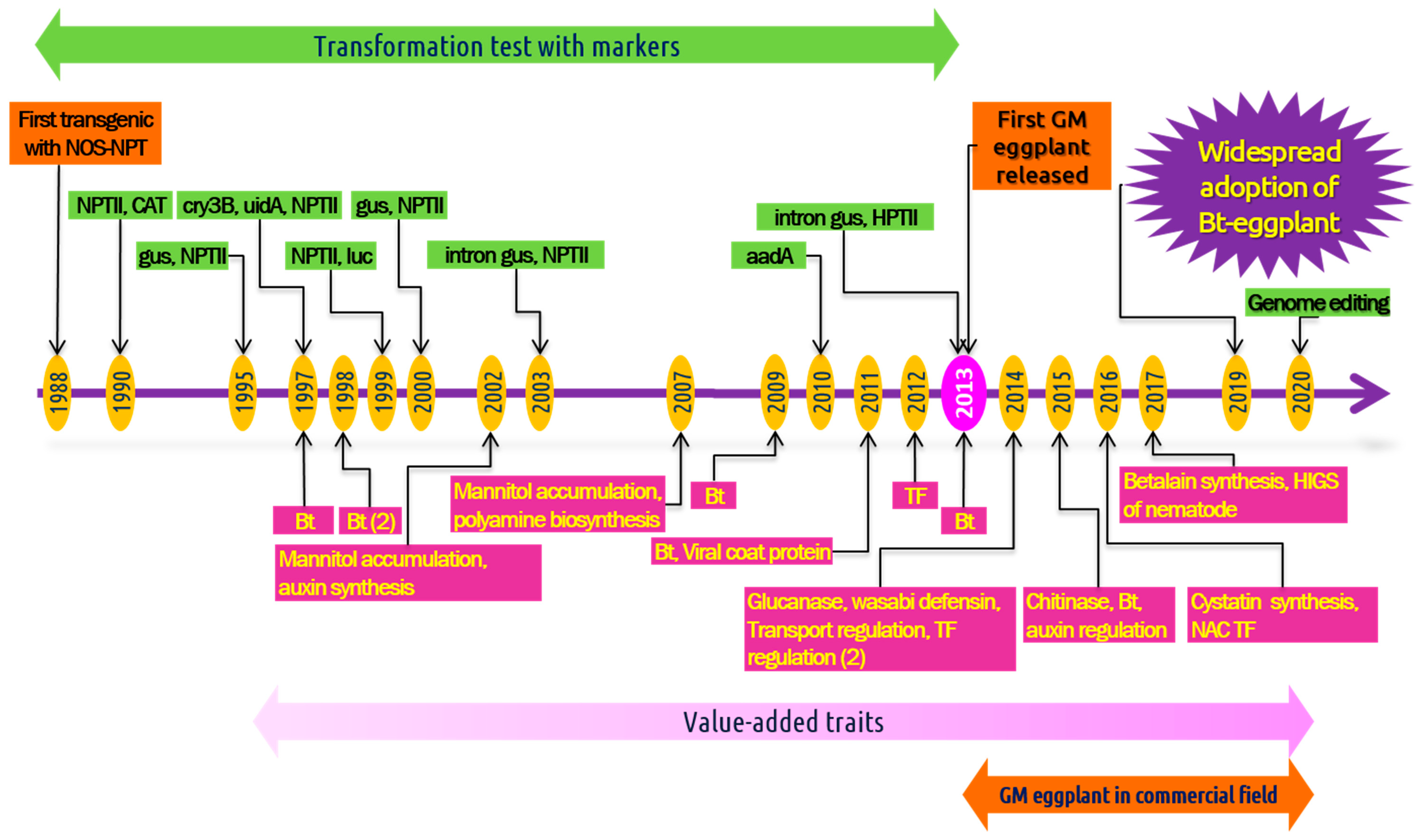
4. Biotechnological Approaches for Genetic Improvement
4.1. Technical Aspects of Genetic Transformation in Eggplant
4.1.1. Mode of Plant Regeneration for Transformation
4.1.2. Choice of Explants for Genetic Transformation
4.1.3. Choice of Vectors for Eggplant Transformation
4.1.4. Targeting Transgene to an Organelle
4.2. Application of Transgenic Technology in Eggplant
4.2.1. Transformation for Insect Resistance
4.2.2. Transformation for Nematode Resistance
4.2.3. Genetic Engineering for Disease Resistance
4.2.4. Transformation for Abiotic Stress Tolerance
4.2.5. Engineering for Other Traits Associated with Quality and Productivity
Control of Fruit Development
Metabolic Engineering
5. Promoters—The Drivers of Transgenes
6. Genome Editing in Eggplant
7. Commercial Cultivation of Transgenic Eggplant: Adoption and Controversy
Updates on Commercial Cultivation of Bt-Eggplant in Bangladesh
8. Eggplant for Science: The Future Model Crop Plant for Gene Functions?
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- BBS. Statistical Database of the Bangladesh Bureau of Statistics; B.B.o. Statistics, Ed.; Bangladesh Bureau of Statistics (BBS): Dhaka, Bangladesh, 2014. [Google Scholar]
- FAOSTAT. Statistical Database of the Food and Agriculture Organization of the United Nations; F.a.A.O.o.t.U. Nations, Ed.; Online database; 2019; Available online: http://www.fao.org/faostat/en/#data (accessed on 10 April 2021).
- Hui, Y.H.; Sherkat, F. Handbook of Food Science, Technology, and Engineering-4 Volume Set; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- FAO. International Treaty on Plant Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- Kumar, S.; Prasanna, P.; Wankhades, S. Potential benefits of bt brinjal in India-an economic assessment. Agric. Econ. Res. Rev. 2011, 24, 83–90. [Google Scholar]
- Jorge, P.A.R.; Neyra, L.C.; Osaki, R.M.; de Almeida, E.; Bragagnolos, N. Effect of eggplant on plasma lipid levels, lipidic peroxidation and reversion of endothelial dysfunction in experimental hypercholesterolemia. Arq. Bras. Cardiol. 1998, 70, 87–91. [Google Scholar] [CrossRef]
- Sidhu, M.; Dhatt, A.; Sidhus, G. Plant regeneration in eggplant (Solanum melongena L.): A review. Afr. J. Biotechnol. 2014, 13, 714–722. [Google Scholar]
- Collonnier, C.; Fock, I.; Kashyap, V.; Rotino, G.; Daunay, M.; Lian, Y.; Mariska, I.; Rajam, M.; Servaes, A.; Ducreux, G.; et al. Applications of biotechnology in eggplant. Plant Cell Tiss. Org. Cult. 2001, 65, 91–107. [Google Scholar] [CrossRef]
- Kashyap, V.; Kumar, S.V.; Collonnier, C.; Fusari, F.; Haicour, R.; Rotino, G.; Sihachakr, D.; Rajam, M. Biotechnology of eggplant. Sci. Hortic. 2003, 97, 1–25. [Google Scholar] [CrossRef]
- Choudhary, B.; Gaur, K. The Development and Regulation of Bt Brinjal in India (Eggplant/Aubergine), in ISAAA Briefs; International Service for the Acquisition of Agri-Biotech Applications: New Delhi, India, 2009. [Google Scholar]
- Mall, N.; Pandey, R.; Singh, S.; Singhs, S. Seasonal incidence of insect-pests and estimation of the losses caused by shoot and fruit borer on brinjal. Indian J. Entomol. 1992, 54, 241–247. [Google Scholar]
- Singh, B.K.; Singh, S.; Yadavs, S.M. Some important plant pathogenic disease of brinjal (Solenum melongena L.) and their management. Plant Patho. J. 2014, 13, 208–213. [Google Scholar] [CrossRef]
- Akinci, I.E.; Akinci, S.; Yilmaz, K.; Dikici, H. Response of eggplant varieties (Solanum melongena) to salinity in germination and seedling stages. N. Z. J. Crop Hortic. Sci. 2004, 32, 193–200. [Google Scholar] [CrossRef]
- Monti, A.; Brugnoli, E.; Scartazza, A.; Amaducci, M.T. The effect of transient and continuous drought on yield, photosynthesis and carbon isotope discrimination in sugar beet (Beta vulgaris L.). J. Exp. Bot. 2006, 57, 1253–1262. [Google Scholar] [CrossRef]
- Brown, K.F.; Messem, A.B.; Dunham, R.J.; Biscoe, P.V. Effect of drought on growth and water use of sugar beet. J. Agric. Sci. 1987, 109, 421–435. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007—The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC); Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Plazas, M.; Andújar, I.; Vilanova, S.; Hurtado, M.; Gramazio, P.; Herráiz, F.J.; Prohens, J. Breeding for chlorogenic acid content in eggplant: Interest and prospects. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 26–35. [Google Scholar] [CrossRef]
- Sánchez-Díaz, M.; Aparicio-Tejo, P.; González-Murua, C.; Peñas, J.I. The effect of NaCl salinity and water stress with polyethylene glycol on nitrogen fixation, stomatal response and transpiration of Medicago sativa, Trifolium repens and Trifolium brachycalycinum (subclover). Physiol. Plant. 1982, 54, 361–366. [Google Scholar] [CrossRef]
- Du, L.; Bao, C.; Hu, T.; Zhu, Q.; Hu, H.; He, Q.; Mao, W. SmARF8, a transcription factor involved in parthenocarpy in eggplant. Mol. Genet. Genom. 2015, 291, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Sękara, A.; Cebula, S.; Kunickis, E. Cultivated eggplants–origin, breeding objectives and genetic resources, a review. Folia Hortic. 2007, 19, 97–114. [Google Scholar]
- Farooqui, M.A.; Rao, A.V.; Jayasree, T.; Sadanandam, A. Induction of atrazine resistance and somatic embryogenesis in Solanum melongena. Theor. Appl. Genet. 1997, 95, 702–705. [Google Scholar] [CrossRef]
- Alicchio, R. Somaclonal variation in eggplant (Solanum melongena L.). In Somaclonal Variation in Crop Improvement I; Springer: Berlin/Heidelberg, Germany, 1990; pp. 416–434. [Google Scholar]
- Rotino, G.; Falavigna, A.; Restainos, F. In vitro selection of eggplant cells resistant to culture filtrate of Verticillium dahliae Kleb. and regeneration of plants. Capsicum Newsl. 1987, 6, 94–95. [Google Scholar]
- Dorica, B. The study of “in vitro” induced somaclonal variations in eggplants (Solanum melongena). Buletinul USAMV ClujNapoca Seria Zootennie şi Biotehnologii 2006, 62, 178–181. [Google Scholar]
- Hitomi, A.; Amagai, H.; Ezura, H. The influence of auxin type on the array of somaclonal variants generated from somatic embryogenesis of eggplant, Solanum melongena L. Plant Breed. 1998, 117, 379–383. [Google Scholar] [CrossRef]
- Vikram, P.; Swamy, B.P.M.; Dixit, S.; Singh, R.; Singh, B.P.; Miro, B.; Kohli, A.; Henry, A.; Singh, N.K.; Kumar, A. Drought susceptibility of modern rice varieties: An effect of linkage of drought tolerance with undesirable traits. Sci. Rep. 2015, 5, 14799. [Google Scholar] [CrossRef] [PubMed]
- Alam, I.; Kim, K.-H.; Sharmin, S.A.; Kim, Y.-G.; Lee, B.-H. Advances in the molecular breeding of forage crops for abiotic stress tolerance. J. Plant Biotechnol. 2010, 37, 425–441. [Google Scholar] [CrossRef]
- Guri, A.; Sinks, K.C. Agrobacterium transformation of eggplant. J. Plant Physiol. 1988, 133, 52–55. [Google Scholar] [CrossRef]
- Başay, S.; Ellialtıoğlus, Ş. Effect of genotypical factors on the effectiveness of anther culture in eggplant (Solanum melongena L.). Turk. J. Biol. 2013, 37, 499–505. [Google Scholar] [CrossRef]
- Corral-Martínez, P.; Seguí-Simarros, J. Efficient production of callus-derived doubled haploids through isolated microspore culture in eggplant (Solanum melongena L.). Euphytica 2012, 187, 47–61. [Google Scholar] [CrossRef]
- Miyoshi, K. Callus induction and plantlet formation through culture of isolated microspores of eggplant (Solanum melongena L.). Plant Cell Rep. 1996, 15, 391–395. [Google Scholar] [CrossRef]
- Zayova, E.; Vassilevska-Ivanova, R.; Kraptchev, B.; Stoeva, D. Indirect shoot organogenesis of eggplant (Solanum melongena L.). J. Cent. Eur. Agric. 2012, 13, 446–457. [Google Scholar]
- Rotino, G.L.; Gleddies, S. Transformation of eggplant (Solanum melongena L.) using a binary Agrobacterium tumefaciens vector. Plant Cell Rep. 1990, 9, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Magioli, C.; Pinheiro, M.M.; Mansurs, E. Establishment of an efficient Agrobacterium-mediated transformation system for eggplant and study of a potential biotechnologically useful promoter. J. Plant Biotechnol. 2000, 2, 43–49. [Google Scholar]
- Fári, M.; Nagy, I.; Csányi, M.; Mitykó, J.; Andrásfalvy, A. Agrobacterium mediated genetic transformation and plant regeneration via organogenesis and somatic embryogenesis from cotyledon leaves in eggplant (Solanum melongena L. cv. ‘Kecskeméti lila’). Plant Cell Rep. 1995, 15, 82–86. [Google Scholar] [CrossRef]
- Billings, S.; Jelenkovic, G.; Chin, C.-K.; Eberhardt, J. The effect of growth regulators and antibiotics on eggplant transformation. J. Am. Soc. Hort. Sci. 1997, 122, 158–162. [Google Scholar] [CrossRef]
- Kumar, P.A.; Mandaokar, A.; Sreenivasu, K.; Chakrabarti, S.K.; Bisaria, S.; Sharma, S.R.; Kaur, S.; Sharma, R.P. Insect-resistant transgenic brinjal plants. Mol. Breed. 1998, 4, 33–37. [Google Scholar] [CrossRef]
- Hanyu, H.; Murata, A.; Park, E.Y.; Okabe, M.; Billings, S.; Jelenkovic, G.; Pedersen, H.; Chin, C.-K. Stability of luciferase gene expression in a long term period in transgenic eggplant, Solanum melongena. Plant Biotechnol. 1999, 16, 403–407. [Google Scholar] [CrossRef][Green Version]
- Franklin, G.; Lakshmis, S.G. Agrobacterium tumefaciens-mediated transformation of eggplant (Solanum melongena L.) using root explants. Plant Cell Rep. 2003, 21, 549–554. [Google Scholar] [CrossRef]
- Singh, A.K.; Verma, S.S.; Bansal, K.C. Plastid transformation in eggplant (Solanum melongena L.). Transgenic Res. 2010, 19, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, K.; Rajesh, M.; Jaganath, B.; Vasuki, A.; Theboral, J.; Elayaraja, D.; Karthik, S.; Manickavasagam, M.; Ganapathi, A. Assessment of factors influencing the Agrobacterium-mediated in planta seed transformation of brinjal (Solanum melongena L.). Appl. Biochem. Biotechnol. 2013, 171, 450–468. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Haicour, R.; Sihachakr, D.; Rajams, M.V. Expression of rice chitinase gene in transgenic eggplant confers resistance to fungal wilts. Indian J. Biotechnol. 2015, 14, 233–240. [Google Scholar]
- Khan, H.; Faisal, M.; Aniss, M. Plant regeneration via somatic embryogensis in callus culuture of Solanum melongena L. Phytomorphology 2008, 58, 153–157. [Google Scholar]
- Rajam, M.V.; Sharmas, P. Genotype, explant and position effects on organogenesis and somatic embryogenesis in eggplant (Solanum melongena L.). J. Exp. Bot. 1995, 46, 135–141. [Google Scholar]
- Beranová, M.; Rakouský, S.; Vávrová, Z.; Skalicky, T. Sonication assisted Agrobacterium-mediated transformation enhances the transformation efficiency in flax (Linum usitatissimum L.). Plant Cell Tissue Organ Cult. 2008, 94, 253–259. [Google Scholar] [CrossRef]
- Keshamma, E.; Rohini, S.; Rao, K.S.; Madhusudhan, B.; Kumars, M.U. Tissue culture-independent in planta transformation strategy: An Agrobacterium tumefaciens-mediated gene transfer method to overcome recalcitrance in cotton (Gossypium hirsutum L.). J. Cotton Sci. 2008, 12, 264–272. [Google Scholar]
- Park, B.-J.; Liu, Z.; Kanno, A.; Kameya, T. Transformation of radish (Raphanus sativus L.) via sonication and vacuum infiltration of germinated seeds with Agrobacterium harboring a group 3 LEA gene from B. napus. Plant Cell Rep. 2005, 24, 494–500. [Google Scholar] [CrossRef]
- Tague, B.W.; Mantis, J. In planta Agrobacterium-mediated transformation by vacuum infiltration. In Arabidopsis Protocols; Springer: Berlin/Heidelberg, Germany, 2006; pp. 215–223. [Google Scholar]
- Weeks, J.T.; Ye, J.; Rommens, C.M. Development of an in planta method for transformation of alfalfa (Medicago sativa). Transgenic Res. 2008, 17, 587–597. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, B.; Yang, Y.; Mei, J.; Zhao, X.; Guo, X.; Huang, X.; Tang, D.; Liu, X. Piercing and vacuum infiltration of the mature embryo: A simplified method for Agrobacterium-mediated transformation of indica rice. Plant Cell Rep. 2009, 28, 1065–1074. [Google Scholar] [CrossRef]
- Yasmeen, A.; Mirza, B.; Inayatullah, S.; Safdar, N.; Jamil, M.; Ali, S.; Choudhry, M.F. In planta transformation of tomato. Plant Mol. Biol. Rep. 2009, 27, 20–28. [Google Scholar] [CrossRef]
- He, Y.; Bai, J.; Wu, F.; Mao, Y. In planta transformation of Brassica rapa and B. napus via vernalization-infiltration methods. Protocol. Exch. 2013. [Google Scholar] [CrossRef]
- Song, L.; Zhao, D.-G.; Wu, Y.-J.; Tian, X.-E. A simplified seed transformation method for obtaining transgenic Brassica napus plants. Agric. Sci. Chin. 2009, 8, 658–663. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Kumar, A.; Desai, N.; Parikh, S. Organelle transformation. Methods Mol. Biol. 2012, 877, 401–406. [Google Scholar]
- Rotino, G.; Perri, E.; Acciarri, N.; Sunseri, F.; Arpaias, S. Development of eggplant varietal resistance to insects and diseases via plant breeding. Adv. Hortic. Sci. 1997, 11, 193–201. [Google Scholar]
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Pal, J.K.; Singh, M.; Rai, M.; Satpathy, S.; Singh, D.V.; Kumars, S. Development and bioassay of CryiAc-transgenic eggplant (Solarium melongena L.) resistant to shoot and fruit borer. J. Hortic. Sci. Biotechnol. 2009, 84, 434–438. [Google Scholar] [CrossRef]
- Rai, N.P.; Rai, G.K.; Kumar, S.; Kumari, N.; Singh, M. Shoot and fruit borer resistant transgenic eggplant (Solanum melongena L.) expressing cry1Aa3 gene: Development and bioassay. Crop Protect. 2013, 53, 37–45. [Google Scholar] [CrossRef]
- Shrivastava, D.; Dalal, M.; Nain, V.; Sharma, P.; Kumars, P.A. Targeted Integration of Bacillus thuringiensis delta-Endotoxin cry1Fa1 in Brinjal (Solanum melongena L.). Curr. Trends Biotechnol. Pharm. 2011, 5, 1149–1156. [Google Scholar]
- Jelenkovic, G.; Billings, S.; Chen, Q.; Lashomb, J.; Hamilton, G.; Ghidiu, G. Transformation of eggplant with synthetic cryIIIA gene produces a high level of resistance to the Colorado potato beetle. J. Am. Soc. Hort. Sci. 1998, 123, 19–25. [Google Scholar] [CrossRef]
- Arpaia, S.; Mennella, G.; Onofaro, V.; Perri, E.; Sunseri, F.; Rotino, G.L. Production of transgenic eggplant (Solanum melongena L.) resistant to Colorado potato beetle (Leptinotarsa decemlineata Say). Theor. Appl. Genet. 1997, 95, 329–334. [Google Scholar] [CrossRef]
- Li, H.; Bouwers, G. Toxicity of Bacillus thuringiensis Cry proteins to Helicoverpa armigera (Lepidoptera: Noctuidae) in South Africa. J. Invertebr. Pathol. 2012, 109, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Perez-Guerrero, S.; Aldebis, H.K.; Vargas-Osunas, E. Toxicity of six Bacillus thuringiensis Cry proteins against the olive moth Prays oleae. Bull. Insectol. 2012, 65, 119–121. [Google Scholar]
- Williams, S.; Friedrich, L.; Dincher, S.; Carozzi, N.; Kessmann, H.; Ward, E.; Rylas, J. Chemical regulation of Bacillus thuringiensis∂-endotoxin expression in transgenic plants. Nat. Biotechnol. 1992, 10, 540–543. [Google Scholar] [CrossRef]
- Vaeck, M.; Reynaerts, A.; Hofte, H.; Jansens, S.; de Beuckeleer, M.; Dean, C.; Zabeau, M.; Montagu, M.V.; Leemanss, J. Transgenic plants protected from insect attack. Nature 1987, 328, 33–37. [Google Scholar] [CrossRef]
- Koziel, M.G.; Beland, G.L.; Bowman, C.; Carozzi, N.B.; Crenshaw, R.; Crossland, L.; Dawson, J.; Desai, N.; Hill, M.; Kadwell, S.; et al. Field performance of elite transgenic maize plants expressing an insecticidal protein derived from Bacillus thuringiensis. Biotechnology 1993, 11, 194–200. [Google Scholar] [CrossRef]
- Jadhav, M.; Jadhav, A.; Pawar, B.; Kale, A.; Kutes, N. Agrobacterium-mediated genetic transformation of Brinjal with cry1F gene for resistance against shoot and fruit borer. J. Crop Improv. 2015, 29, 518–527. [Google Scholar] [CrossRef]
- Shruti, B.K.; Roy, R.; Kumar, P.A.; Roy, S.P. Gene integration of cry1f in, brinjal for resistance against fruit and shoot borer. J. Adv. Zool 2015, 36, 83–89. [Google Scholar]
- Firoz, A.; P, S.; P, P.S.; Anwar, F.; Sharmila, P.; Saradhi, P.P. No more recalcitrant: Chickpea regeneration and genetic transformation. Afr. J. Biotechnol. 2010, 9, 782–797. [Google Scholar] [CrossRef]
- Koenning, S.R.; Overstreet, C.; Noling, J.W.; Donald, P.A.; Becker, J.O.; Fortnum, B.A. Fortnums, Survey of crop losses in response to phytoparasitic nematodes in the United States for 1994. J. Nematol. 1999, 31, 587–618. [Google Scholar]
- Papolu, P.K.; Dutta, T.K.; Tyagi, N.; Urwin, P.E.; Lilley, C.J.; Rao, U. Expression of a cystatin transgene in eggplant provides resistance to root-knot nematode, Meloidogyne incognita. Front. Plant Sci. 2016, 7, 1122. [Google Scholar] [CrossRef] [PubMed]
- Shivakumara, T.N.; Chaudhary, S.; Kamaraju, D.; Dutta, T.K.; Papolu, P.K.; Banakar, P.; Sreevathsa, R.; Singh, B.; Manjaiah, K.M.; Rao, U. Host-induced silencing of two pharyngeal gland genes conferred transcriptional alteration of cell wall-modifying enzymes of meloidogyne incognita vis-á-vis perturbed nematode infectivity in eggplant. Front. Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Veluthakkal, R.; Dasguptas, M.G. Pathogenesis-related genes and proteins in forest tree species. Trees Struct. Funct. 2010, 24, 993–1006. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivels, N. Plant β-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Karr, A.L. β-1,3-Glucanase and chitinase activities in soybean root nodules. J. Plant Physiol. 2002, 159, 245–256. [Google Scholar] [CrossRef]
- Qiu, Z.; Yan, S.; Xia, B.; Jiang, J.; Yu, B.; Lei, J.; Chen, C.; Chen, L.; Yang, Y.; Wang, Y.; et al. The eggplant transcription factor MYB44 enhances resistance to bacterial wilt by activating the expression of spermidine synthase. J. Exp. Bot. 2019, 70, 5343–5354. [Google Scholar] [CrossRef]
- Bhat, S.G.; Arulananthu, G.; Rajesh, G.; Rameshs, N. Agrobacterium-mediated transformation of brinjal (Solanum melongena L.) using fungal resistant gene. Electron. J. Plant Breed. 2020, 11, 160–168. [Google Scholar]
- Singh, D.; Ambroise, A.; Haicour, R.; Sihachakr, D.; Rajam, M.V. Increased resistance to fungal wilts in transgenic eggplant expressing alfalfa glucanase gene. Physiol. Mol. Biol. Plants 2014, 20, 143–150. [Google Scholar] [CrossRef]
- Darwish, N.A.; Khan, R.S.; Ntui, V.O.; Nakamura, I.; Miis, M. Generation of selectable marker-free transgenic eggplant resistant to Alternaria solani using the R/RS site-specific recombination system. Plant Cell Rep. 2014, 33, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Prabhavathi, V.; Rajam, M. Mannitol-accumulating transgenic eggplants exhibit enhanced resistance to fungal wilts. Plant Sci. 2007, 173, 50–54. [Google Scholar] [CrossRef]
- Pratap, D.; Kumar, S.; Raj, S.K.; Sharmas, A.K. Agrobacterium-mediated transformation of eggplant (Solanum melongena L.) using cotyledon explants and coat protein gene of Cucumber mosaic virus. Indian J. Biotechnol. 2011, 10, 19–24. [Google Scholar]
- Stoop, J.M.; Williamson, J.D.; Pharr, D.M. Mannitol metabolism in plants: A method for coping with stress. Trends Plant Sci. 1996, 1, 139–144. [Google Scholar] [CrossRef]
- Shen, B.; Jensen, R.G.; Bohnert, H.J. Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol. 1997, 113, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.S.; Reddys, A.S.N. Inhibition of fungal and bacterial plant pathogens by synthetic peptides: In vitro growth inhibition, interaction between peptides and inhibition of disease progression. Mol. Plant-Microbe Interact. 2000, 13, 847–859. [Google Scholar] [CrossRef] [PubMed]
- López-García, B.; González-Candelas, L.; Pérez-Payá, E.; Marcos, J.F. Identification and characterization of a hexapeptide with activity against phytopathogenic fungi that cause postharvest decay in fruits. Mol. Plant-Microbe Interact. 2000, 13, 837–846. [Google Scholar] [CrossRef] [PubMed]
- López-García, B.; Pérez-Payá, E.; Marcos, J.F. Identification of novel hexapeptides bioactive against phytopathogenic fungi through screening of a synthetic peptide combinatorial library. Appl. Environ. Microbiol. 2002, 68, 2453–2460. [Google Scholar] [CrossRef]
- Allefs, S.J.H.M.; De Jong, E.R.; Florack, D.E.A.; Hoogendoorn, C.; Stiekema, W.J. Erwinia soft rot resistance of potato cultivars expressing antimicrobial peptide tachyplesin I. Mol. Breed. 1996, 2, 97–105. [Google Scholar] [CrossRef]
- Ponti, D.; Mangoni, M.L.; Mignogna, G.; Simmaco, M.; Barra, D. An amphibian antimicrobial peptide variant expressed in Nicotiana tabacum confers resistance to phytopathogens. Biochem. J. 2003, 370, 121–127. [Google Scholar] [CrossRef]
- Alan, A.R.; Blowers, A.; Earle, E.D. Expression of a magainin-type antimicrobial peptide gene (MSI-99) in tomato enhances resistance to bacterial speck disease. Plant Cell Rep. 2004, 22, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Osusky, M.; Zhou, G.; Osuska, L.; Hancock, R.E.; Kay, W.W.; Misra, S. Transgenic plants expressing cationic peptide chimeras exhibit broad-spectrum resistance to phytopathogens. Nat. Biotech. 2000, 18, 1162–1166. [Google Scholar] [CrossRef]
- Yevtushenko, D.P.; Misras, S. Comparison of pathogen-induced expression and efficacy of two amphibian antimicrobial peptides, MsrA2 and temporin A, for engineering wide-spectrum disease resistance in tobacco. Plant Biotechnol. J. 2007, 5, 720–734. [Google Scholar] [CrossRef]
- Na, C.; Shuanghua, W.; Jinglong, F.; Bihao, C.; Jianjun, L.; Changming, C.; Jin, J. Overexpression of the eggplant (Solanum melongena) NAC family transcription factor smnac suppresses resistance to bacterial wilt. Sci. Rep. 2016, 6, 31568. [Google Scholar] [CrossRef]
- Prins, M.; Laimer, M.; Noris, E.; Schubert, J.; Wassenegger, M.; Tepfer, M. Strategies for antiviral resistance in transgenic plants. Mol. Plant Pathol. 2008, 9, 73–83. [Google Scholar] [CrossRef]
- Ziegler, A.; Torrances, L. Applications of recombinant antibodies in plant pathology. Mol. Plant Pathol. 2002, 3, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Abel, P.P.; Nelson, R.S.; De, B.; Hoffmann, N.; Rogers, S.G.; Fraley, R.T.; Beachy, R.N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science 1986, 232, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Lomonossoff, G.P. Pathogen-derived resistance to plant viruses. Annu. Rev. Phytopathol. 1995, 33, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Blumwald, E.; Aharon, G.S.; Apse, M.P. Sodium transport in plant cells. Biochim. Biophys. Acta 2000, 1465, 140–151. [Google Scholar] [CrossRef]
- Yarra, R.; Kirti, P.B. Expressing class I wheat NHX (TaNHX2) gene in eggplant (Solanum melongena L.) improves plant performance under saline condition. Funct. Integr. Genom. 2019, 19, 541–554. [Google Scholar] [CrossRef]
- Kumar, S.K.; Sivanesan, I.; Murugesan, K.; Jeong, B.R.; Hwang, S.J. Enhancing salt tolerance in eggplant by introduction of foreign halotolerance gene, HAL1 isolated from yeast. Hortic. Environ. Biotechnol. 2014, 55, 222–229. [Google Scholar] [CrossRef]
- Wan, F.; Pan, Y.; Li, J.; Chen, X.; Pan, Y.; Wang, Y.; Tian, S.; Zhang, X. Heterologous expression of Arabidopsis C-repeat binding factor 3 (AtCBF3) and cold-regulated 15A (AtCOR15A) enhanced chilling tolerance in transgenic eggplant (Solanum melongena L.). Plant Cell Rep. 2014, 33, 1951–1961. [Google Scholar] [CrossRef]
- Sagare, D.B.; Mohantys, I. Development of moisture stress tolerant brinjal cv. Utkal Anushree (Solanum melongena L.) using Agrobacterium mediated gene transformation. J. Agric. Sci. 2012, 4, 141. [Google Scholar] [CrossRef][Green Version]
- Prabhavathi, V.R.; Rajams, M.V. Polyamine accumulation in transgenic eggplant enhances tolerance to multiple abiotic stresses and fungal resistance. Plant Biotechnol. 2007, 24, 273–282. [Google Scholar] [CrossRef]
- Prabhavathi, V.; Yadav, J.; Kumar, P.; Rajam, M. Abiotic stress tolerance in transgenic eggplant (Solanum melongena L.) by introduction of bacterial mannitol phosphodehydrogenase gene. Mol. Breed. 2002, 9, 137–147. [Google Scholar] [CrossRef]
- Alam, I.; Sharmin, S.A.; Kim, K.-H.; Kim, Y.-G.; Lee, J.J.; Bahk, J.D.; Lee, B.-H. Comparative proteomic approach to identify proteins involved in flooding combined with salinity stress in soybean. Plant Soil 2011, 346, 45–62. [Google Scholar] [CrossRef]
- Serrano, R. Salt tolerance in plants and microorganisms: Toxicity targets and defense responses. Int. Rev. Cytol. 1996, 165, 1–52. [Google Scholar]
- Bordas, M.; Montesinos, C.; Dabauza, M.; Salvador, A.; Roig, L.A.; Serrano, R.; Moreno, V. Transfer of the yeast salt tolerance gene HAL1 to Cucumis melo L. cultivars and in vitro evaluation of salt tolerance. Transgenic Res. 1997, 6, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, C.; Rus, A.M.; Bolarín, M.C.; López-Coronado, J.M.; Arrillaga, I.; Montesinos, C.; Caro, M.; Serrano, R.; Moreno, V. The yeast HAL1 gene improves salt tolerance of transgenic tomato. Plant Physiol. 2000, 123, 393–402. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonaks, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Bray, E.A. Molecular responses to water deficit. Plant Physiol. 1993, 103, 1035–1040. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought- from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Guilfoyle, T.J.; Hagens, G. Auxin response factors. Curr. Opin. Plant Biol. 2007, 10, 453–460. [Google Scholar] [CrossRef]
- Karapanos, I.C.; Mahmood, S.; Thanopouloss, C. Fruit set in solanaceous vegetable crops as affected by floral and environmental factors. Eur. J. Plant Sci. Biotechnol. 2008, 2, 88–105. [Google Scholar]
- Mishiba, K.-I.; Nishida, K.; Inoue, N.; Fujiwara, T.; Teranishi, S.; Iwata, Y.; Takeda, S.; Koizumi, N. Genetic engineering of eggplant accumulating β-carotene in fruit. Plant Cell Rep. 2020, 39, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Maioli, A.; Gianoglio, S.; Moglia, A.; Acquadro, A.; Valentino, D.; Milani, A.M.; Prohens, J.; Orzaez, D.; Granell, A.; Lanteri, S.; et al. Simultaneous CRISPR/Cas9 editing of three PPO genesreduces fruit flesh browning in Solanum melongena L. Front. Plant Sci. 2020, 11, 1883. [Google Scholar] [CrossRef] [PubMed]
- Acciarri, N.; Restaino, F.; Vitelli, G.; Perrone, D.; Zottini, M.; Pandolfini, T.; Spena, A.; Rotino, G.L. Genetically modified parthenocarpic eggplants: Improved fruit productivity under both greenhouse and open field cultivation. BMC Biotechnol. 2002, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, S.; Frova, C.; Torti, G.; Soressi, G.P. Relationship between set, development and activities of growth regulators in tomato fruits. Plant Cell Physiol. 1978, 19, 1281–1288. [Google Scholar]
- Tian, S.B.; Liu, F.Z.; Wang, Y.Q.; Luo, Z.Y.; Chen, Y.K.; Liu, J.S.; Lian, Y. Genetic analysis of parthenocarpy in eggplant. Acta Hortic. Sin. 2003, 30, 413–416. [Google Scholar]
- Archbold, D.D.; Denniss, F.G. Strawberry receptacle growth and endogenous IAA content as affected by growth regulator application and achene removal. J. Am. Soc. Hort. Sci. 1985, 110, 816–820. [Google Scholar]
- Rotino, G.L.; Perri, E.; Zottini, M.; Sommer, H.; Spena, A. Genetic engineering of parthenocarpic plants. Nat. Biotech. 1997, 15, 1398–1401. [Google Scholar] [CrossRef] [PubMed]
- Donzella, G.; Spena, A.; Rotino, G.L. Transgenic parthenocarpic eggplants: Superior germplasm for increased winter production. Mol. Breed. 2000, 6, 79–86. [Google Scholar] [CrossRef]
- Kumar, R.; Tyagi, A.K.; Sharma, A.K. Genome-wide analysis of auxin response factor (ARF) gene family from tomato and analysis of their role in flower and fruit development. Mol. Genet. Genom. 2011, 285, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Hooper, L.C.; Johnson, S.D.; Rodrigues, J.C.M.; Vivian-Smith, A.; Koltunow, A.M. Expression of Aberrant Forms of AUXIN RESPONSE FACTOR8 Stimulates Parthenocarpy in Arabidopsis and Tomato. Plant Physiol. 2007, 145, 351–366. [Google Scholar] [CrossRef]
- Polturak, G.; Grossman, N.; Vela-Corcia, D.; Dong, Y.; Nudel, A.; Pliner, M.; Levy, M.; Rogachev, I.; Aharoni, A. Engineered gray mold resistance, antioxidant capacity, and pigmentation in betalain-producing crops and ornamentals. Proc. Natl. Acad. Sci. USA 2017, 114, 9062–9067. [Google Scholar] [CrossRef]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotech. 2004, 22, 746–754. [Google Scholar] [CrossRef]
- Prohens, J.; Rodríguez-Burruezo, A.; Raigón, M.D.; Nuezs, F. Total phenolic concentration and browning susceptibility in a collection of different varietal types and hybrids of eggplant: Implications for breeding for higher nutritional quality and reduced browning. J. Am. Soc. Hort. Sci. 2007, 132, 638–646. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Bomblies, K.; Yoo, S.K.; Yang, J.W.; Choi, M.S.; Lee, J.S.; Weigel, D.; Ahn, J.H. The 35S promoter used in a selectable marker gene of a plant transformation vector affects the expression of the transgene. Planta 2005, 221, 523–530. [Google Scholar] [CrossRef]
- Yin, K.; Gao, C.; Qiu, J.-L. Progress and prospects in plant genome editing. Nat. Plants 2017, 3, 17107. [Google Scholar] [CrossRef]
- Krishna, V.V.; Qaims, M. Potential impacts of Bt eggplant on economic surplus and farmers’ health in India. Agric. Econ. 2008, 38, 167–180. [Google Scholar] [CrossRef]
- Mesnage, R.; Clair, E.; Gress, S.; Then, C.; Szekacs, A.; Séralini, G.-E. Cytotoxicity on human cells of Cry1Ab and Cry1Ac Bt insecticidal toxins alone or with a glyphosate-based herbicide. J. Appl. Toxicol. 2013, 33, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Feng, Y.; Ge, Y.; Tetreau, G.; Chen, X.; Dong, X.; Shi, W. The cultivation of Bt corn producing Cry1Ac toxins does not adversely affect non-target arthropods. PLoS ONE 2014, 9, e114228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Xu, L.; Gao, S.; Guo, J.; Luo, J.; You, Q.; Ques, Y. Cry1Ac transgenic sugarcane does not affect the diversity of microbial communities and has no significant effect on enzyme activities in rhizosphere soil within one crop season. Front. Plant Sci. 2016, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Shelton, A.M.; Hossain, M.J.; Paranjape, V.; Azad, A.K.; Rahman, M.L.; Khan, A.S.M.M.R.; Prodhan, M.Z.H.; Rashid, M.A.; Majumder, R.; Hussain, S.S.; et al. Bt Eggplant project in Bangladesh: History, present status, and future direction. Front. Bioeng. Biotechnol. 2018, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Mondal, R.I.; Quamruzzaman, A.K.M.; Hasan, K.; Khanam, D. The journey of Bt eggplant in Bangladesh. In Proceedings of the 4th Annual South. Asia Biosafety Conference, Hyderabad, India, 19–21 September 2016. [Google Scholar]
- Prodhan, M.Z.H.; Hasan, M.T.; Chowdhury, M.M.I.; Alam, M.S.; Rahman, M.L.; Azad, A.K.; Hossain, M.J.; Naranjo, S.E.; Shelton, A.M. Bt eggplant (Solanum melongena L.) in Bangladesh: Fruit production and control of eggplant fruit and shoot borer (Leucinodes orbonalis Guenee), effects on non-target arthropods and economic returns. PLoS ONE 2018, 13, e0205713. [Google Scholar] [CrossRef]
- Hirakawa, H.; Shirasawa, K.; Miyatake, K.; Nunome, T.; Negoro, S.; Ohyama, A.; Yamaguchi, H.; Sato, S.; Isobe, S.; Tabata, S.; et al. Draft genome sequence of eggplant (Solanum melongena L.): The representative Solanum species indigenous to the Old World. DNA Res. 2014, 21, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Gramazio, P.; Yan, H.; Hasing, T.; Vilanova, S.; Prohens, J.; Bombarely, A. Whole-Genome Resequencing of Seven Eggplant (Solanum melongena) and One Wild Relative (S. incanum) Accessions Provides New Insights and Breeding Tools for Eggplant Enhancement. Front. Plant. Sci. 2019, 10, 1220. [Google Scholar] [CrossRef]


| Country | Production (ton) | Contribution (%) | Acreage (ha) | Yield (kg/ha) § |
|---|---|---|---|---|
| China | 32,883,567 | 62.9 | 784,966 | 41,892 (12) |
| India | 12,510,000 | 23.9 | 733,000 | 17,067 (46) |
| Egypt | 1,307,793 | 2.5 | 48,253 | 27,103 (29) |
| Turkey | 883,917 | 1.7 | 25,592 | 34,538 (17) |
| Iran | 654,149 | 1.3 | 21,225 | 30,776 (20) |
| Indonesia | 535,436 | 1.0 | 43,905 | 12,195 (59) |
| Japan | 307,800 | 0.6 | 9160 | 33,603 (18) |
| Italy | 286,473 | 0.5 | 9449 | 30,318 (21) |
| Philippines | 241,901 | 0.5 | 21,446 | 11,280 (64) |
| Bangladesh * | 226,000 | 0.4 | 31,556 | 7924 (71) |
| Global | 52,309,119 | 100 | 1,858,253 | 28,149 |
| Cultivar | Explant | Agrobacterium Strain | Vector | Gene | Selection Marker | Frequency | Ref |
|---|---|---|---|---|---|---|---|
| Black Beauty | In vitro leaves | 715 and pCIB10 | pMON200 | NOS-NPT | Kanamycin | nc | [28] |
| Picenfiia | Leaves (callus) | GV3101 | pBCATI | NPTII, CAT | Kanamycin, 100 mg/L | 8% | [33] |
| Kecskeméti lila | Somatic embryo from cotyledon | C58C1 RifR | pGSGluc1 | gus, NPTII | Kanamycin, 100 mg/L | nc | [35] |
| Hibush | Stem, leaves | Q10, Q20, Q30, Q40, Q201-204 | pRU01, PRU02, PRU03 | cryIIIB, uidA, NPTII | Kanamycin, 50 mg/L | 21% | [36] |
| Pusa Purple Long | Cotyledonary leaves | EHA 105 | pBT 1291, pBinAR | cry1Ab | Kanamycin, 100 mg/L | nc | [37] |
| Hibush | Leaves (callus) | LBA4404 | pBI121 | NPTII, luc | Kanamycin, 100 mg/L | 3% | [38] |
| F-100, Emb | Cotyledon, leaves | C58C1 | pTSM-3.1800 | gus, NPTII | Kanamycin, 50 mg/L | 5–23% | [34] |
| MEBH 11, MEBH 9, Kalpatru, Rohini | Root | LBA4404 | pBAL2 | Gus-intron, NPTII | Kanamycin, 100 mg/L | nc | [39] |
| Eggplant | Stem (chloroplast) | Biolistic | pPRV111A | aadA | Spectinomycin, 300 mg/L | 9% | [40] |
| Jamuni Gola, Pusa Kranti, Azad Kranti, Arka Samhitha, Hisar Shyamal | Seed (in planta) | EHA 105 | CAMBIA 1301-bar | gusA-intron, hptII | BASTA®, 100 mg/L | 46% | [41] |
| Pusa purple long | Cotyledone, leaves | LBA4404 | pCAMBARchi11 | Endochitinase (I) | Hygromycin, 20 mg/L | 10–20% | [42] |
| Promoter::Gene | Target Insect | Cry Protein Level | Key Results | Ref |
|---|---|---|---|---|
| CaMV 35s::cry1Aa3 | EFSB (Leucinodes orbonalis) Lepidoptera | 30.9–44.3 ng/g (fresh leaves); 20.5–35.7 ng/g (fruits) |
| [58] |
| CaMV 35s::cry1Fa1 | EFSB (Leucinodes orbonalis) Lepidoptera | Not determined |
| [59] |
| CaMV 35s::cry1Ac | EFSB (Leucinodes orbonalis) Lepidoptera | 2.46–4.33 ng/mL in leaves |
| [57] |
| CaMV 35s::cry1Ab | EFSB (Leucinodes orbonalis) Lepidoptera | 125–142 ng/mg in soluble protein |
| [37] |
| CaMV 35s::cry3A | Colorado potato beetle (Leptinotarsa decemlineata) | Not determined |
| [60] |
| CaMV 35s::cry3A | Colorado potato beetle (Leptinotarsa decemlineata) | Not determined |
| [61] |
| Promoter::Gene | Target Trait | Key Results | Ref |
|---|---|---|---|
| CaMV 35s:: SmMYB44 | Bacterial wilt |
| [76] |
| not mentioned::hevein | Fungal wilts tolerance |
| [77] |
| CaMV 35s::chi | Fungal wilts tolerance |
| [42] |
| CaMV 35s::glu | Fungal wilts tolerance |
| [78] |
| CaMV 35s:: WD | Early blight (Alternaria solani) tolerance |
| [79] |
| CaMV35s::mtlD | Fungal wilt resistance |
| [80] |
| CaMV 35s::CMV-CP | Virus resistance (Cucumber mosaic virus) |
| [81] |
| Promoter::Gene | Target Trait | Key Results | Ref |
|---|---|---|---|
| CaMV35s::HAL1 | Salt tolerance |
| [99] |
| AtRD29A::AtCBF3 and AtRD29A::AtCOR15A | Chilling tolerance |
| [100] |
| rd29A::DREB1A | Moisture stress tolerance |
| [101] |
| CaMV35s::adc | Multiple abiotic stresses tolerance |
| [102] |
| CaMV35s::mtlD | Multiple abiotic stresses tolerance |
| [103] |
| Promoter::Gene | Target Trait | Key Results | Ref |
|---|---|---|---|
| EEF48::crtB | β-carotene accumulation |
| [113] |
| CaMV 35s:: Cas9-SmPPO | Fruit flesh browning |
| [114] |
| CaMV 35s:: SmARF8 [RNAi] | Parthenocarpic fruit |
| [19] |
| pDefH9:: DefH9-iaaM | Parthenocarpic fruit |
| [115] |
| Traits | Arabidopsis thaliana | Nicotiana tabacum | Solanum melongena |
|---|---|---|---|
| Genome size | 125 Mb | 4400–4600 Mb | ~1155 Mb |
| Ploidy | Diploid | Allotetraploid | Diploid |
| Chromosome number | 10 (n = 5) | 48 (n = 12) | 24 (n = 12) |
| Life cycle | 6 weeks | 12 weeks | 16 weeks |
| Seed production | Numerous | Numerous | Numerous |
| Space requirement | Very low | Moderate | Moderate |
| Genetic transformation efficiency | High | High | Moderate to high |
| Whole genome sequence | Available | Available | Available |
| Availability of mutant lines | Very high | Limited | Few |
| True-to-type micropropagation | Not easy | Very handy | Very handy |
| Commercial significance | No | Yes | Yes |
| Edible fruits | No | No | Yes |
| Research community | All over the world | All over the world | Limited countries |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, I.; Salimullah, M. Genetic Engineering of Eggplant (Solanum melongena L.): Progress, Controversy and Potential. Horticulturae 2021, 7, 78. https://doi.org/10.3390/horticulturae7040078
Alam I, Salimullah M. Genetic Engineering of Eggplant (Solanum melongena L.): Progress, Controversy and Potential. Horticulturae. 2021; 7(4):78. https://doi.org/10.3390/horticulturae7040078
Chicago/Turabian StyleAlam, Iftekhar, and Md Salimullah. 2021. "Genetic Engineering of Eggplant (Solanum melongena L.): Progress, Controversy and Potential" Horticulturae 7, no. 4: 78. https://doi.org/10.3390/horticulturae7040078
APA StyleAlam, I., & Salimullah, M. (2021). Genetic Engineering of Eggplant (Solanum melongena L.): Progress, Controversy and Potential. Horticulturae, 7(4), 78. https://doi.org/10.3390/horticulturae7040078






