Grafting Improves Fruit Yield of Cucumber Plants Grown under Combined Heat and Soil Salinity Stresses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Growing Conditions and Experimental Design
2.3. Vegetative Growth, Fruit, and Yield Traits
2.4. Chlorophyll, Enzymatic Antioxidants, Proline, and Electrolyte Leakage
2.5. Mineral Concentrations
2.6. Statistical Analyses
3. Results
3.1. Vegetative Growth
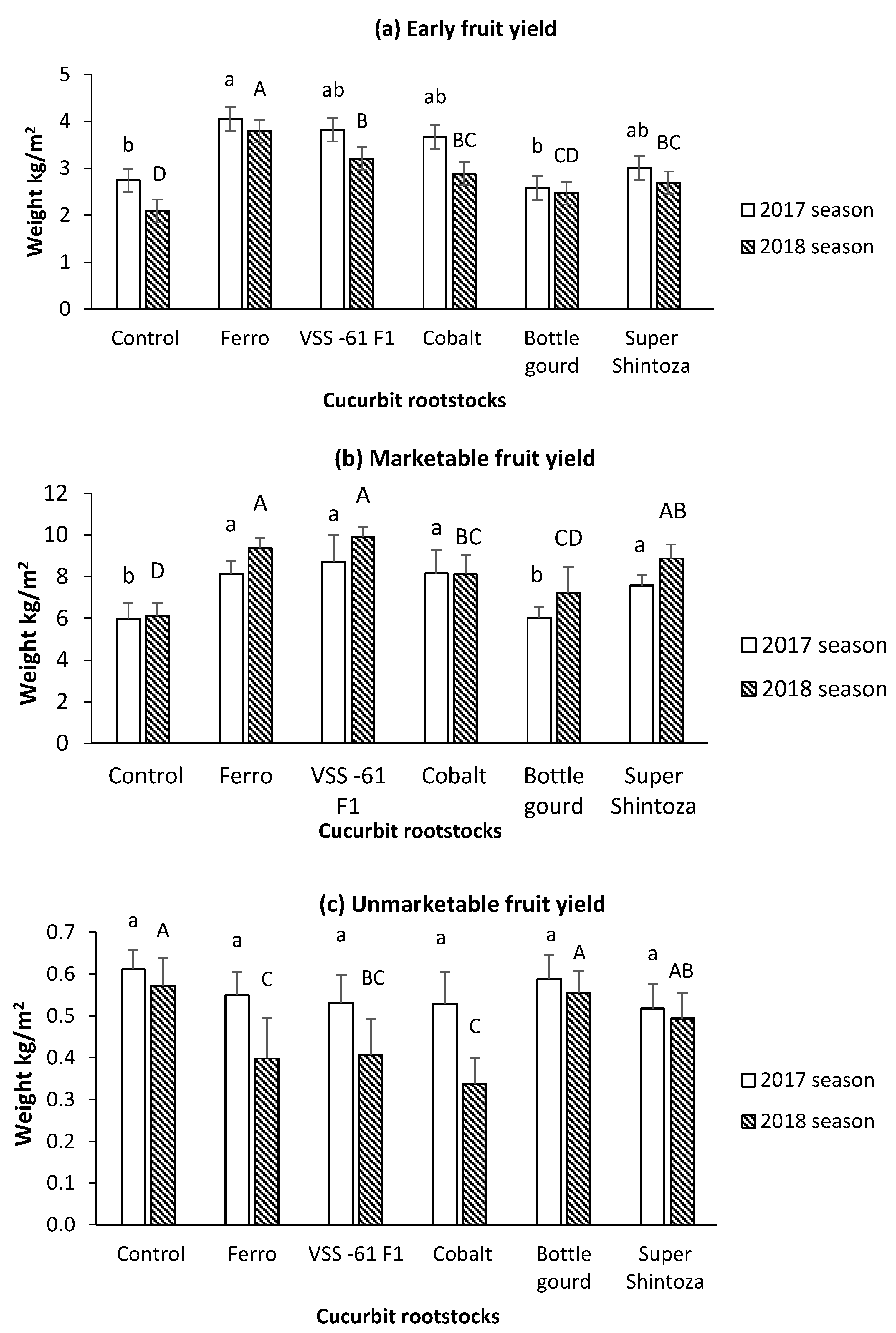
3.2. Yield and Fruit Quality
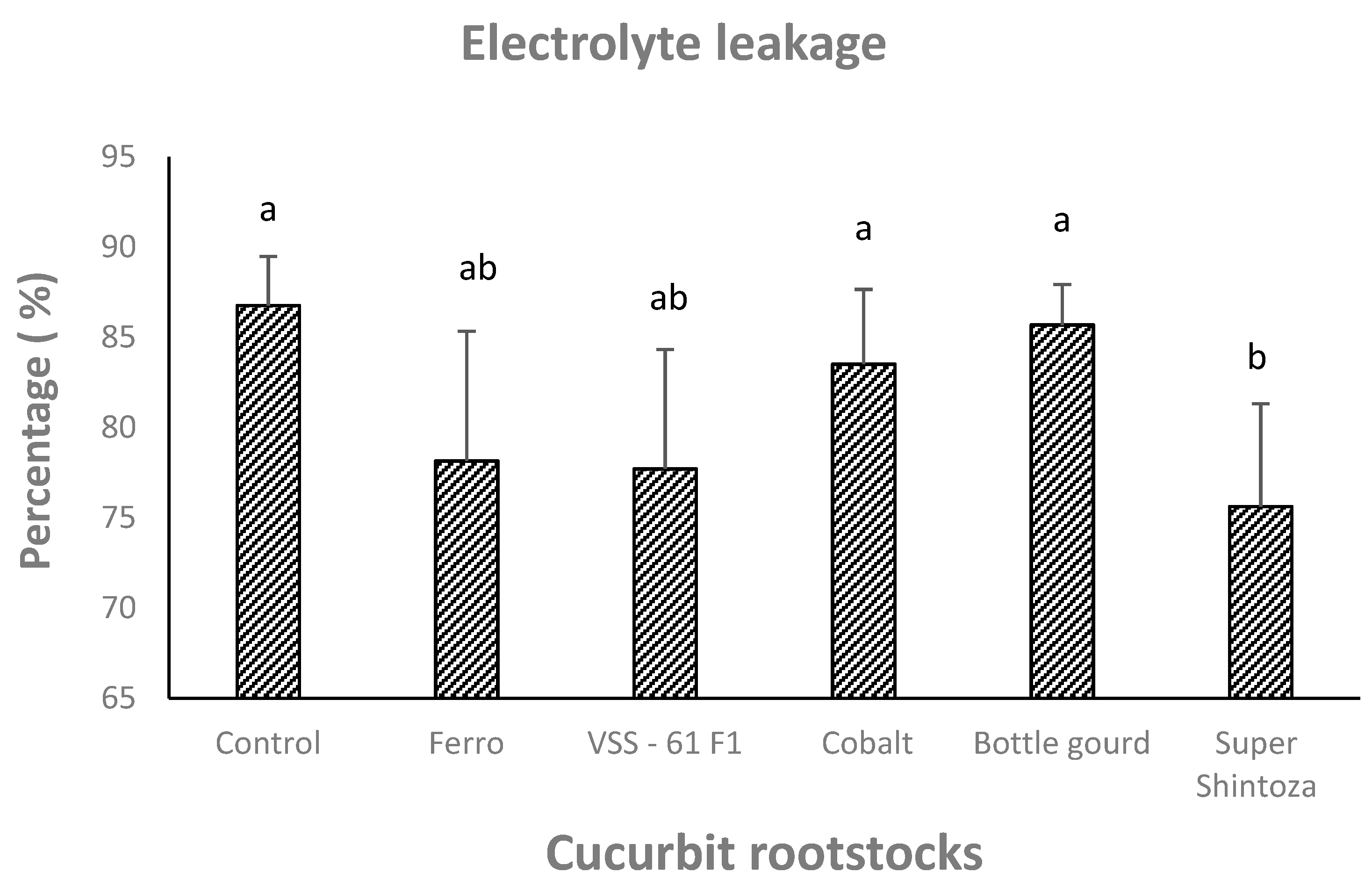
3.3. Chlorophyll, Enzymatic Antioxidants, Proline, and Electrolyte Leakage
3.4. Mineral Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, B.J.; Filho, A.B.C.; La Scala, N. Greenhouse gas emissions and carbon footprint of cucumber, tomato and lettuce production using two cropping systems. J. Clean. Prod. 2021, 282, 124517. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Wu, X.; Shu, S.; Sun, J.; Guo, S. Redox and thylakoid membrane proteomic analysis reveals the momordica (Momordica charantia L.) rootstock-induced photoprotection of cucumber leaves under short-term heat stress. Plant Physiol. Biochem. 2019, 136, 98–108. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, Y.; Hess, F.; Huang, B.; Chen, Z. Nutrient balance and soil changes in plastic net-house vegetable production. Nutr. Cyc. Agroecosys. 2020, 117, 77–92. [Google Scholar] [CrossRef]
- Phogat, V.; Mallants, D.; Cox, J.W.; Šimůnek, J.; Oliver, D.P.; Awad, J. Management of soil salinity associated with irrigation of protected crops. Agric. Water Manag. 2020, 227, 105845. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Kailash Chandra Naga, K.C.; Kumar, R.; Chourasia, K.N.; Subhash, S.; Kumar, D.; Sharma, S. Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci. Hort. 2020, 272, 109592. [Google Scholar] [CrossRef]
- Nie, W.; Gong, B.; Chen, Y.; Wang, J.; Wei, M.; Shi, Q. Photosynthetic capacity, ion homeostasis and reactive oxygen metabolism were involved in exogenous salicylic acid increasing cucumber seedlings tolerance to alkaline stress. Sci. Hort. 2018, 235, 413–423. [Google Scholar] [CrossRef]
- Li, C.; Bian, B.; Gong, T.; Liao, W. Comparative proteomic analysis of key proteins during abscisic acid-hydrogen peroxide-induced adventitious rooting in cucumber (Cucumis sativus L.) under drought stress. J. Plant Physiol. 2018, 229, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Elsheery, N.I.; Helaly, M.N.; Omar, S.A.; John, S.V.S.; Zabochnicka-Swiątek, M.; Kalaji, H.M.; Rastogi, A. Physiological and molecular mechanisms of salinity tolerance in grafted cucumber. S. Afr. J. Bot. 2020, 130, 90–102. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Z.; Zhang, X.; Zheng, S.; Wang, J.; Mo, J. Alleviating effects of exogenous melatonin on salt stress in cucumber. Sci. Hort. 2020, 262, 109070. [Google Scholar] [CrossRef]
- Ali, A.H.; Abdelrahman, M.; Radwan, U.; El-Zayat, S.; El-Sayed, M.A. Effect of Thermomyces fungal endophyte isolated from extreme hot desert-adapted plant on heat stress tolerance of cucumber. Appl. Soil Ecol. 2018, 124, 155–162. [Google Scholar] [CrossRef]
- Pandey, P.; Ramegowda, V.; Senthil-Kumar, M. Shared and unique responses of plants to multiple individual stresses and stress combinations: Physiological and molecular mechanisms. Front. Plant Sci. 2015, 6, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas-Barry, G.; Martin, C.S.; Lynch, M.D.J.; Ramsubhag, A.; Miller, J.; Charles, T.C. Driving factors influencing the rhizobacteriome community structure of plants adapted to multiple climatic stressors in edaphic savannas. Sci. Total Environ. 2021, 769, 145214. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Singh, A.; Zander, K.K.; Mathew, S.; Kumar, A. Measuring successful processes of knowledge co-production for managing climate change and associated environmental stressors: Adaptation policies and practices to support Indian farmers. J. Environ. Manag. 2021. [Google Scholar] [CrossRef]
- Tomaz de Oliveira, M.M.; Lu, S.; Zurgil, U.; Raveh, E.; Tel-Zur, N. Grafting in Hylocereus (Cactaceae) as a tool for strengthening tolerance to high temperature stress. Plant Physiol. Biochem. 2021, 160, 94–105. [Google Scholar] [CrossRef]
- Bithell, S.L.; Condè, B.; Traynor, M.; Donald, E.C. Grafting for soilborne disease management in Australian vegetable production systems—a review. Australas. Plant Pathol. 2013, 42, 329–336. [Google Scholar] [CrossRef]
- Reddy, P.P. Grafted vegetables for management of soilborne pathogens. In Sustainable Crop. Protection under Protected Cultivation; Reddy, P.P., Ed.; Springer Nature: Singapore, 2016; pp. 83–97. [Google Scholar] [CrossRef]
- Usanmaz, S.; Abak, K. Plant growth and yield of cucumber plants grafted on different commercial and local rootstocks grown under salinity stress. Saudi J. Biol. Sci. 2019, 26, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Men, L.; Gao, L.; Tian, Y. Effect of grafting and gypsum application on cucumber (Cucumis sativus L.) growth under saline water irrigation. Agric. Water Manag. 2017, 188, 79–90. [Google Scholar] [CrossRef]
- Al-Harbi, A.R.; Al-Omran, A.M.; Alharbi, K. Grafting improves cucumber water stress tolerance in Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 298–304. [Google Scholar] [CrossRef]
- Sallaku, G.; Sandén, H.; Babaj, I.; Kaciu, S.; Balliu, A.; Rewald, B. Specific nutrient absorption rates of transplanted cucumber seedlings are highly related to RGR and influenced by grafting method, AMF inoculation and salinity. Sci. Hort. 2019, 243, 177–188. [Google Scholar] [CrossRef]
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A.; Kempen, B.; de Sousa, L. Global mapping of soil salinity change. Remote Sens. Environ. 2020, 111, 111260. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, X.; Zhang, J.; He, Y.; Zhu, X.; Zhou, X.; Gong, H.; Yin, J.; Liu, Y. Silicon confers cucumber resistance to salinity stress through regulation of proline and cytokinins. Plant Physiol. Biochem. 2020, 156, 209–220. [Google Scholar] [CrossRef]
- Alpaslan, M.; Gunes, A. Exploring the communities of bacteria, fungi and ammonia oxidizers in rhizosphere of Fusarium-diseased greenhouse cucumber. Plant Soil 2001, 236, 123–128. [Google Scholar] [CrossRef]
- Huang, Y.; Bie, Z.; He, S.; Hua, B.; Zhen, A.; Liu, Z. Improving cucumber tolerance to major nutrients induced salinity by grafting onto Cucurbita ficifolia. Environ. Expt. Bot. 2010, 69, 32–38. [Google Scholar] [CrossRef]
- Van Velthuizen, H.; Huddleston, B.; Fischer, G.; Salvatore, M.; Ataman, E.; Nachtergaele, F.O.; Zanetti, M.; Bloise, M.; Antonicelli, A.; Bel, J.; et al. Mapping Biophyical Factors That Influence Agricultural Production and Rural Vulnerability; Food and Agriculture Organization of the United Nations: Rome, Italy; International Institute for Applied Systems Analysis: Rome, Italy, 2007; pp. 1–84. [Google Scholar]
- Wang, Y.; Guo, S.; Wang, L.; Wang, L.; He, X.; Shu, S.; Sun, J.; Lu, N. Identification of microRNAs associated with the exogenous spermidine-mediated improvement of high-temperature tolerance in cucumber seedlings (Cucumis sativus L.). BMC Genom. 2018, 19, 285. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yuan, Y.; Du, N.; Wang, Y.; Shu, S.; Sun, J.; Guo, S. Proteomic analysis of heat stress resistance of cucumber leaves when grafted onto Momordica rootstock. Hort. Res. 2018, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Parvathi, M.S.; Dhanyalakshmi, K.H.; Nataraja, K.N. Molecular mechanisms associated with drought and heat tolerance in plants and options for crop improvement for combined stress tolerance. In Agronomic Crops; Hasanuzzaman, M., Ed.; Springer Nature: Singapore, 2020; pp. 481–502. [Google Scholar] [CrossRef]
- Lopez-Delacalle, M.; Silva, C.J.; Mestre, T.C.; Martinez, V.; Blanco-Ulate, B.; Rivero, R.M. Synchronization of proline, ascorbate and oxidative stress pathways under the combination of salinity and heat in tomato plants. Environ. Expt. Bot. 2021, 183, 104351. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Brenes, M.; Pérez, J.; González-Orenga, S.; Solana, A.; Boscaiu, M.; Prohens, J.; Plazas, M.; Fita, A.; Vicente, O. Comparative studies on the physiological and biochemical response of salt stress of eggplant (Solanum melongena) and its rootstock S. torvum. Agriculture 2020, 10, 328. [Google Scholar] [CrossRef]
- Sevgican, A. Örtüaltı Sebzeciliği; Ege Üniversitesi Ziraat Fakültesi Yayınları: İzmir, Turkey, 2002; pp. 1–476. [Google Scholar]
- Upadhyaya, A.; Sankhla, D.; Davis, T.D.; Sankhla, N.; Smith, B.N. Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J. Plant Physiol. 1985, 121, 453–461. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, C.; Ormrod, D.P.; Murr, D.P.; Watkins, C.B. Influence of salicylic acid on H2O2 production, oxidative stress and H2O2-metabolizing enzymes: Salicylic acid-mediated oxidative damage requires H2O2. Plant Physiol. 1997, 115, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J. Exp. Bot. 1995, 46, 1843–1852. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, K.Q.; Duan, B.W.; Sun, C.Z.; Zheng, K.L.; Cai, R.; Zhuang, J.Y. Rapid determination of silicon content in rice. Rice Sci. 2005, 12, 145–147. [Google Scholar]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant. Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989; pp. 1–491. [Google Scholar]
- Cui, B.-J.; Niu, W.-Q.; Du, Y.D.; Zhang, Q. Response of yield and nitrogen use efficiency to aerated irrigation and N application rate in net-house cucumber. Sci. Hort. 2020, 265, 109220. [Google Scholar] [CrossRef]
- Dong, J.; Gruda, N.; Li, X.; Tang, Y.; Zhang, P.; Duan, Z. Sustainable vegetable production under changing climate: The impact of elevated CO2 on yield of vegetables and the interactions with environments–A review. J. Clean. Prod. 2020, 253, 119920. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Rea, E.; Colla, G. Improving melon and cucumber photosynthetic activity, mineral composition, and growth performance under salinity stress by grafting onto Cucurbita hybrid rootstocks. Photosynthetica 2012, 50, 180–188. [Google Scholar] [CrossRef]
- Fan, H.F.; Ding, L.; Xu, Y.L.; Du, C.X. Antioxidant system and photosynthetic characteristics responses to short-term PEG-induced drought stress in cucumber seedling leaves. Russ. J. Plant Physiol. 2017, 64, 162–173. [Google Scholar] [CrossRef]
- Amaro, A.C.E.; Macedo, A.C.; Ramos, A.R.P.; Goto, R.; Ono, E.O.; Rodrigues, J.D. The use of grafting to improve the net photosynthesis of cucumber. Theor. Exp. Plant Physiol. 2014, 26, 241–249. [Google Scholar] [CrossRef]
- Lu, X.; Liu, W.; Wang, T.; Zhang, J.; Li, X.; Zhang, W. Systemic long-distance signaling and communication between rootstock and scion in grafted vegetables. Front. Plant Sci. 2020, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, S.S.; Yi, C.Y.; Wang, F.; Zhou, J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Hydrogen peroxide mediates abscisic acid-induced HSP70 accumulation and heat tolerance in grafted cucumber plants. Plant Cell Environ. 2014, 37, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Giné, A.; González, C.; Serrano, L.; Sorribas, F.J. Population dynamics of Meloidogyne incognita on cucumber grafted onto the Cucurbita hybrid RS841 or ungrafted and yield losses under protected cultivation. Eur. J. Plant. Pathol. 2017, 148, 795–805. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Duan, Z.; Mao, J.; LI, X.; Dong, F. Low root zone temperature limits nutrient effects on cucumber seedling growth and induces adversity physiological response. J. Integr. Agric. 2013, 12, 1450–1460. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, D.; Zhang, Y. Investigating the effects of net-house vegetable cultivation on soil fertility in Lhasa, Tibetan Plateau. Chin. Geogr. Sci. 2020, 30, 456–465. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Mao, X.; Zhang, M.; Yang, W.; Di, H.J.; Ma, L.; Liu, W.; Li, B. Response of soil microbial communities to continuously mono-cropped cucumber under net-house conditions in a calcareous soil of north China. J. Soils Sediments 2020, 20, 2446–2459. [Google Scholar] [CrossRef]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawany, M.; Elhawat, N.; Al-Otaib, A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef]
| Rootstock Name | Rootstock Species | Characteristics | Seed Source |
|---|---|---|---|
| Ferro | Cucurbita maxima × C. moschata | Resistant to Fusarium wilt and Verticillium wilt | Rijk Zwaan Agro Company, Cairo, Egypt |
| Cobalt | C. maxima × C. moschata | Resistant to Fusarium wilt and tolerant to low and high temperatures | Rijk Zwaan Agro Company, Cairo, Egypt |
| VSS-61 F1 | Cucurbita pepo (squash) | Resistant to Fusarium wilt and nematode | Meroe seeds, Techno Green Seed Company, Cairo, Egypt |
| Bottle gourd | Lagenaria siceraria | Resistant to Fusarium wilt and tolerant to low temperatures | El-Nada and El-Nour Company, Nubaria, Egypt |
| Super Shintoza | C. maxima × C. moschata | Resistant to Fusarium wilt and tolerant to low and high temperatures | G.S.I. Exports Seed Company, Amsterdam, The Netherlands |
| 2017 | 2018 | |||||
|---|---|---|---|---|---|---|
| Rootstock | 30 DAT | 50 DAT | 70 DAT | 30 DAT | 50 DAT | 70 DAT |
| Plant height (cm) a | ||||||
| Control (ungrafted) | 111 c | 152 b | 244 b | 105 c | 203 b | 267 b |
| Ferro | 163 a | 213 a | 286 ab | 129 a | 224 ab | 345 a |
| VSS-61 F1 | 140 ab | 197 a | 324 a | 124 ab | 211 b | 368 a |
| Cobalt | 156 a | 227 a | 274 ab | 111 bc | 204 b | 325 a |
| Bottle gourd | 145 ab | 218 a | 264 ab | 112 bc | 224 ab | 339 a |
| Super Shintoza | 123 bc | 219 a | 313 ab | 116 bc | 248 a | 343 a |
| Prob. F b | ** | ** | ** | ** | ** | ** |
| Leaf area (dm2 plant−1) a | ||||||
| Control (ungrafted) | 37.40 b | 45.23 b | 65.40c | 33.97 b | 63.35 b | 83.42 b |
| Ferro | 40.82 a | 61.52 a | 82.11a | 38.12 ab | 83.27 a | 91.76 ab |
| VSS-61 F1 | 38.14 ab | 64.64 a | 83.40 a | 39.56 a | 76.02 ab | 96.53 a |
| Cobalt | 40.11 ab | 59.05 a | 76.55 ab | 39.52 a | 71.13 ab | 88.64 ab |
| Bottle gourd | 37.63 b | 55.13 ab | 72.46 bc | 34.93 ab | 74.32 ab | 85.21 ab |
| Super Shintoza | 38.63 ab | 64.23 a | 79.64 ab | 37.53 ab | 76.84 ab | 91.75 ab |
| Prob. F b | * | ** | ** | ** | * | * |
| 2017 | 2018 | |||||
|---|---|---|---|---|---|---|
| Rootstock | 30 DAT | 50 DAT | 70 DAT | 30 DAT | 50 DAT | 70 DAT |
| Abortion rate (%) a | ||||||
| Control (ungrafted) | 2.46 b | 10.68 | 18.18 | 2.78 b | 10.76 ab | 20.76 ab |
| Ferro | 1.15 c | 10.90 | 18.91 | 1.49 c | 7.16 c | 15.44 bc |
| VSS-61 F1 | 1.20 c | 10.86 | 18.15 | 1.67 c | 6.62 c | 13.89 c |
| Cobalt | 2.65 b | 11.16 | 20.77 | 2.34 ab | 7.40 bc | 15.43 bc |
| Bottle gourd | 2.60 b | 10.21 | 18.25 | 2.36 ab | 9.42 abc | 18.08 abc |
| Super Shintoza | 3.75 a | 11.12 | 20.16 | 2.84 a | 11.84 a | 22.44 a |
| Prob. F b | ** | ns | ns | * | ** | ** |
| Fruit firmness (g cm−2) a | ||||||
| Control (ungrafted) | 430 a | 491 b | 423 c | 447 | 421 | 407 |
| Ferro | 369 c | 540 ab | 450 abc | 435 | 417 | 427 |
| VSS-61 F1 | 384 bc | 555 a | 469 a | 447 | 457 | 444 |
| Cobalt | 334 a | 524 ab | 460 ab | 421 | 441 | 423 |
| Bottle gourd | 431 ab | 524 ab | 434 bc | 435 | 388 | 423 |
| Super Shintoza | 417 ab | 401 c | 450 abc | 477 | 416 | 437 |
| Prob. F b | * | ** | ** | ns | ns | ns |
| Total soluble solids (%) a | ||||||
| Control (ungrafted) | 3.06 c | 2.93 | 3.21 | 3.27 | 3.31 | 3.11 b |
| Ferro | 3.38 ab | 3.07 | 3.25 | 3.47 | 3.47 | 3.51 a |
| VSS-61 F1 | 3.45 a | 2.95 | 3.53 | 3.67 | 3.34 | 3.60 a |
| Cobalt | 3.34 ab | 3.10 | 3.32 | 3.62 | 3.58 | 3.20 ab |
| Bottle gourd | 3.41 ab | 3.02 | 3.32 | 3.63 | 3.37 | 3.39 ab |
| Super Shintoza | 3.29 b | 3.22 | 3.31 | 3.45 | 3.53 | 3.39 ab |
| Prob. F b | ** | ns | ns | ns | ns | * |
| 2017 | 2018 | |||||
|---|---|---|---|---|---|---|
| Rootstock | 30 DAT | 50 DAT | 70 DAT | 30 DAT | 50 DAT | 70 DAT |
| Chlorophyll content (SPAD units) a | ||||||
| Control (ungrafted) | 39.47 | 39.07 b | 37.53 b | 35.60 b | 37.66 | 34.63 b |
| Ferro | 39.48 | 39.46 ab | 39.67 a | 38.50 ab | 37.63 | 37.65 ab |
| VSS-61 F1 | 39.74 | 40.54 a | 40.09 a | 37.33 ab | 37.10 | 39.66 a |
| Cobalt | 39.75 | 39.82 ab | 39.61 a | 37.36 ab | 39.71 | 38.63 ab |
| Bottle gourd | 39.44 | 39.29 b | 39.55 a | 37.75 ab | 38.94 | 36.70 ab |
| Super Shintoza | 39.29 | 39.24 b | 39.81 a | 39.27 a | 38.95 | 39.59 a |
| Prob. F b | ns | * | ** | * | ns | ** |
| 2017 a | 2018 a | |||||
|---|---|---|---|---|---|---|
| Rootstock | Catalase | Peroxidase | Proline | Catalase | Peroxidase | Proline |
| Control (ungrafted) | 0.033 b | 0.080 | 0.16 b | 0.037 b | 0.070 b | 0.17 c |
| Ferro | 0.036 ab | 0.088 | 0.17 b | 0.043 ab | 0.113 a | 0.18 c |
| VSS-61 F1 | 0.045 a | 0.099 | 0.31 a | 0.054 a | 0.078 b | 0.29 b |
| Cobalt | 0.040 a | 0.084 | 0.22 b | 0.040 ab | 0.078 b | 0.20 c |
| Bottle gourd | 0.041 a | 0.104 | 0.37 a | 0.040 ab | 0.120 a | 0.40 a |
| Super Shintoza | 0.045 a | 0.081 | 0.22 b | 0.038 ab | 0.074 b | 0.20 c |
| Prob. F b | * | ns | ** | * | ** | ** |
| 2017 a | 2018 a | |||||
|---|---|---|---|---|---|---|
| Rootstock | N | P | K | N | P | K |
| Control (ungrafted) | 3.84 bc | 0.53 bc | 4.61 ab | 4.82 ab | 0.48 | 3.72 b |
| Ferro | 3.62 bc | 0.71 a | 4.74 a | 4.99 ab | 0.43 | 4.29 a |
| VSS-61 F1 | 3.69 bc | 0.64 ab | 4.48 ab | 4.73 b | 0.44 | 3.29 c |
| Cobalt | 3.92 b | 0.62 ab | 4.62 ab | 5.01 ab | 0.47 | 3.67 b |
| Bottle gourd | 3.48 c | 0.62 ab | 4.12 bc | 5.14 a | 0.49 | 4.03 a |
| Super Shintoza | 4.99 a | 0.47 c | 4.02 c | 5.15 a | 0.52 | 4.15 a |
| Prob. F b | ** | ** | ** | * | ns | ** |
| 2017 a | 2018 a | |||
|---|---|---|---|---|
| Rootstock | Si | Se | Si | Se |
| Control (ungrafted) | 6.42 b | 255 c | 4.56 b | 279 c |
| Ferro | 7.71 a | 301 bc | 5.86 a | 308 bc |
| VSS-61 F1 | 7.55 ab | 507 a | 5.70 ab | 474 a |
| Cobalt | 7.62 a | 349 b | 5.77 a | 406 ab |
| Bottle gourd | 7.80 a | 328 bc | 6.15 a | 402 ab |
| Super Shintoza | 7.47 ab | 332 b | 5.61 ab | 413 ab |
| Prob. F b | * | ** | * | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayoumi, Y.; Abd-Alkarim, E.; El-Ramady, H.; El-Aidy, F.; Hamed, E.-S.; Taha, N.; Prohens, J.; Rakha, M. Grafting Improves Fruit Yield of Cucumber Plants Grown under Combined Heat and Soil Salinity Stresses. Horticulturae 2021, 7, 61. https://doi.org/10.3390/horticulturae7030061
Bayoumi Y, Abd-Alkarim E, El-Ramady H, El-Aidy F, Hamed E-S, Taha N, Prohens J, Rakha M. Grafting Improves Fruit Yield of Cucumber Plants Grown under Combined Heat and Soil Salinity Stresses. Horticulturae. 2021; 7(3):61. https://doi.org/10.3390/horticulturae7030061
Chicago/Turabian StyleBayoumi, Yousry, Emad Abd-Alkarim, Hassan El-Ramady, Farouk El-Aidy, El-Samahy Hamed, Naglaa Taha, Jaime Prohens, and Mohamed Rakha. 2021. "Grafting Improves Fruit Yield of Cucumber Plants Grown under Combined Heat and Soil Salinity Stresses" Horticulturae 7, no. 3: 61. https://doi.org/10.3390/horticulturae7030061
APA StyleBayoumi, Y., Abd-Alkarim, E., El-Ramady, H., El-Aidy, F., Hamed, E.-S., Taha, N., Prohens, J., & Rakha, M. (2021). Grafting Improves Fruit Yield of Cucumber Plants Grown under Combined Heat and Soil Salinity Stresses. Horticulturae, 7(3), 61. https://doi.org/10.3390/horticulturae7030061








