Abstract
The low-node pinching order at a high-density plant cultivation system (LN&HD) is now widely adopted for increasing tomato yield and fruit quality. The LN&HD cultivation period spans 70–120 days, employs the use of a small amount of substrate (low substrate volume), and plants are usually topped between the first and the fourth truss. Using a small amount of substrate in cultivation induces root restriction. Increasing the extent of root restriction in small pots has been adopted for increasing the fruit quality of tomato in some advanced countries. However, improving fruit quality at the expense of yield becomes a major drawback for adopting the LN&HD in Ghana. The LN&HD was introduced into Ghana mainly to increase tomato yield sustainably at a cost-effective level. This study aimed to manipulate or reduce the extent of root restriction to increase tomato yield. Information related to manipulating or reducing the extent of root restriction has not been extensively reported. Thus, an experiment was conducted (between 21 April 2019 and 11 August 2019) in the greenhouse of the University of Ghana Forest and Horticultural Research Centre, Kade-Ghana. Plants of two tomato cultivars (Jaguar and Momotaro York) were subjected to four root restriction conditions. The extent of root restriction were (1) complete root restriction in a 1.0 L volume capacity pot, (2) complete root restriction in a 1.5 L volume capacity pot, (3) partial root restriction in Rockwool-like cultivation, otherwise referred to as Cocowool, and (4) No root restriction in a trough containing 1.5 L of the substrate. The experiment was laid out in a 2 x 4 factorial in a randomized complete block. Results showed that partial root restriction in Cocowool and unrestricted roots in the trough produced the highest tomato yield and total dry matter compared to the plants that received complete root restrictions in the 1.0 and 1.5 L pots. However, the tomato’s total soluble solids increased with a complete root restriction in the 1.0 L pot. Reducing the extent of root restriction increased the yield and total dry matter of tomato. With the LN&HD, a small amount of substrate could be used (at a reduced cost) with a partial root restriction to increase the yield of tropical tomato cultivars grown in Ghana.
1. Introduction
The abysmal tomato production in Ghana has been a congenital challenge. Therefore, efforts are geared toward increasing tomato yield sustainably, using a relatively affordable, adoptable, and practicable cultivation system. In recent times, a cost-effective cultivation system known as the low-node pinching order at high-density planting (LN&HD) has been widely adopted for tomato production [1,2,3]. The LN&HD is a cultivation system in which tomato plants are grown at high density and usually pinched (topped) between the first and the fourth node. Usually, the cultivation period is 70–120 days, while fruits are harvested between the first and the fourth truss. Plants’ growing points are eventually pinched (topped) after the first, second, third, or fourth truss(es) is/are set. This practice reduces the cultivation period. Additionally, this cultivation system has been adopted to sustain a high yield and fruit quality of tomato in Japan [4]. Recently, the LN&HD has been recommended for Ghana to improve the productivity of tropical tomato cultivars at economic levels [5].
The LN&HD requires a small amount of substrate (low substrate volume) for growing plants. Incidentally, the total cost of production could be reduced with a low substrate volume. However, many other cultivation systems require a large amount of substrate. In effect, the total cost of production is increased [6]. Substrate volume influences the crop’s performance. Zhang et al. [7] performed three-truss tomato cultivation in a 0.25 L pot. A yield of 1.3–1.5 kg per plant was reported. Pires et al. [6] cultivated tomato in 5–10 L of a substrate in pots. Results showed that the yield and dry shoot mass were not affected by substrate volume. Substrate volume does not affect plant height [8]. Different kinds of substrates have been used to cultivate tomato [9,10]. Coconut shell fiber (Cocopeat) is suggested to be more efficient for tomato production [11].
The LN&HD involves planting in a small-sized pot, which contains a small amount of substrate. When plants are grown (confined) in the pot, the roots become restricted [12]. Root confinement in the pot induces a complete root restriction in that pot. This is because the root consummates its growth and other physiological activities strictly in the pot. However, the extent of root restriction reduces in Rockwool cultivation compared to the pot. Therefore, Rockwool cultivation techniques could be extrapolated. In this case, the Rockwool substrate is replaced with coconut shell fiber (Cocopeat). Therefore, root restriction in the Cocopeat is denoted as “Cocowool” in this study. The extent of root restriction affects the plant’s performance in terms of growth, yield, and fruit quality. Root-zone restriction in vegetable cultivation is now widespread [13]. Root-zone restriction reduces shoot growth and fruit yield [11], reduces plant height, leaf area, and dry plant mass [14], increases the root-to-shoot ratio [15], and reduces root growth [16]. In addition, root-restricted plants show a reduced shoot fresh weight and reduced fruit yield of tomato [11].
Tomato production, using the LN&HD cultivation system in some previous studies, adopted the nutrient film techniques. In recent studies, plants are now grown in pots specifically to induce root-zone restriction. The main objective of the root-zone restriction in those studies was to increase the fruit quality of tomato. However, increasing the fruit quality of tomato at the expense of yield is a potential drawback for adopting LN&HD in Ghana. Despite the primary focus on fruit quality, the yields obtained are usually fifteen times more than Ghana’s yields. With the LN&HD, this study aimed to manipulate or reduce the extent of root restriction to increase the yield of tomato.
2. Materials and Methods
The study was carried out (between 21 April 2019 and 11 August 2019) at the greenhouse of the University of Ghana, Forest and Horticultural crops Research Centre, Kade, Ghana.
2.1. The Recirculating Hydroponic System
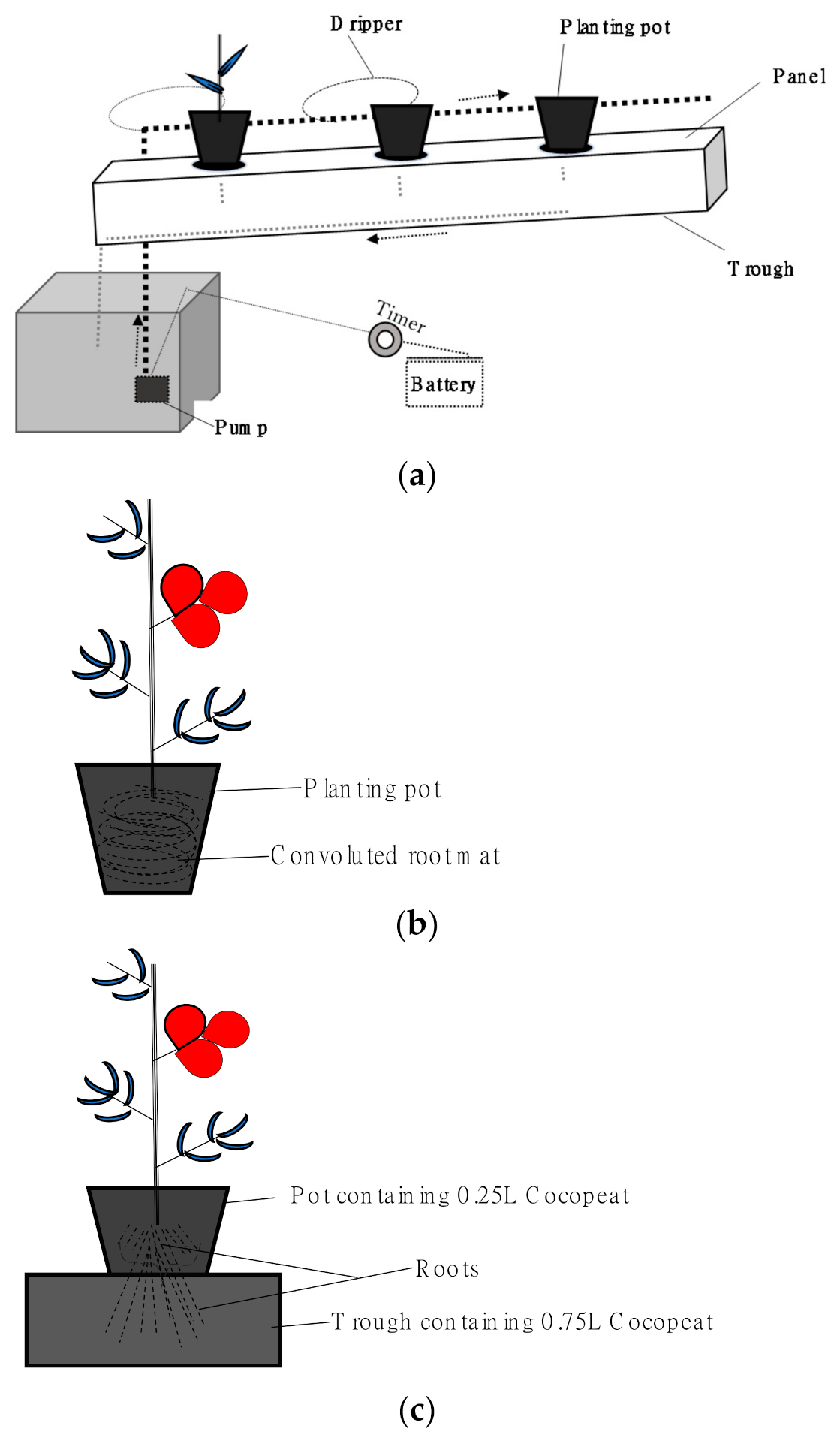
The recirculating hydroponic system was constructed using locally available materials (Figure 1a). Materials obtained for the construction include plastic Tongue & Groove ceiling panels, coconut shell fiber, irrigation tubes and drippers, 12v water pumps, 12 V car batteries, a timer (CN101A) switch, battery charger, wooden slabs, reserve tank, and plastic mulch. The total cost of these materials was 1042 US dollars.
Figure 1.
(a) Recirculating hydroponic system, (b) complete root restriction in 1.0–1.5 L pot, and (c) Partial root restriction in Cocowool (0.25 L pot + 0.75 L trough).
2.2. Treatments
Four root restriction treatments were studied: (1) The first is confining plants in 1.0 L of the substrate in pots to induce complete root restriction (Figure 1b). (2) The second is confining other plants in 1.5 L of the substrate in pots (Figure 1b). The substrate volume is increased by 50% in this treatment. (3) The third is partial root restriction in Rockwool-like cultivation (Figure 1c). This treatment was termed “Cocowool”. The roots in the Cocowool are expected to initially undergo a partial root restriction in a 0.25 L pot. The roots are expected to grow further from the pot into the trough’s main substrate. (4) The fourth is cultivation in a trough with no root restriction. Each plant is exposed to 1.5 L of the substrate in the trough.
Two cultivars of tomato were evaluated in the four root-restricted conditions. The two cultivars were Jaguar and Momotaro York, obtained from Techisem, Savanna Seed Company limited (Longué-Jumelles, France), and TAKII & Co., Ltd., (Kyoto, Japan).
2.3. Cultivation
The seeds were sown in cell trays using the coconut shell fiber (obtained from FibreWealth Limited, Tema, Ghana). Emerged seedlings were manually supplied with 0.1 S m−1 of a nutrient solution according to Enshi standard formula by the ebb and flow method. Seedlings were raised on a moveable bench in the greenhouse. The seedlings were transplanted on the third week after germination.
An automated recirculating drip system was set to feed the transplants. Plants were fed between 8:00 am and 5:00 pm. for two minutes per hour. The nutrient solution recipe adopted according to Enshi standard [17] was 0.7 mM NH4-N, 8 mM NO3-N, 1.3 mM PO4-P, 4 mM K, 2 mM Ca, 1 mM Mg, 2 mM SO4-S, 3 ppm Fe, 0.5 ppm B, 0.5 ppm Mn, 0.02 ppm Cu, 0.05 ppm Zn, 0.01 ppm Mo. The nutrients solution supplied was maintained at electrical conductivity of 0.12 S m−1 and pH 5.5–6.5. The spacing adopted was 1.2 by 0.2 m. Application of 1.0 mL L−1 4-chlorophenoxyacetic acid was carried out on the flowers to enhance fruit set. Plants of the Jaguar cultivar were pinched (topped) on the seventh week after transplanting, while the Momotaro York plants were pinched on the eighth week after transplanting. Pinching was carried out on the third leaf above the third truss to discontinue further plant growth.
2.4. Data Collection
This study determined some growth parameters, yield, dry matter partitioning, and total soluble solids.
Plant height was measured between the second and the eighth weeks after transplanting (WAT). The plant’s tallness was measured from the base to the plant’s uppermost part with a ruler. Leaf number per plant was counted on the eighth week after transplanting. Leaves were detached from three randomly selected plants per treatment and photo-scanned with a photo camera. Leaf area was obtained using Lia25 software (https://www.agr.nagoya-u.ac.jp/~shinkan/LIA32/, accessed on 20 July 2019) to analyze the scanned leaves. The leaf area ratio (LAR) was calculated as the total leaf area divided by the total plant dry mass at eight WAT.
Plant dry matter production and partitioning to fruits were determined at the last harvest (16 WAT). Three plants were sampled at random from each treatment and destructively collected. Each sample (all plant parts and all harvested fruits) was oven-dried for seven days at 60 °C. The root-to-shoot ratio was determined as a ratio of the plant’s root dry mass to the dry shoot mass. Root tissue density (RTD) was determined, according to the formula of Birouste et al. [18], as the ratio between the plant’s root dry mass and fresh root mass. RTD was determined at the last harvest.
Fruits were harvested from the three trusses per plant to determine the fruit number and fresh fruit weight. Yield per unit area was obtained as the fresh fruit weight per plant, multiplied by 4.1. The total soluble solids (TSS) of the fruit were measured using the K-BA100R spectrophotometer (Kubota, Yao, Japan) to scan the fruits.
2.5. Experimental Design and Data Analysis
The experiment was laid out in a 2 × 4 factorial in randomized complete block design with three replications. Data collected were analyzed using GenStat (Rothamsted Research, Harpenden, UK) while Tukey’s honest significant difference (HSD(0.05)) was used to separate the means at p < 0.05.
3. Results
3.1. Plant Height
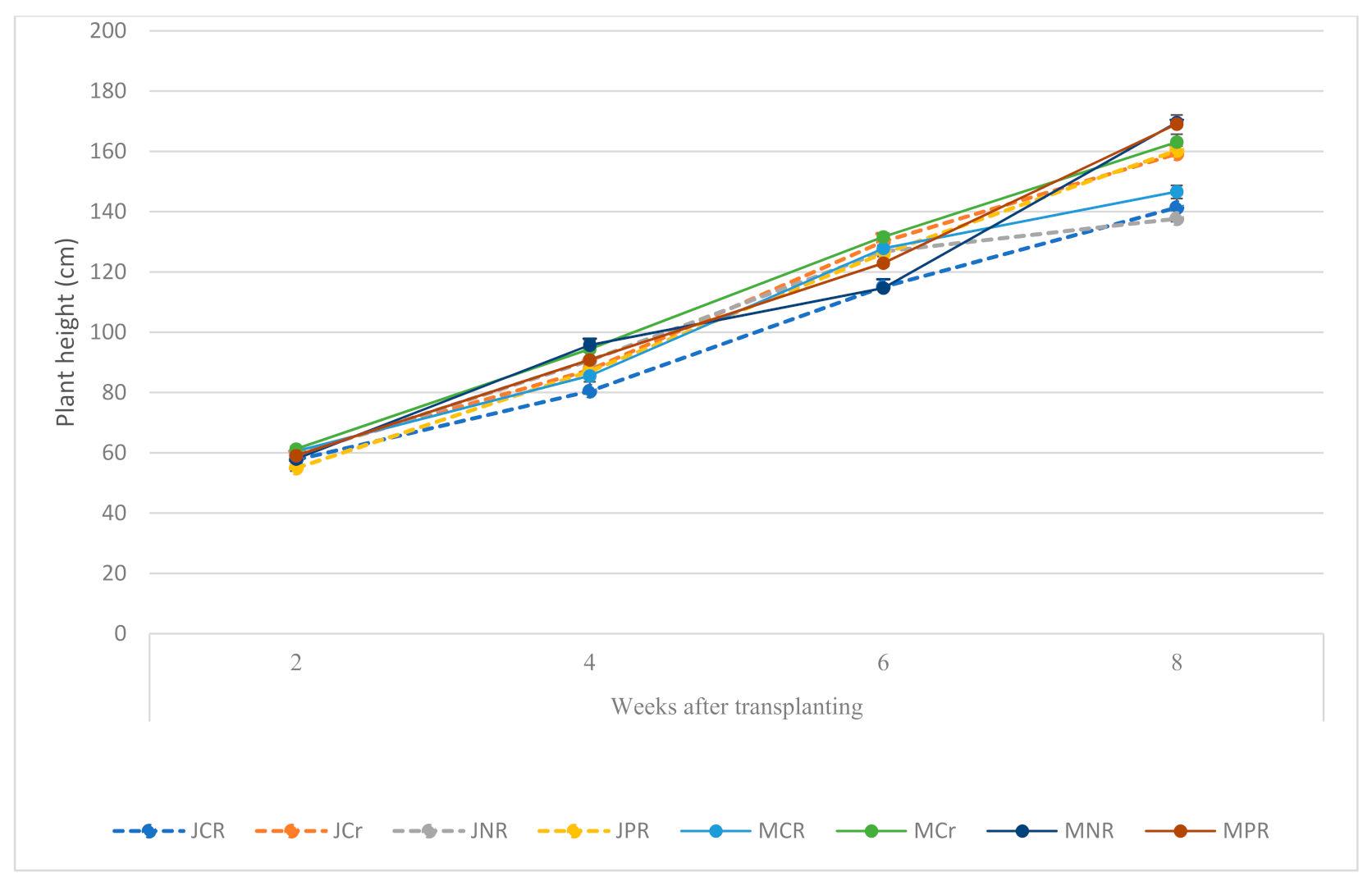
Upon final pinching on the eighth week after transplanting, the Momotaro plants were significantly taller than the Jaguar plants (Figure 2). Plant height was markedly influenced by root restriction. In the two cultivars, growth (plant height) was reduced for plants subjected to complete root restriction in the pots compared to the partial and the unrestricted plants.

Figure 2.
Plant height of tomato cultivars in response to root restriction J, M = Jaguar, Momotaro York cultivars; CR = complete root restriction in 1.0 L pot; Cr = complete root restriction in 1.5 L pot; PR = partial root restriction in Cocowool; NR = no root restriction; Every point represents the mean of three replications ±SE.
3.2. Growth Parameters
3.2.1. Leaf Number
The number of leaves varied significantly between the two cultivars at eight WAT. Momotaro York (MY) plants produced a larger number of leaves than Jaguar (Table 1). Root restriction affected the number of leaves produced per plant significantly. Plants that received complete root restriction in the pots recorded fewer leaves than those in the other treatments. Root restriction with cultivar interactions affected leaf number. The unrestricted MY plants produced the highest number of leaves per plant.

Table 1.
Effect of root restriction (RR) on plant growth parameters of tomato cultivars at 8 weeks after transplanting (WAT).
3.2.2. Leaf Area
The cultivars varied markedly in terms of leaf area (Table 1). MY recorded 946 cm2 of leaf area more than Jaguar. The unrestricted plants recorded a higher leaf area than those in the other treatments. The highest leaf area was recorded in MY plants, which were unrestricted in the trough. For the two cultivars, complete root restriction reduced leaf area than partial or no root restriction.
3.2.3. Leaf Area Ratio
The two cultivars differed in terms of leaf area ratio (Table 1). MY plants recorded 7.1 cm2 g−1 of leaf area ratio more than Jaguar. The leaf area ratio recorded in the partial or unrestricted plants was higher than plants subjected to complete root restriction. Complete root restriction in the two cultivars had a lower leaf area ratio than the partial and the unrestricted.
3.3. Dry Matter Partitioning
3.3.1. Total Plant Dry Matter
There was a variation in the total plant dry matter (TPDM) produced per plant in the cultivars (Table 2). The TPDM recorded in MY was 30.3 g more than Jaguar. Dry matter production was markedly affected by root restriction. Plants that received partial root restriction in the Cocowool produced the highest TPDM. Potted plants with a complete root restriction recorded the lowest TPDM. The interaction of root restriction with the cultivars influenced the TPDM significantly. For the Jaguar, the highest TPDM was observed in the plants that were partially root restricted in the Cocowool. Momotaro plants, which received complete root restrictions in the 1.0 L and the 1.5 L pots, showed no variation in TPDM production.

Table 2.
Effect of root restriction (RR) on plant dry matter partitioning and root tissue density of tomato cultivars at 16 WAT.
3.3.2. Dry Matter Partitioned to Root
Dry matter partitioned to root (RDM) was influenced by root restriction (Table 2). Plants subjected to partial root restriction recorded the lowest RDM compared to other treatments. Complete root restriction in the 1.0 and 1.5 L pots was similar in terms of RDM. RDM in the two cultivars was affected by the extent of root restriction. The cultivars recorded a low RDM when partially restricted in Cocowool. Plants that received complete root restriction in the pots showed a similar RDM. Conversely, plants cultivated in unrestricted conditions recorded the highest RDM in the two cultivars.
3.3.3. Dry Matter Partitioned to Fruit
Dry matter partitioned to fruit in the two cultivars varied significantly. The MY plants partitioned 21.3 g more of dry matter to fruit than Jaguar (Table 2). Partitioning of dry matter to fruit in the complete root restricted plants reduced by 20 g and 13 g compared to the Cocowool and the trough cultivation. Dry matter allocation to fruit was affected markedly by root restriction and cultivar interactions. For Jaguar, plants partially root-restricted in Cocowool allocated more dry matter to fruit than the pots’ complete root-restricted plants. Similarly, the dry matter allocated to fruit in the complete restricted MY plants was lower than the unrestricted plants.
3.3.4. Root to Shoot Ratio
The root-to-shoot ratio varied in the two cultivars (Table 2). Jaguar cultivar recorded 56 mg g−1 of root-to-shoot ratio more than MY. The root-to-shoot ratio was markedly influenced by root restriction. The unrestricted roots recorded the highest root-to-shoot ratio. However, plants that received complete root restriction in the pots recorded a higher root-to-shoot ratio than those partially restricted. The root-to-shoot ratio in the two cultivars was influenced by the extent of root restriction. Plants grown with partial root restriction for the two cultivars recorded the lowest root-to-shoot ratio than the other treatments.
3.3.5. Root Tissue Density
The two cultivars varied markedly in terms of root tissue density (Table 2). The MY recorded 18.6 mg g−1 of root tissue density more than Jaguar. The extent of root restriction influenced the root tissue density of the plants. Plants that received partial root restriction in the Cocowool recorded the lowest root tissue density compared to the other treatments. The unrestricted plants recorded the highest root tissue density. In terms of an interaction between cultivars and root restriction, root tissue density was significantly affected. A partial root restriction of Jaguar plants in the Cocowool recorded the lowest root tissue density. The MY plants, on the other hand, responded differently to root restriction.
3.4. Yield and Total Soluble Solids
3.4.1. Fruit Number Per Plant
The number of fruits produced was not affected by cultivar, root restriction, or their interactions (Table 3).

Table 3.
Effect of root restriction (RR) on the yield and total soluble solids of tomato cultivars.
3.4.2. Yield Per Unit Area
The fresh fruit weight (yield) per unit area differed markedly in the two cultivars (Table 3). The MY cultivar produced 800 g more yield than Jaguar. The unrestricted plants recorded the highest yield and followed by the partially restricted plants. Plants that received complete root restriction in pots produced the lowest yield. Cultivar and root restriction interaction affected the yield of tomato. The unrestricted or partially restricted Jaguar plants recorded the highest yield compared to those subjected to complete root restriction. The highest yield in the MY cultivar was recorded in the unrestricted plants and followed by the partially restricted plants.
3.4.3. Total Soluble Solids
The two cultivars varied in terms of total soluble solids (Table 3). Plants of MY produced higher total soluble solids than Jaguar. Plants cultivated in the pots with complete root restriction were higher in total soluble solids than the partial and the unrestricted. In the two cultivars, plants subjected to complete root restriction in pots were higher in total soluble solids than the partial or the unrestricted.
4. Discussion
4.1. Plant Growth Parameters
The difference in the plant height between the two cultivars was that Momotaro York (MY) had a longer internode length. Due to the longer internode length, pinching was delayed until the eighth week, hence the observed taller plants in the Momotaro York. Plants subjected to complete root restriction in the 1.0 and 1.5 L pots grew shorter than plants in the other treatments. This was associated with a reduced root growth, which consequently reduced the shoot growth. This result agreed with Mugnai and Al-Debei [14], who reported that plant height significantly reduces with root restriction.
The leaf area recorded in MY was higher than that in Jaguar. MY produced a larger number of leaves with a broader leaf surface than Jaguar. The pots’ complete root restriction reduced leaf area compared to the unrestricted and the partially restricted plants. A 9–14% reduction in leaf area for plants subjected to complete root restriction was observed compared to the partial and the unrestricted. This study’s findings agreed with that of Mugnai and Al-Debei, and Kharkina et al. [14,19], which reported that leaf area reduces with root restriction. The capacity for plants to accumulate dry matter (leaf area ratio) was higher in MY than in Jaguar. Such observation was due to a higher leaf area recorded in the former. The plant’s capacity to accumulate dry matter decreased with an increase in the extent of root restriction. Complete root restriction reduced 10–14% of the plant’s capacity to accumulate dry matter compared to partial or no root restriction.
4.2. Dry Matter Partitioning
MY plants were taller, with individual broader leaves and larger leaf number. That accounted for the higher total plant dry matter (TPDM) production compared to Jaguar. The taller plants might have had a higher amount of photosynthates stored in the stems. Complete root restriction in the 1.0 L and 1.5 L pots were similar in terms of TPDM produced. TPDM reduced by 10% and 7% in the completely root-restricted plants compared to the partial and the unrestricted plants. This observation might be associated with a low sink demand in the completely restricted roots. A low sink demand is capable of reducing the accumulation of photosynthates in the sink organs. It was emphasized by Franck et al., Nakano et al., Scofield et al., and Velez-Ramirez et al. [20,21,22,23] that assimilates produced in root-restricted plants exceed their utilization; therefore, the assimilates are usually stored in the leaves and the stems.
Conversely, it was observed that plants subjected to partial root restriction in the Cocowool method produced the highest TPDM compared to the other treatments. Initial root growth in the 0.25 L pot of the Cocowool method was very limited due to extreme initial root restriction. There was a further growth of young finer roots into the substrate of the underlying trough. The young finer roots did not add significantly to the root dry matter due to the low root tissue density. The finer roots are believed to be efficient in nutrient and water uptake for rapid plant expansion and more dry matter production in the Cocowool method. However, the two cultivars responded differently in terms of TPDM.
Partial root restriction in Cocowool reduced RDM compared to the complete and the unrestricted plants. Plants in the complete restriction formed a larger mass of convoluted roots than in the partial. RDM was reduced by 17–20% for partial root restriction than the complete and the unrestricted. An increase in substrate volume by 50% in the pot condition did not affect RDM, as indicated in Ismail and Noor [16].
Dry matter partitioned to MY’s fruit was 22% more than Jaguar due to the higher TPDM produced in the former. A 13–20% reduction in dry matter allocated to fruit was observed for complete root restriction in pots compared to the unrestricted or partial restriction in Cocowool. Cocowool cultivation, with a partial root restriction, favored the highest allocation of dry matter to fruit. That was an indication of a higher translocation of photosynthates to fruits, as sink organs.
4.3. Yield and Total Soluble Solids
The yield per unit area in terms of fresh fruit weight recorded in MY was higher than in Jaguar. That was due to a higher partitioning of dry matter to fruit in the former. Dueck et al., Li et al., and Qian et al. [24,25,26] stressed that tomato fruit sink strength differs at different stages of growth and in different cultivars. Plants subjected to complete root restriction in the 1.0 L and the 1.5 L pots encountered a yield reduction of 9–10% per unit area, compared to the other treatments. The unrestricted and the partially restricted plants might have impacted little or partial limitation on the roots’ growth, thus enhancing the translocation of more dry matter to fruit. Pires et al. [6] indicated that root restriction did not affect tomato yield. Contrarily, results from this study showed that the yield of tomato increased with the extent of root restriction. The result supports the findings of Saito et al. [8] that tomato yield decreases as root restriction increases. The yield recorded in this study was similar to 9.6–10.0 kg m−2 reported by Ayarna et al. [5] in four-truss cultivation. This comparison further explains that root-zone restriction and the extent of restriction affect tomato yield.
The total soluble solids were higher in the MY fruit than Jaguar due to genetic discrepancies between the two cultivars. The MY might be one of the cultivars originally bred for high fruit quality in Japan [27]. Complete root restriction in the small pots recorded the highest total soluble solids compared to the other treatments. This might be attributed to a reduced root growth, which perhaps limits the uptake of water and nutrients. This study’s results contradict the findings of Saito et al. [8] that root restriction has no significant effect on tomato’s total soluble solids. A 50% increase in the substrate volume of a 1.0 L pot with complete root restriction reduced the total soluble solids by 9%, depending on the cultivar. The total soluble solids of tomato, as influenced by root restriction, vary in different cultivars. The extent of root restriction influences the performance of tomato.
5. Conclusions
Cultivation of plants in different root restricted conditions affected tomato’s performance in terms of growth, dry mass partitioning, and the total soluble solids. Complete root restriction of plants in small pots of 1.0–1.5 L of substrate reduced tomato yield by 9–10% per unit area. This root restriction method instead, increased the total soluble solids at the expense of yield. A partial restriction of the root in 1.0 L of a substrate in a Cocowool method increased tomato yield at the total soluble solids’ expense. Additionally, the roots of plants cultivated in 1.5 L of a substrate in a trough (with no root restriction) increased tomato yield. Plants varied in response when cultivated in the same substrate root volume with differing root restriction degrees. Tomato cultivars responded differently to the cultivation conditions of root restriction. The low substrate volume cultivation of tomato can be adopted to increase Ghana’s tomato yield and reduce production costs. Incidentally, Ghana is currently placing more emphasis on the yield of tomato. Therefore, it is worthwhile to recommend the partial root restriction in Cocowool for increasing tomato yield at economic levels. The LN&HD is a useful and effective tool for improving the yield of tomato cultivars grown in Ghana. However, with less root restriction (Cocowool cultivation), potential drawbacks of LN&HD are resolved.
Author Contributions
Conceptualization, A.W.A. and S.T.; methodology, A.W.A.; S.T. and G.O.N. software, A.W.A.; validation, S.T., G.O.N.; formal analysis, A.W.A. and S.T.; investigation, A.W.A. and S.T. And G.O.N.; resources, S.T. and G.O.N.; data curation, A.W.A. and S.T.; writing—original draft preparation, A.W.A.; writing—review and editing, S.T and G.O.N.; visualization, A.W.A., S.T. and G.O.N.; supervision, S.T.; project administration, A.W.A.; funding acquisition, S.T. and G.O.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Takahashi, T.; Ishigami, Y.; Goto, E.; Niibori, K.; Goto, K. Modeling the growth and yield of tomatoes cultivated with a low node-order pinching system at high plant density. Environ. Control Biol. 2012, 54, 53–61. [Google Scholar] [CrossRef]
- Tamai, D. The practical cultivation and technologist training in tomato low node order pinching and high-density planting cultivation. Shisetsu-To-Engei 2014, 165, 62–65. [Google Scholar]
- Watanabe, S. New growing system for tomato with low node-order pinching and high-density planting. Proceed. Vege. Tea Sci. 2006, 3, 91–98. [Google Scholar]
- Johkan, M.; Ishii, M.; Maruo, T.; Na, L.; Tsukagoshi, S.; Hojoh, M.A.; Nakaminami, A.; Shinohara, Y. Improved light conditions at the fruit truss accelerate harvest time and enhance ascorbic acid concentration in a low-truss, high-density tomato production system. J. Jpn. Soc. Hort. Sci. 2013, 82, 317–321. [Google Scholar] [CrossRef][Green Version]
- Ayarna, A.W.; Tsukagoshi, S.; Nkansah, G.O.; Lu, N.; Maeda, K. Evaluation of Tropical Tomato for Growth, Yield, Nutrient, and Water Use Efficiency in Recirculating Hydroponic System. Agriculture 2020, 10, 252. [Google Scholar] [CrossRef]
- Pires, R.C.d.M.; Furlani, P.R.; Ribeiro, R.V.; Décio, B.J.; Sakai, E.; Lourenção, A.L.; Neto, A.T. Irrigation frequency and substrate volume affect the growth and yield of tomato plants under greenhouse conditions. Sci. Agric. (Piracicaba Braz.) 2011, 68, 400–405. [Google Scholar] [CrossRef]
- Zhang, Y.; Kiriiwa, Y.; Nukaya, A. Influence of Nutrient Concentration and Composition on the Growth, Uptake Patterns of Nutrient Elements, and Fruit Coloring Disorder for Tomatoes Grown in Extremely Low-volume Substrate. Jpn. Soc. Hortic. Sci. 2015, 84, 37–45. [Google Scholar]
- Saito, T.F.; Iikubo, N.; Inai, T.; Fuji, S.T.; Konishi, C.; Ezura, H. Effects of Root-volume Restriction and Salinity on the Fruit Yield and Quality. J. Japan. Soc. Hort. Sci. 2008, 77, 165–172. [Google Scholar] [CrossRef][Green Version]
- Sampaio, R.A.; Ramos, S.J.; Guilherme, D.O.; Costa, C.A.; Fernandes, L.A. Tomato seedlings production using substrates with coconut fiber and rock waste. Hort. Bras. 2008, 26, 499–503, (In Portuguese, with abstract in English). [Google Scholar] [CrossRef]
- Sezen, S.M.; Celikel, G.; Yazar, A.; Tekin, S.; Kapur, B. Effect of irrigation management on yield and quality of tomatoes grown in different soilless media in a glasshouse. Sci. Res. Essay 2010, 5, 41–48. [Google Scholar]
- Pires, R.C.M.; Furlani, P.R.; Sakai, E.; Lourenção, A.L.; Silva, E.A.; Torre, N.A.; Melo, A.M.T. Tomato development and yield under different irrigation frequencies in the greenhouse. Hort. Bras. 2009, 27, 228–234, (In Portuguese, with abstract in English). [Google Scholar] [CrossRef]
- Mugnai, S.; Ferrante, A.; Petrognani, L.; Serra, G.; Vernieri, P. Stress-induced variation in leaf gas exchange and chlorophyll a fluorescence in Callistemon plants. Res. J. Biol. Sci. 2009, 4, 913–919. [Google Scholar]
- Shi, K.; Fu, L.J.; Ding, X.T.; Dong, D.K.; Zhou, Y.H.; Yu, J.Q. Root restriction-induced limitation to photosynthesis in tomato leaves. Sci. Hort. 2008, 117, 197. [Google Scholar] [CrossRef]
- Mugnai, S.; Al-Debei, H.S. Growth reduction in root-restricted tomato plants is linked to photosynthetic impairment and starch accumulation in the leaves. Adv. Hortic. Sci. 2011, 25, 99–105. [Google Scholar]
- Mugnai, S.; Vernieri, P.; Tognoni, F. Container volume effects on morphology and physiology of tomato seedlings. Acta Hortic. 2000, 516, 49–56. [Google Scholar] [CrossRef]
- Ismail, M.R.; Noor, K.M. Growth, water relations and physiological processes of starfruit plants under root growth restriction. Sci. Hort. 1996, 66, 51–55. [Google Scholar] [CrossRef]
- Hori, H. Gravel culture of vegetable and ornamental crops. (Japanese text). Agric. Hortic. 1966, 210. [Google Scholar]
- Birouste, M.; Zamora Ledezma, E.; Bossard, C.; Pérez-Ramos, I.M.; Roumet, C. Measurement of fine root tissue density: A comparison of three methods reveals the potential of root dry matter content. Plant Soil 2014, 374, 299–313. [Google Scholar] [CrossRef]
- Kharkina, T.G.; Ottosen, C.O.; Rosenqvist, E. Effects of root restriction on growth and physiology of cucumber plants. Physiol. Plant. 1999, 105, 434. [Google Scholar] [CrossRef]
- Franck, N.; Vaast, P.; Génard, M.; Dauzat, J. Soluble sugars mediate sink feedback down-regulation of leaf photosynthesis in field-grown Coffea arabica. Tree Physiol. 2006, 26, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Muramatsu, S.; Makino, A.; Mae, T. Relationship between the suppression of photosynthesis and starch accumulation in the pod-removed bean. Funct. Plant Biol. 2000, 27, 167–173. [Google Scholar] [CrossRef]
- Scofield, G.N.; Ruuska, S.A.; Aoki, N.; Lewis, D.C.; Tabe, L.M.; Jenkins, C.L.D. Starch storage in the stems of wheat plants: Localization and temporal changes. Ann. Bot. 2009, 103, 859–868. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; van Ieperen, W.; Vreugdenhil, D.; van Poppel, P.M.; Heuvelink, E.; Millenaar, F.F. A single locus confers tolerance to continuous light and allows substantial yield increase in tomato. Nat. Commun. 2014, 5, 4549. [Google Scholar] [CrossRef] [PubMed]
- Dueck, T.A.; Janse, J.; Schapendonk, A.H.C.M.; Kempkes, F.L.K.; EveleensClark, B.A.; Scheffers, C.P. Lichtbenutting van Tomaat onder LED en SON-T Belichting; Wageningen UR Glastuinbouw/Plant Dynamics BV, Rapporten GTB: Wageningen, The Netherlands, 2010; p. 1040. [Google Scholar]
- Li, T.; Heuvenlink, E.; Marcelis, L.F.M. Quantifying the source-sink balance and carbohydrate contents in three tomato cultivars. Front. Plant Sci. 2015, 6, 416. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Dieleman, J.A.; Elings, A.; Marcelis, L.F.M. Leaf photosynthetic and morphological responses to elevated CO2 concentration and altered fruit number in the semi-closed greenhouse. Sci. Hortic. 2012, 145, 1–9. [Google Scholar] [CrossRef]
- Higashide, T.; Yasuba, K.I.; Suzuki, K.; Nakano, A.; Ohmori, H. Yield of Japanese tomato cultivars has been hampered by a breeding focus on flavor. HortScience 2012, 47, 1408–1411. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).