1. Introduction
Senescence is a highly regulated process that represents a developmental transition from anabolism and growth to catabolism and cell death in organs, tissues, or whole plants. Developmental age, in combination with various endogenous and exogenous signals, determines the timing of senescence [
1]. Ethylene is the primary hormonal signal regulating the initiation and progression of senescence in both leaves and flowers. External signals that influence senescence include environmental stresses like drought, nutrient limitations, low light levels, and temperature extremes, as well as biotic factors like pathogens [
2,
3].
Leaf and petal senescence are both accompanied by the upregulation of thousands of genes, and the progression of senescence in these organs requires de novo protein synthesis. Many of the upregulated genes encode enzymes involved in the degradation of proteins, nucleic acids, cell wall carbohydrates, and membrane lipids [
4,
5,
6,
7]. While organic molecules are degraded, organelles like the mitochondria and the nucleus must be maintained until late in the senescence process to allow for transcription of senescence-associated genes (SAGs) and to provide energy for cellular catabolism. This organized disassembly of macromolecules allows nutrients to be remobilized from older leaves and flowers to new sink tissues [
8,
9,
10]. In pollinated flowers, senescence allows nutrients to be reallocated from the dying petals to the developing ovary before the corolla is shed [
11,
12]. In both leaves and petals, cell death and associated macromolecule degradation starts in the margins and spreads inward, allowing for the maintenance of the vascular system until the final stages of senescence to facilitate nutrient remobilization via the phloem [
2,
13,
14,
15].
A characteristic feature of senescence is the hydrolysis of nucleic acids. Leaf and petal senescence are accompanied by large decreases in extractable RNA and DNA. Programmed cell death (PCD) at the advanced stages of senescence involves the fragmentation of nuclear DNA, which can be visualized as internucleosomal fragments or DNA ladders [
2,
16]. Senescence-associated nucleases hydrolyze the phosphodiester linkages in nucleic acids, releasing nucleotide bases that can serve as a source of nitrogen and phosphorus for remobilization to developing tissues [
17,
18]. Most of the nucleases that are involved in PCD are endonucleases, and activities against RNA, double-stranded DNA (dsDNA) and single-stranded DNA (ssDNA) are upregulated during petal and leaf senescence [
19,
20,
21,
22,
23,
24,
25,
26].
Plants have two major types of endonucleases, which are distinguished by their divalent cation cofactors and pH optima. Zn
2+-dependent endonucleases (type I) require Zn
2+ for catalytic activity and typically have an acidic pH optimum, while Ca
2+-dependent endonucleases (type II) have a requirement for Ca
2+ and are more active in the neutral pH range. Many Ca
2+-dependent endonucleases preferentially degrade ssDNA over RNA, and Zn
2+-dependent endonucleases prefer RNA and ssDNA to dsDNA as substrate. Both types of endonucleases are upregulated during senescence [
17,
27].
While most of the work on characterizing endonuclease activity and gene expression during senescence has been in leaves, endonucleases are also upregulated during the senescence of flower petals [
7,
19,
21,
23,
28]. The upregulation of endonucleases with activity against RNA, ssDNA and dsDNA is observed during the age-related senescence of petals from unpollinated flowers and during pollination-induced senescence in petunias. This increased nuclease activity occurs later in the senescence program concomitant with DNA fragmentation, ethylene production, and corolla wilting [
21,
23]. Multiple senescence induced endonucleases with activity against RNA, ssDNA, and dsDNA are enhanced by Ca
2+ in
Petunia inflata petals [
21]. In-gel activity assays show that some nucleases are detected constitutively in non-senescing petals with increasing activity during senescence, while some senescence-specific nucleases are detected only in senescing petals [
21,
23]. In
Petunia ×
hybrida, a senescence-specific endonuclease was identified that has activity against ssDNA, dsDNA, and RNA [
23]. This 43-kDa bifunctional endonuclease, which was identified from in-gel activity assays, was named PhNUC1. PhNUC1 activity is detected in the presence of Ca
2+ and activity is inhibited by Zn
2+, suggesting it could be a Ca
2+ -dependent endonuclease. Interestingly, the activity of PhNUC1 is greatly enhanced by the addition of Co
2+, and Co
2+ can overcome inhibition of the activity by Zn
2+. The activity of PhNUC1 is induced by ethylene, and it is a glycoprotein with a pH optimum around 7.5 [
23]. Only a few other endonuclease activities have been shown to be enhanced by Co
2+ [
25,
26]. Senescence-associated genes encoding endonucleases have also been identified from daylily (
Hemerocallis sp.) and carnation (
Dianthus caryophyllus) flowers [
29,
30].
The senescence specific activity of PhNUC1 and its regulation by ethylene suggest that this endonuclease may catalyze the degradation of nucleic acids during petal senescence, but further investigation is needed to confirm this role. A proteomic analysis of pollination-induced corolla senescence identified an endonuclease (protein 14-12) with an observed molecular mass (
Mr) of 43-kDa, that was detected only in senescing petals at 48 and 72 h after pollination [
31]. The goal of this paper was to clone the gene encoding the senescence specific protein (14-12) and determine if the cobalt-enhanced, bifunctional endonuclease PhNUC1 is a product of that gene. The gene was named
PhENDO1 according to the nomenclature recommendations by Triques et al. [
32]. Down-regulating
PhENDO1 in petunia using virus induced gene silencing (VIGS) demonstrated that PhNUC1 was a product of the
PhENDO1 gene. Down regulating this senescence specific activity was not sufficient to delay flower senescence
. PhENDO1 gene expression was upregulated during petal senescence, by ethylene treatment, and during phosphorus (P) starvation, supporting a role in nutrient remobilization during developmental and stress induced PCD.
2. Materials and Methods
2.1. Plant Material
Petunia × hybrida ‘Mitchell Diploid’ (MD) were used in all experiments unless otherwise stated. Comparative analyses using MD plants transformed with 35S:etr1-1 (line 44568; referred to as etr1-1 petunias) were conducted to evaluate the role of ethylene in gene expression. Etr1-1 petunia seeds were obtained from Dr. David Clark (University of Florida). Seeds were treated with 100 mg L−1 GA3 for 24 h and sown in cell-packs on top of soil-less mix (Promix BX, Premier Horticulture, Quakertown, PA, USA). All plants were established in the greenhouse after germination and plants were transferred to 16-cm pots after 4 weeks. Plants were fertilized at each watering with 150 mg L−1 Nitrogen (N) from Scott’s Excel 15N-2.2P-12.5K-3.6Ca-1.2Mg (The Scotts Co., Marysville, OH, USA). A one-time treatment of Soluble Trace Element Mix (S.T.E.M., The Scotts Co.) was applied four weeks after transferring to 16-cm pots. Temperature in the greenhouse was set at 24/16 °C (day/night) with a 13-h photoperiod supplemented by high pressure sodium and metal halide lights.
2.2. Collection of Senescing Corollas
Flowers were emasculated 1 d before opening to prevent self-pollination. To study pollination-induced senescence, flowers were pollinated on the day of flower opening by brushing pollen from freshly dehisced anthers onto the stigma. Alternatively, flowers were emasculated and left unpollinated to senesce naturally. Zero h after pollination (hap) and 0 d represent unpollinated flowers on the day of flower opening. Petunia corollas (the fused petals on a petunia flower are collectively called the corolla) were collected from pollinated flowers at 0, 24, 48, and 72 h after flower opening, flash frozen in liquid nitrogen, and stored at −80 °C for subsequent RNA or protein extraction. Corollas from unpollinated flowers were collected from MD and etr1-1 petunias on various days from flower opening through corolla wilting to evaluate natural senescence. Four replicates, each containing eight corollas from at least three different plants, were collected at each time point. Tissue was flash frozen in liquid nitrogen and stored at −80 °C prior to RNA extraction.
2.3. Ethylene and Cycloheximide Treatment of Flowers
Flowers were removed from plants 1 d after flower opening and placed in vials of deionized water or 50 µM cycloheximide, an inhibitor of protein synthesis. Flowers were then sealed in 24-L chambers and treated with air (control 0 µL L−1 ethylene) or 0.1 µL L−1 ethylene for 4 h (n = 12). The control chambers contained potassium permanganate (Ethylene Control, Selma, CA, USA) to absorb any ethylene produced by the flowers. After 4 h of treatment, corollas were harvested, flash frozen in liquid nitrogen, and stored at −80 °C. Four biologicals replicates, each containing three corollas were pooled for subsequent RNA extraction.
2.4. Nutrient Deficiency Treatments
Petunia seeds were germinated as previously described. Four-week-old seedlings were transplanted, three seedlings per pot, into 11-cm pots containing coarse perlite mixed with a wetting polymer (Soil Moist, JRM Chemicals, Cleveland, OH, USA). Treatments included complete nutrient solution, -N, -P and -K. Six pots received the different treatments. Pots contained a capillary wick and nutrient solutions were applied to trays for capillary uptake as well as top watered with fresh solution daily for 4 weeks. Nutrient solutions were prepared according to the recipes in Machlis and Torrey [
33] and as previously described in Quijia Pilajio et al. [
34]. After four weeks of treatment, all the leaves from each plant were harvested, and the leaves from the three plants in a single pot were pooled, frozen in liquid nitrogen, and stored at −80 °C for RNA extraction.
2.5. Cloning the Gene Encoding the Senescence-Specific Endonuclease PhNUC1
Total proteins were extracted from corollas, separated by two-dimensional gel electrophoresis (2-DE), and identified by mass spectrometry as described previously [
31]. Protein profiles of pollinated corollas were compared to unpollinated corollas at the same developmental age and during the progression of pollination-induced senescence to identify differentially expressed proteins for sequence analysis. Mass spectrometry was conducted at the Cleveland Clinic Proteomics Laboratory (Cleveland Clinic Foundation, Cleveland, OH, USA). Protein abundance was calculated using PDQuest v7.40 (Bio-Rad Laboratories, Hercules, CA, USA) as described in Bai et al. [
31].
RT-PCR was used to clone the gene (
PhENDO1) encoding a senescence-specific endonuclease (14-12) previously identified in a large-scale proteomic analysis of senescing versus non senescing petunia corollas [
31]. Total RNA was isolated from senescing petunia corollas at 72 h after pollination and first-strand cDNA was synthesized using the Omniscript Reverse Transcriptase kit (Qiagen, Valencia, CA, USA). Additional details on RNA extraction and cDNA synthesis can be found below in the section RNA extraction and gene expression analysis. Specific primers were designed based on the peptide sequences of 14-12 and other senescence-associated nucleases (
Table S1). Using 2 µg cDNA as template, a fragment of a petunia endonuclease was amplified. The remaining 5′ and 3′ cDNA sequences were isolated by rapid amplification of cDNA ends (RACE) (SMART RACE kit, Clontech, Mountain View, CA, USA), and the full-length
PhENDO1 cDNA was then isolated by RT-PCR. Primer sequences can be found in
Table S1. Sequence information for the full-length cDNA was obtained by capillary sequencing at the Molecular and Cellular Imaging Center (The Ohio State University/OARDC, Wooster, OH, USA) and analyzed with ChromasPro and the BLAST algorithm from NCBI non-redundant database. Sequence for
PhENDO1 can be found in the GenBank database Accession No. MW247148.
2.6. RNA Extraction and Gene Expression Analysis
Total RNA was extracted from petunia tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and RNA was treated with RQ1 RNase-free DNase (Promega, Madison, WI, USA). Complementary DNA (cDNA) was synthesized from 2 µg RNA using the Omniscript Reverse Transcriptase kit (Qiagen). Quantitative PCR was performed in a 20 µL reaction volume using B-R SYBR Green Master Mix (Quanta BioSciences, Gaithersburg, MD, USA). One microliter cDNA was used as template, and all reactions were performed in triplicate. PCR was conducted for 40 cycles of 94 °C for 10 s, 60 °C for 30 s, 72 °C for 15 s using the iQ5 Thermocycler (BioRad, Hercules, CA, USA).
PhCP10, a senescence-specific cysteine protease from petunia (Genbank #AY662996; [
35]), was used as a molecular marker for senescence [
36]. Primers (
Table S1) were designed to amplify transcripts using IDT primer Quest.
Melt curves were generated to check amplification specificity, and standard curves were generated to determine reaction efficiencies. An optimized delta delta CT method was used to determine relative target gene expression by using the reaction efficiencies and normalizing target gene expression to that of the reference gene
PhACTIN (Actin2/7 GenBank #CV299322). In the second VIGS experiment (described below), the normalized relative expression was calculated using arithmetic means of relative quantity for the target gene and two reference genes:
PhACTIN and
PhRPS13 (SGN# U209515). Both genes have previously been shown to be stable targets for normalizing gene expression in senescing corollas and leaves from petunia [
36,
37].
2.7. Down Regulating PhENDO1 in Petunias Using Virus Induced Gene Silencing (VIGS)
Two independent experiments were conducted in petunia to characterize the function of
PhENDO1 in petal senescence. The TRV1 (TRV RNA1) and TRV2 (TRV RNA2) vectors from tobacco rattle virus were used for VIGS in petunia [
38]. The pTRV2:
PhCHS (or pTRV2:
PhCHS:
GFP) construct was used as a control to visualize successful silencing in the corollas. Silencing the chalcone synthase gene (
CHS) causes purple flowers to turn fully white or have white sectors [
39].
In the first experiment, to generate the pTRV2:
PhCHS:
PhENDO1 construct, a 303 bp fragment of
PhENDO1 was amplified by RT-PCR from petunia corolla cDNA using a forward primer with a
NcoI restriction site and a reverse primer with a
KpnI restriction site (
Table S1). The fragment was chosen from the region corresponding to bases 304–606 to include both DNase and RNase active sites. The amplified fragment was ligated into pTRV2:
PhCHS following double digestion using the respective restriction enzymes. The constructed plasmids, pTRV1, pTRV2, pTRV2:
PhCHS, and pTRV2:
PhCHS:
PhENDO1 were transformed into
Agrobacterium tumefaciens GV3101 by heat shock.
In the second VIGS experiment RNAi designer (
https://rnaidesigner.lifetechnologies.com/rnaiexpress/design.do, accessed on 15 March 2018) was used to identify siRNA regions in
PhENDO1 to increase silencing efficiency, and a second construct with a 245-bp fragment was amplified using a forward primer with a
BamHI site and a reverse primer with an
XbaI restriction site (
Table S1). PCR products were ligated into pTRV2:
PhCHS following double digestion with the respective restriction enzymes. A pTRV2:
PhCHS construct including a 265-bp fragment of the
GFP gene was used as the control in the second experiment [
40]. The constructed plasmids, pTRV1, pTRV2:
PhCHS:
GFP, and pTRV2:
PhCHS:
PhENDO1 were transformed into
Agrobacterium tumefaciens GV3101 by electroporation.
To prepare for plant inoculation, the transformed Agrobacterium cultures were grown overnight at 30 °C in liquid LB media containing 25 mg·L−1 gentamicin, 10 mg·L−1 rifampin, and 50 mg·L−1 kanamycin. The overnight cultures were used to inoculate (1:100 ratio) the LB-MESA media containing 10 mM MES pH 5.7 and 20 μM acetosyringone in addition to the antibiotics mentioned above. Once the OD600 of the cultures reached 0.8–1.0, the cells were harvested at 4 °C and resuspended to an OD600 of 2.0 in agroinduction media containing 10 mM MES pH 5.7, 10 mM MgCl2, and 200 µM acetosyringone. The cultures were incubated overnight at room temperature and the cultures containing pTRV1 and pTRV2 or its derivatives were mixed at a 1:1 ratio as inoculum.
In the first experiment,
Petunia × hybrida ‘Fantasy Blue’ 4-week old seedlings were inoculated via leaf infiltration using a needless syringe. Treatments included mock inoculation (agroinduction media only), pTRV1+pTRV2 (empty vector), pTRV1+pTRV2:
PhCHS (control), and pTRV1+pTRV2:
PhCHS:
PhENDO1 inoculation. In the second experiment,
Petunia × hybrida ‘Picobella Blue’ 4-week old seedlings were inoculated via apical meristem inoculation following optimized methods in Broderick and Jones [
40]. Syngenta Flowers (formerly Goldsmith Seeds) replaced ‘Fantasy Blue’ with the improved variety ‘Picobella Blue’ that has the same plant profile, but different genetic background. Treatments for the second experiment included pTRV1+pTRV2:
PhCHS:
GFP (control) and pTRV1+pTRV2:
PhCHS:
PhENDO1.
The inoculated plants were maintained in a growth chamber (Conviron, Winnipeg, Canada) at Ohio Agricultural Research and Development Center, The Ohio State University, Wooster, OH for the duration of these experiments. Growing conditions in the growth chamber were 24 °C/20 °C (day/night) with a 13-h photoperiod from high pressure sodium lights for the first experiment and 20 °C/18 °C (day/night) with a 16-h photoperiod supplemented by metal halide lights for the second experiment.
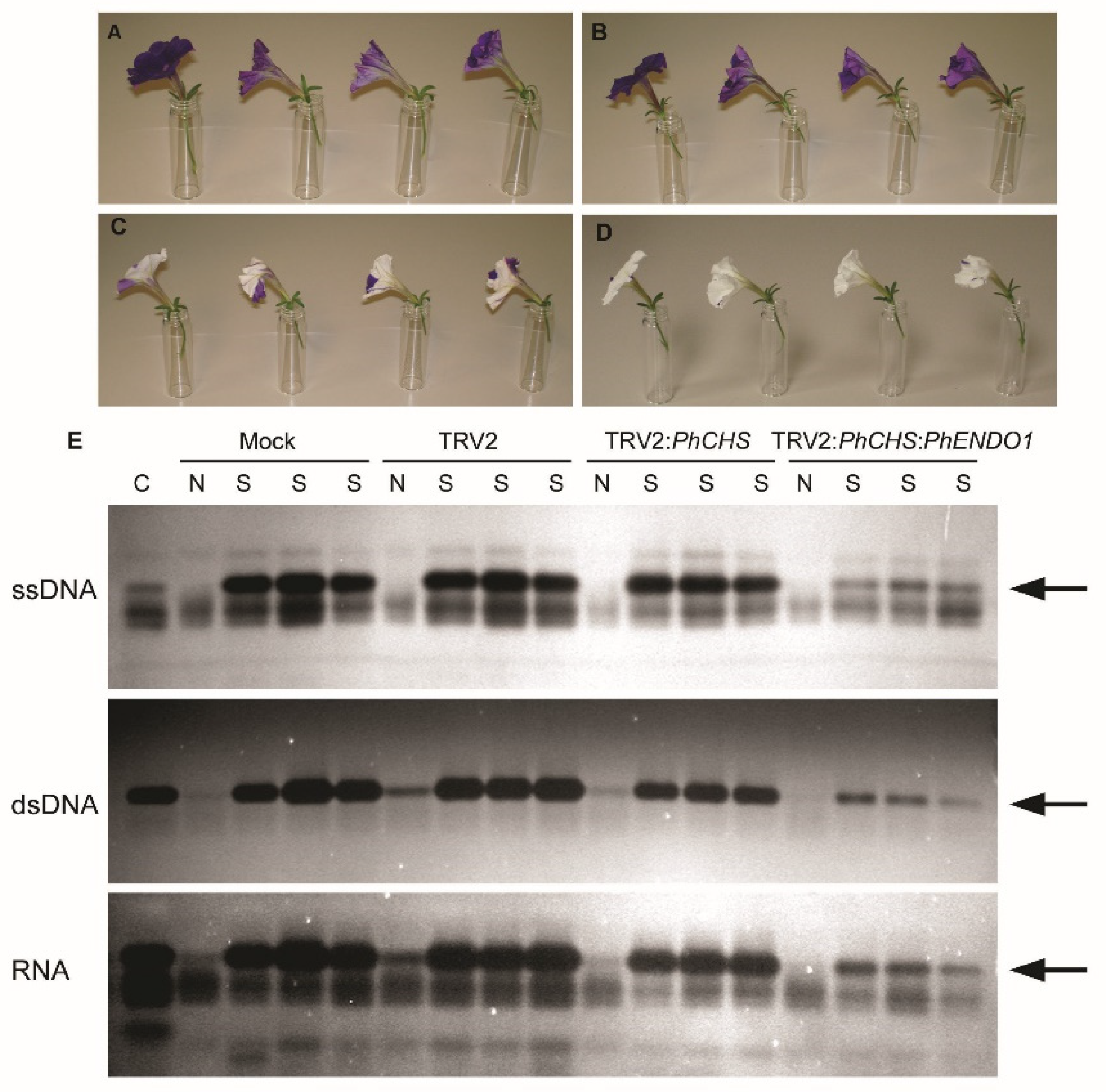
To analyze the longevity of flowers from PhENDO1-silenced plants, flowers were emasculated one day prior to flower opening to avoid self-pollination. Flower longevity was defined as the number of days from flower opening until the flower had senesced and the corolla was wilted. Flower longevity was measured using 48 flowers per treatment in the first experiment and 24 flowers per treatment in the second experiment. Corollas from senescing and non-senescing flowers were also harvested (n = 4), flash frozen in liquid nitrogen, and stored at −80 °C for extraction of RNA to confirm the down regulation of the PhENDO1 transcript levels and for extraction of total protein to determine endonuclease activity. RNA extraction and qPCR were conducted as previously described.
2.8. Protein Extraction and Nuclease Activity Assays
Total protein was extracted from corollas collected from VIGS plants to use for both in-gel nuclease assays to separate individual activities and activity assays to quantify total nuclease activity. Corollas were collected at 0 d (non-senescing) and on the day of wilting (senescing) from mock inoculated, pTRV2 empty vector, pTRV2:PhCHS control, and pTRV2:PhCHS:PhENDO1 plants for in-gel nuclease activity assays. A protein sample contained one corolla, and all collections, extractions, and gels were replicated five times. For quantifying total nuclease activity, corollas were collected from pTRV2:PhCHS:GFP controls and pTRV2:PhCHS:PhENDO1 plants. Three corollas from each treatment were pooled together for each of the four biological replicates. Corollas were flash frozen and stored at −80 °C. Frozen corollas were ground to a fine powder in a chilled mortar and pestle and extracted in 50 mM Tris-HCl (pH 7.4) amended with 20 mM DTT. Total protein was quantified using Coomassie Bradford Protein Assay (Pierce Biotechnology Thermo Scientific, Rockford, IL, USA).
In-gel nuclease activity assays were conducted as previously reported in Langston et al. [
23]. Briefly, total proteins were resolved on a 15% (
w/v) SDS-PAGE gel containing 100 µg mL
−1 BSA. To identify DNase activities, the gels contained either 15 µg mL
−1 double-stranded salmon sperm DNA (Invitrogen, Grand Island, NY, USA) or DNA that had been boiled for 3 min to make it single stranded. To identify RNase activities, gels contained 40 µg mL
−1 total RNA from petunia corollas. SDS-PAGE was run at 120 V for 2 h at 25 °C. After separation, nucleases were renatured by incubating the gels in renaturation buffer [0.1 M Tris-HCl (pH 7.4), 1% Triton X-100] with shaking at 37 °C for 1 h. Following two rinses in 0.1 M Tris-HCl (pH 7.4), gels were incubated overnight at 37 °C in development buffer supplemented with cobalt to optimize activity of the senescence specific endonuclease previously identified [
23] [50 mM Tris-HCl (pH 7.5), 20 mM NaCl, 100 µM CoCl
2]. Gels were stained for 1 h at room temperature in 50 mM Tris (pH 7.0) containing 0.5 µg mL
−1 ethidium bromide to visualize the bands of nuclease activity.
Total nuclease activity was measured for each corolla protein extract in nuclease reaction buffer containing 50 mM Tris-HCl (pH7.5), 20 mM NaCl, 20 µM CaCl2, 10 µM MgCl2, 20 µM CoCl2, 10 µM MnCl2 and either heat-treated, single-stranded salmon sperm DNA (Invitrogen) or petunia leaf RNA as substrate. Each reaction consisted of 50 µL protein extract, 0.2 µg mL−1 heat-treated DNA or 0.5 µg mL−1 RNA, 0.1 mg mL−1 BSA and nuclease reaction buffer to a final volume of 350 µL. The reaction was incubated at 37 °C for 20 min and terminated with the addition of 350 µL chilled 3.4% (w/v) perchloric acid followed by 10 min incubation on ice. Precipitates were removed by centrifugation (5000× g for 20 min at 4 °C). Supernatant was assayed for change in absorbance at 260 nm compared to the blank sample. One unit of DNase or RNase activity is the amount of enzyme liberating acid soluble material at a rate of 1.0 absorbance unit per min. Four biological replicates were assayed for each treatment and each sample was run in triplicate.
2.9. Statistical Analysis
All statistical analyses in this study were conducted in R 3.3.1. An analysis of variance (ANOVA) was performed followed by mean separation via Fisher’s Least Significant Difference (LSD) test using R package agricolae (α = 0.05). When data was not normally distributed, a log-transformation of the data was conducted. A statistical model was used for analyses: Y = μ + Treat + Rep + ε, where μ is the mean across all experiments and treatments, Treat is the effect of treatment (e.g., buffer or VIGS construct), Rep is the effect of replicates in each experiment, and ε is the uncontrolled error. All effects in the model were considered fixed.
4. Discussion
The programmed senescence of petunia petals is accompanied by increases in DNA fragmentation and the induction of various nuclease activities as evidenced by in-gel activity assays. An increase in total endonuclease activity accompanies the decrease in RNA and DNA content of the petals, which occurs late in the senescence program concomitant with corolla wilting (
Figure 6 [
21,
23]). The degradation of nucleic acids provides N and P that can be reallocated during nutrient starvation or during the senescence of plant organs. The N and P content of petals decreases during the senescence of pollinated and unpollinated flowers, supporting the remobilization function of programmed cell death in the corolla [
9,
10,
11,
12,
37,
43].
Multiple constitutive and senescence upregulated endonuclease activities have been reported in flowers that could be involved in the large-scale degradation of genomic DNA and RNA during programmed cell death in petals [
19,
21,
23]. Xu and Hanson [
21] reported the activity of two constitutive and two upregulated activities against RNA, five upregulated activities against ssDNA, and four upregulated activities against dsDNA during the pollination-induced senescence of
Petunia inflata corollas. One of these was a senescence-specific endonuclease (46-kDa) with activity against only ssDNA. The divalent cation requirements and pH optimum of this endonuclease were not reported. In
Petunia ×
hybrida, a senescence-specific, 43-kDa endonuclease (PhNUC1) was identified in corollas that had activity against DNA (both ss and ds) and RNA. This bifunctional endonuclease has low levels of activity in the presence of Ca
2+, but the addition of Co
2+ enhances activity against all substrates much beyond that of Ca
2+. Cobalt restores the activity that is inhibited by the chelating agent EDTA, while Ca
2+ does not [
23]. The inhibition by Zn
2+ and pH optimum around 7.5 suggest that PhNUC1 is a type II Ca
2+-dependent endonuclease. Similar bifunctional endonucleases, that are upregulated during leaf senescence in parsley (
Petroselinum crispum) (PcNUC1, 43-kDa and PcNUC2, 40-kDa) and tomato (
Solanum lycopersicum) (LeNUC1, 41-kDa) also have neutral pH optima and Co
2+-enhanced activity [
25,
26].
Two-dimensional gel electrophoresis (2-DE) was used to identify proteins that were differentially expressed between senescing and nonsenescing petunia corollas [
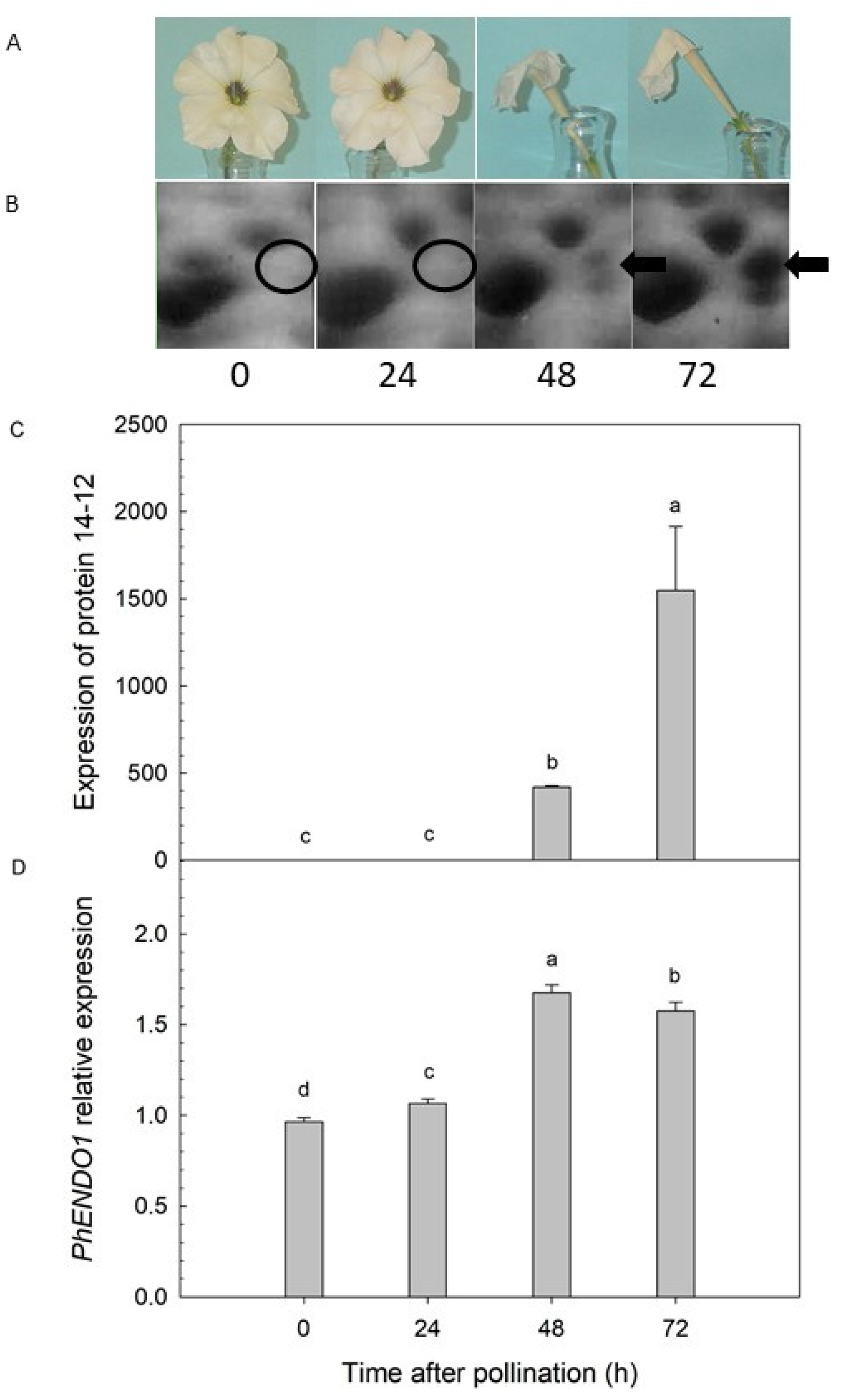
31]. A protein with a predicted molecular mass of 43-kDa was detected only in senescing petals at 48 and 72 h after pollination (
Figure 1B). Liquid chromatography-tandem mass spectrometry (LC-MS/MS) identified this senescence-specific protein as a putative endonuclease (protein 14-12) [
31]. The peptide sequence for that putative endonuclease was successfully used to clone the corresponding cDNA, which was named
PhENDO1 (
Figure S1). PhENDO1 has all the features of an endonuclease, and it has high amino acid identity to other senescence-associated endonucleases like BFN1 (now called AtENDO1) from Arabidopsis (
Arabidopsis thaliana) (
Figure S2). The gene expression pattern for
PhENDO1 corresponded with the senescence-associated expression of the endonuclease protein 14-12 [
31] and with the activity of the bifunctional endonuclease PhNUC1 [
23] in senescing corollas from pollinated flowers (
Figure 1).
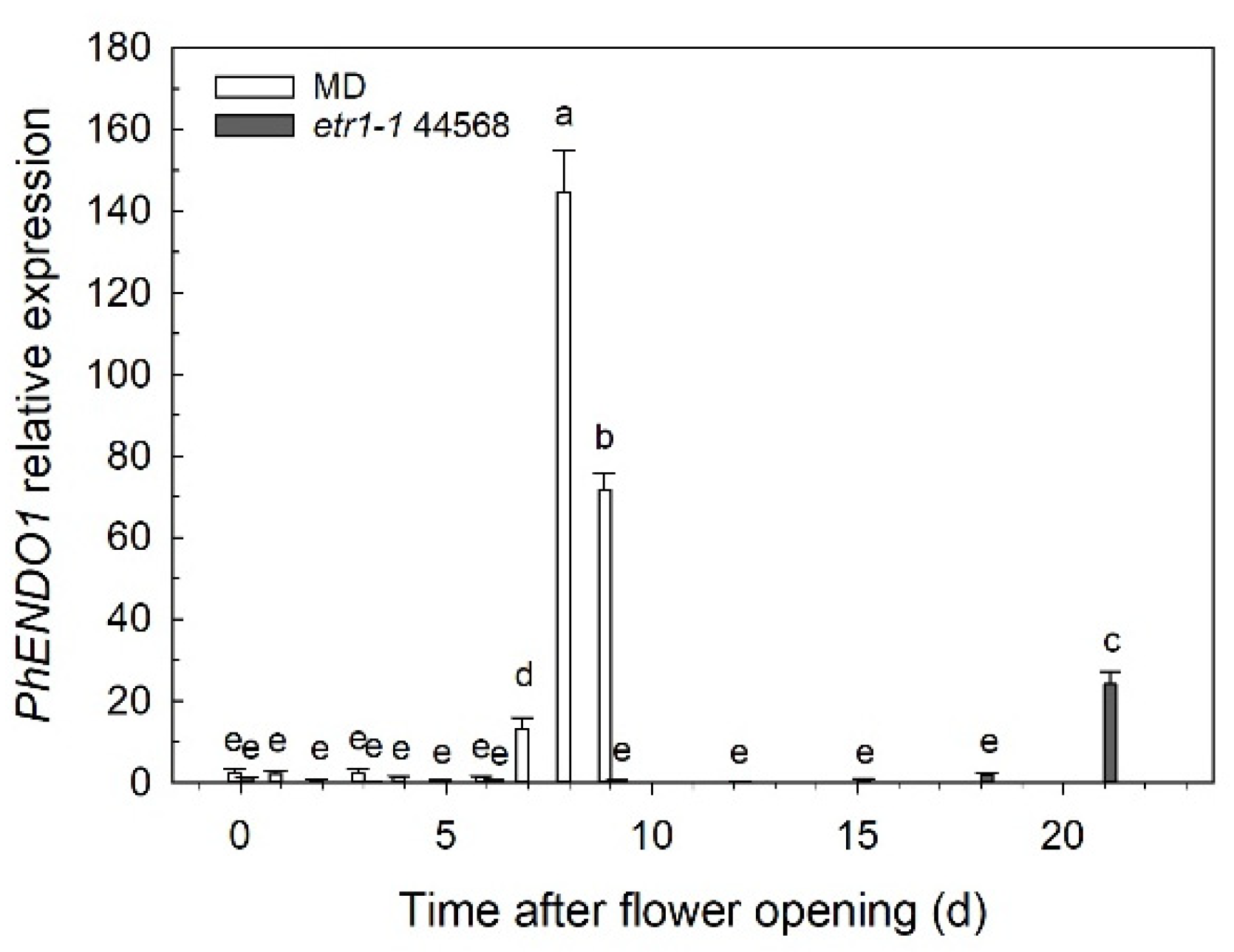
PhENDO1 expression was also upregulated during the age-related senescence of unpollinated petunia corollas, peaking at 8 days after flower opening when the corolla was visibly wilting (
Figure 2). This also mirrors the pattern of PhNUC1 activity observed against both DNA and RNA in unpollinated corollas [
23]. The activities of the bifunctional endonucleases, LeNUC1 (tomato), AtENDO1/BFN1 (Arabidopsis), PcNUC1 and 2 (parsley), and PVN2 and 5 (French bean,
Phaseolus vulgaris) are all upregulated during natural leaf senescence [
20,
24,
25,
26]. Few papers report specifically on expression in senescing petals, but AtENDO1/BFN1 and AtENDO3 are expressed in petals, with expression of AtENDO1 increasing during flower aging [
20,
28,
44].
The predicted molecular mass of PhENDO1 is 34.1-kDa, while the observed
Mr of both the endonuclease 14-12 [
31] and the bifunctional endonuclease activity PhNUC1 [
23] is 43-kDa. Arabidopsis endonucleases have observed molecular masses of 30–40 kDa in SDS-PAGE activity gels, which is also higher than the molecular masses estimated from their amino acid sequences [
44]. The electrophoretic mobility of AtENDO1 and AtENDO2 were shown to be reduced due to the glycosylation state of the proteins [
45]. PhNUC1 and the other cobalt-enhanced endonucleases from tomato and parsley are also glycoproteins [
23,
25,
26], which can explain the difference between the observed size of the activity bands and the predicted molecular masses of the proteins.
The expression pattern of
PhENDO1 (and endonuclease 14-12) corresponded well with the activity of PhNUC1 during corolla senescence, suggesting that this endonuclease activity is encoded by
PhENDO1. Down regulating the expression of
PhENDO1 using VIGS confirmed that PhNUC1 activity was a product of the
PhENDO1 gene (
Figure 5). Overexpressing recombinant AtENDO1 and AtENDO2 in protoplasts reveals that some of the Arabidopsis endonucleases appear as more than one activity band, and that there can be multiple post-translationally modified forms of the proteins with endonuclease activity [
44]. It is possible that there are also other variants of PhENDO1 that we did not observe were down-regulated in petunia corollas, because the conditions used for the in-gel activity assays were those optimized previously for PhNUC1 activity (i.e., pH 7.5, plus Co
2+). The Arabidopsis
ENDO2 knockout mutant (
endo2) was used to confirm which nuclease activities are products of the
AtENDO2 gene [
46]. In-gel activity assays show that
endo2 protoplasts lack activity corresponding to both a 34-kDa (N1) and a 36-kDa (N2) endonuclease [
46]. The N2 variant of AtENDO2 is not glycosylated, while the N1 variant is glycosylated. AtENDO2-N1 is localized in the cytoplasm in healthy cells, and the de-glycosylated form (AtENDO2-N2) is localized in the nucleus where it is believed to play a role in DNA fragmentation [
46].
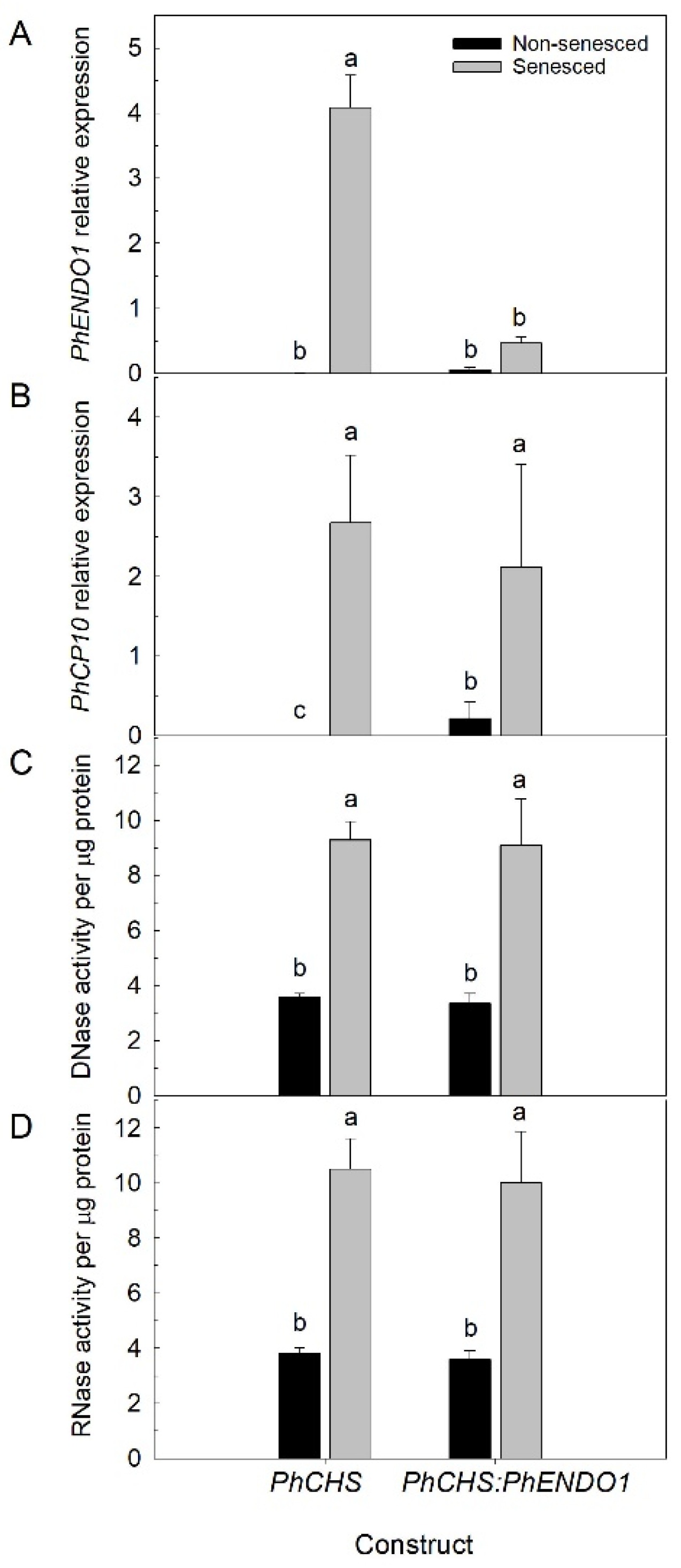
Down regulating the
PhENDO1 gene did not result in any visible differences in growth and development.
PhENDO1-deficient petunias had similar plant biomass, flower size, flower numbers, and flower longevity as control plants (
Table 1 and data not shown). Corolla senescence in these flowers was also accompanied by upregulation of the senescence marker,
PhCP10, to similar levels as the control corollas (
Figure 6B).
PhCP10 encodes a cysteine protease believed to be involved in protein degradation during petal senescence. It is an ortholog of
AtSAG12, which serves as a molecular marker for senescence [
47]. Arabidopsis plants lacking a functional AtENDO1/BFN1 do not have any visible differences in leaf senescence or other aspects of growth and development [
48]. Similarly, overexpressing
AtENDO1 has no effect on leaf senescence [
20].
PhENDO1 transcript levels in senescing flowers were reduced, as was the specific nuclease activity corresponding to the 43-kDa bifunctional endonuclease PhNUC1 (
Figure 5 and
Figure 6A and data not shown). In contrast, the total endonuclease activity against RNA and ssDNA was not reduced in senescing corollas from
PhENDO1-deficient flowers (
Figure 6C,D), suggesting that there were other nucleases that were upregulated during corolla senescence to compensate for the reduced expression of
PhENDO1 and its product PhNUC1. Searching the
Petunia axillaris predicted protein database at the Sol Genomics Network (
https://solgenomics.net/, accessed on 4 February 2021) identified two putative endonucleases (Peaxi162Scf00527g00026.1 and Peaxi162Scf00753g00440.1) that had 81% and 44.8% amino acid identity, respectively, with PhENDO1.
Plant endonucleases are encoded by a multi gene family, and studies show some tissue specificity and differential expression during plant development and stress responses among the individual family members [
44,
49,
50]. In French bean,
PVN4 and
PVN5 are upregulated during cotyledon senescence and
PVN2 and
PVN5 are upregulated during the natural and dark-induced senescence of leaves, suggesting that there are also some functional redundancies between the family members [
49,
50]. Senescence involves a complex network of genes involved in a highly coordinated program of cellular disassembly and resource remobilization. Manipulating individual components of this network often does not alter the initiation or progression of senescence [
51].
Ethylene is a key regulator of senescence in both leaves and flowers [
52,
53]. In ethylene-insensitive transgenic petunias (35S:
etr1-1), where petal senescence is delayed, expression of
PhENDO1 (
Figure 2) and PhNUC1 activity [
23] were similarly delayed until the corollas of those flowers were wilted. A similar pattern of delayed expression in senescing petals from 35S:
etr1-1 transgenic petunias was observed with the senescence-associated gene
PhCP10 [
35]. Treating petunia flowers with ethylene induced expression of
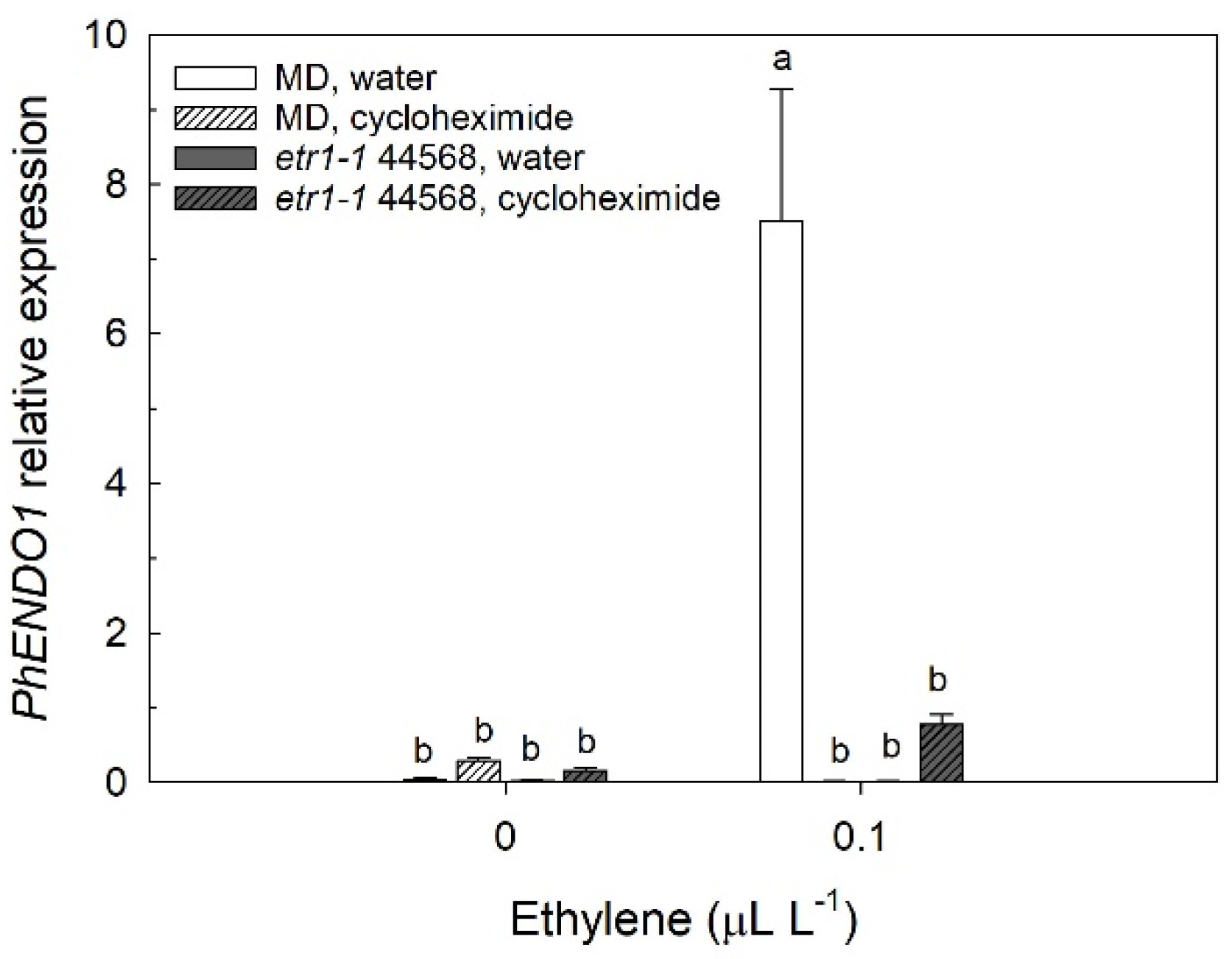
PhENDO1 (
Figure 3) and the activity of PhNUC1 in the corollas [
23]. The progression of senescence requires the de novo synthesis of proteins and treating flowers with cycloheximide delays senescence [
54]. Labeling experiments in morning glory (
Ipomea tricolor) provide evidence that ribonuclease is de novo synthesized during the senescence of the corolla [
55]. The upregulation of
PhENDO1 by ethylene was prevented by treating flowers with the protein synthesis inhibitor, cycloheximide (
Figure 3). Similarly, the induction of
PhCP10 is also prevented by treating petunia flowers with cycloheximide [
36]. These experiments suggest that both the upregulation of
PhENDO1 and
PhCP10 are secondary responses to the ethylene stimulus. The activities of LeNUC1 and PcNUC1 and PcNUC2 are induced by treating young, attached leaves or green, detached leaves, respectively, with ethylene [
25,
26]. Senescence-associated endonuclease genes are also upregulated by other plant hormones including jasmonic acid, salicylic acid, gibberellic acid, and abscisic acid [
29,
56,
57].
Many senescence-associated nucleases are also induced by phosphate starvation [
18,
49,
50,
58,
59,
60]. In tomato,
LE and
LX RNase genes are induced at the late stage of senescence and during phosphate starvation in cell cultures, suggesting a dual role in nutrient remobilization during developmental senescence and phosphate scavenging during nutrient stress [
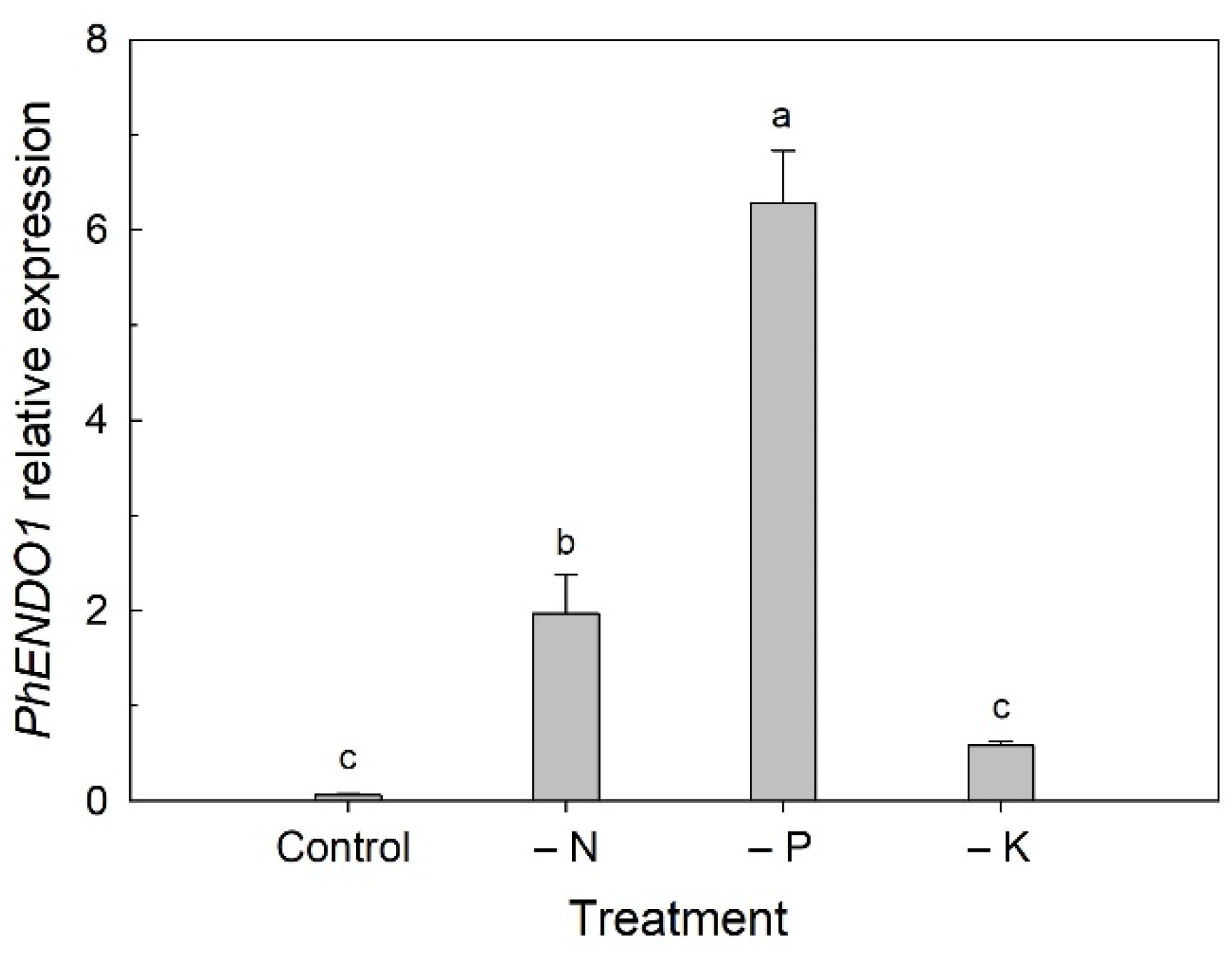
18]. We found that the expression of
PhENDO1 was also upregulated when plants were grown under nutrient deficient conditions (
Figure 4). The greatest upregulation was seen under P-deficient conditions, but N deficiency also resulted in upregulation of
PhENDO1 expression in leaves to a lesser extent. Interestingly, expression of
PhCP10 is highly upregulated in petunia leaves under N-deficient conditions and to a lesser extent under P-deficient conditions [
34].
PVN4 and
PVN5, endonucleases in French bean that are upregulated during cotyledon senescence, are also upregulated when seedlings are grown under nutrient deficient conditions (minus P and N) [
50]. Other senescence-associated bifunctional nucleases, like
AtENDO1/BFN1 are not induced by phosphate starvation [
20].
A decrease in nucleic acid content and increased DNA fragmentation during corolla senescence is accompanied by an increase in endonuclease activities against both DNA and RNA in petunias. A senescence-specific, bifunctional endonuclease, whose activity is enhanced by cobalt was identified in petunia corollas (PhNUC1). In the present study, we cloned the gene (PhENDO1) encoding this endonuclease activity. PhENDO1 gene expression mirrored activity of the endonuclease PhNUC1 during corolla senescence. PhENDO1 was upregulated in corollas from both pollinated and unpollinated flowers, and its induction by ethylene required de novo protein synthesis. Virus induced silencing of PhENDO1 proved an effective way to confirm that PhNUC1 activity was a product of the PhENDO1 gene, and it demonstrated that down regulating this endonuclease did not delay senescence or decrease the total endonuclease activity in senescing corollas. PhENDO1 expression was also upregulated in leaves of plants grown under P-deficient and to a lesser extent N-deficient conditions, confirming a functional role in both nutrient remobilization during senescence and nutrient salvaging during starvation responses. This work increases our understanding of the molecular and biochemical changes accompanying flower petal senescence and their role in programmed cell death and nutrient recycling.