Somatic Embryogenesis in Vitis for Genome Editing: Optimization of Protocols for Recalcitrant Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Media and Culture Conditions
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRISPR/CAS | clustered regularly interspaced short palindromic repeats (CRISPR)-Cas (CRISPR-associated proteins); |
| 2,4-D | 2,4-dichlorophenoxyacetic acid; |
| BA | 6-benzylaminopurine; |
| KOH | potassium hydroxide; |
| NOA | 2-naphthoxyacetic acid; |
| IAA | indole-3-acetic acid; |
| HCl | hydrochloric acid; |
| ANOVA | analysis of variance; |
| NaCl | sodium chloride. |
References
- International Organisation of Vine and Wine Intergovernmental Organisation (OIV). 2019 Statistical Report on World Vitiviniculture; OIV: Paris, France, 2019; pp. 3–10. [Google Scholar]
- Registro Nazionale delle Varietà di Vite. Available online: http://catalogoviti.politicheagricole.it (accessed on 16 November 2021).
- Reisch, B.I.; Pratt, C. Grapes. In Fruit Breeding; Vine and Small Fruits; Janick, J., Moore, J.N., Eds.; Wiley: New York, NY, USA, 1996; Volume 2, pp. 297–369. [Google Scholar]
- Emershad, R.L.; Ramming, D.W. Somatic embryogenesis, and plant development from immature zygotic embryos of seedless grapes (Vitis vinifera L.). Plant Cell Rep. 1994, 14, 6–12. [Google Scholar] [CrossRef]
- Tian, L.; Wang, Y. Seedless grape breeding for disease resistance by using embryo rescue. Vitis 2008, 47, 15–19. [Google Scholar]
- Dalla Costa, L.; Malnoy, M.; Gribaudo, I. Breeding next generation tree fruits: Technical and legal challenges. Hortic. Res. 2017, 4, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, H.; Figueiredo, A.; Serrazina, S.; Pires, R.; Peixe, A. Genome modification approaches to improve performance, quality, and stress tolerance of important Mediterranean fruit species (Olea europaea L., Vitis vinifera L., and Quercus suber L.). In Advances in Plant Transgenics: Methods and Applications; Sathishkumar, R., Kumar, S., Hema, J., Baskar, V., Eds.; Springer: Singapore, 2019; pp. 273–312. [Google Scholar]
- Schaart, J.G.; van de Wiel, C.C.M.; Lotz, L.A.P.; Smulders, M.J.M. Opportunities for products of new plant breeding techniques. Trends Plant Sci. 2016, 21, 438–449. [Google Scholar] [CrossRef]
- Giudice, G.; Moffa, L.; Varotto, S.; Cardone, M.F.; Bergamini, C.; De Lorenzis, G.; Velasco, R.; Nerva, L.; Chitarra, W. Novel and emerging biotechnological crop protection approaches. Plant Biotechnol. J. 2021, 19, 1495–1510. [Google Scholar] [CrossRef]
- Forleo, L.R.; Moffa, L.; Giudice, G.; D’Amico, M.; Nerva, L.; Chitarra, W.; Bergamini, C.; Cardone, M.F.; Velasco, R. Verso un Glera Resistente. CVV 2020, 11, 4–7. [Google Scholar]
- Nakano, M.; Sakakibara, T.; Watanabe, Y.; Mii, M. Establishment of embryogenic cultures in several cultivars of Vitis vinifera and V. × labruscana. Vitis 1997, 36, 141–145. [Google Scholar]
- Lopez-Perez, A.J.; Carreño, J.; Martinez-Cutillas, A.; Dabauza, M. High embryogenic ability and plant regeneration of table grapevine cultivars (Vitis vinifera L.) induced by activated charcoal. Vitis 2005, 44, 79–85. [Google Scholar]
- Gribaudo, I.; Gambino, G.; Vallania, R. Somatic embryogenesis from grapevine anthers: The optimal developmental stage for collecting explant. Am. J. Enol. Vitic. 2004, 55, 427–430. [Google Scholar]
- Hartmann, H.T.; Kester, D.E. Basi teoriche della coltura di tessuti per la micropropagazione. In Propagazione delle Piante; Edizioni Agricole: Bologna, Italy, 1990; pp. 513–554. [Google Scholar]
- Kansas State University. Available online: https://www.k-state.edu/wgrc/electronic_lab/aceto_stain.html (accessed on 16 November 2021).
- Yim, B.; Mun, J.-H.; Jeong, Y.-M.; Hur, Y.Y.; Yu, H.-J. Flower and microspore development in ‘Campbell Early’ (Vitis Labruscana) and ‘Tamnara’ (V. spp.) grapes. Hortic. Sci. Technol. 2015, 33, 420–428. [Google Scholar]
- Gambino, G.; Ruffa, P.; Vallania, R.; Gribaudo, I. Somatic embryogenesis from whole flowers, anthers and ovaries of grapevine (Vitis spp.). Plant Cell Tiss. Organ Cult. 2007, 90, 79–83. [Google Scholar] [CrossRef]
- Onay, A.; Pirinç, V.; Tilkat, E.; Aktürk, Z.; Yildirim, H. Somatic embryogenesis of pistachio from female flowers. J. Hortic. Sci. Biotechnol. 2004, 79, 960–964. [Google Scholar] [CrossRef]
- Franks, T.; He, D.G.; Thomas, M. Regeneration of transgenic Vitis vinifera L. Sultana plants: A genotypic and phenotypic analysis. Mol. Breed 1998, 4, 321–333. [Google Scholar] [CrossRef]
- Pinto-Sintra, A.L. Establishment of embryogenic cultures and plant regeneration in the Potuguese cultivar ‘Touriganacional’ of Vitis vinifera L. Plant Cell Tiss. Organ Cult. 2007, 88, 253–265. [Google Scholar] [CrossRef]
- Nitsch, J.P.; Nitsch, C. Haploid plants from pollen grains. Science 1969, 163, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- De Paoli, G.; Rossi, V.; Scorzoli, A. I substrati di coltura. In Micropropagazione delle Piante Ortoflorofrutticole; Edizioni Agricole: Bologna, Italy, 1994; pp. 39–69. [Google Scholar]
- Bouquet, A.; Torregrosa, L.; Iocco, P.; Thomas, M.R. Grapevine (Vitis vinifera L.). In Agrobacterium Protocols, (Methods in Molecular Biology, vol. 344), 2nd ed.; Wang, K., Ed.; Humana Press: Totowa, NJ, USA, 2006; Volume 2, pp. 273–285. [Google Scholar]
- Martinelli, L.; Gribaudo, I. Strategies for effective somatic embryogenesis in grapevine: An appraisal. In Grapevine Molecular Physiology & Biotechnology; Roubelakis-Angelakis, K.A., Ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; pp. 461–493. [Google Scholar]
- Martinelli, L.; Gribaudo, I.; Bertoldi, D.; Candioli, E.; Poletti, V. High efficiency somatic embryogenesis and plant germination in grapevine cultivars Chardonnay and Brachetto a grappolo lungo. Vitis 2001, 40, 111–115. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: http://www.R-project.org/ (accessed on 16 November 2021).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: A Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Jin, X.L.; Wu, F.B.; Zhang, G.P. Genotypic differences in callus induction and plant regeneration from mature embryos of barley (Hordeum vulgare L.). J. Zhejiang Univ. Sci. B 2011, 12, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Iocco, P.; Franks, T.; Thomas, M. Genetic transformation of major wine grape cultivars of Vitis vinifera L. Transgenic Res. 2001, 10, 105–112. [Google Scholar] [CrossRef]
- Dhekney, S.A.; Li, Z.T.; Compton, M.E.; Gray, D.J. Optimizing initiation and maintenance of Vitis embryogenic cultures. HortScience 2009, 44, 1400–1406. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Hernández, H.A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; De-la-Peña, C.; Loyola-Vargas, V.M. Signaling overview of plant somatic embryogenesis. Front. Plant Sci. 2019, 10, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]

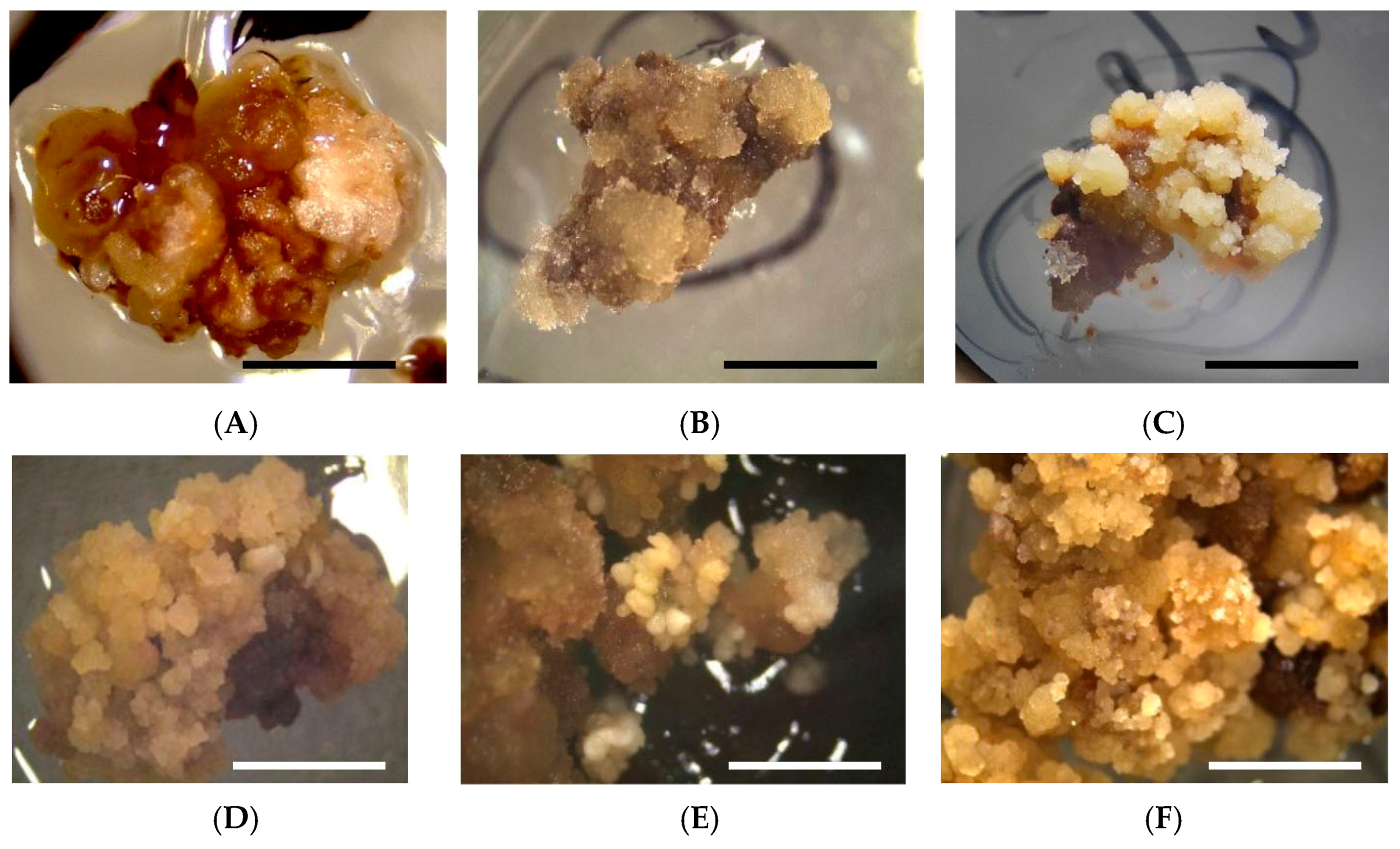


| Variety Name | Explants Number | Embryogenic Callus (%) |
|---|---|---|
| Aglianico N. | 2030 | 0.34 |
| Autumn Royal N. | 560 | 0.89 |
| Chardonnay B. | 1050 | 4.1 |
| Crimson Rs. | 560 | 0.71 |
| Glera B. | 2100 | 0.05 |
| Italia B. | 5670 | 0.09 |
| Negromaro N. | 1120 | 0.09 |
| Palieri N. | 1260 | 0.16 |
| Primitivo N. | 1680 | 0 |
| Red Globe Rs. | 1750 | 0.06 |
| Sultanina B. | 1120 | 3.7 |
| Summer Royal N. | 1190 | 1.3 |
| Victoria B. | 1540 | 0.06 |
| Cultivar | Callus Types (%) | Substrate | Sterilization | ||||
|---|---|---|---|---|---|---|---|
| C | NC | LS | HS | ||||
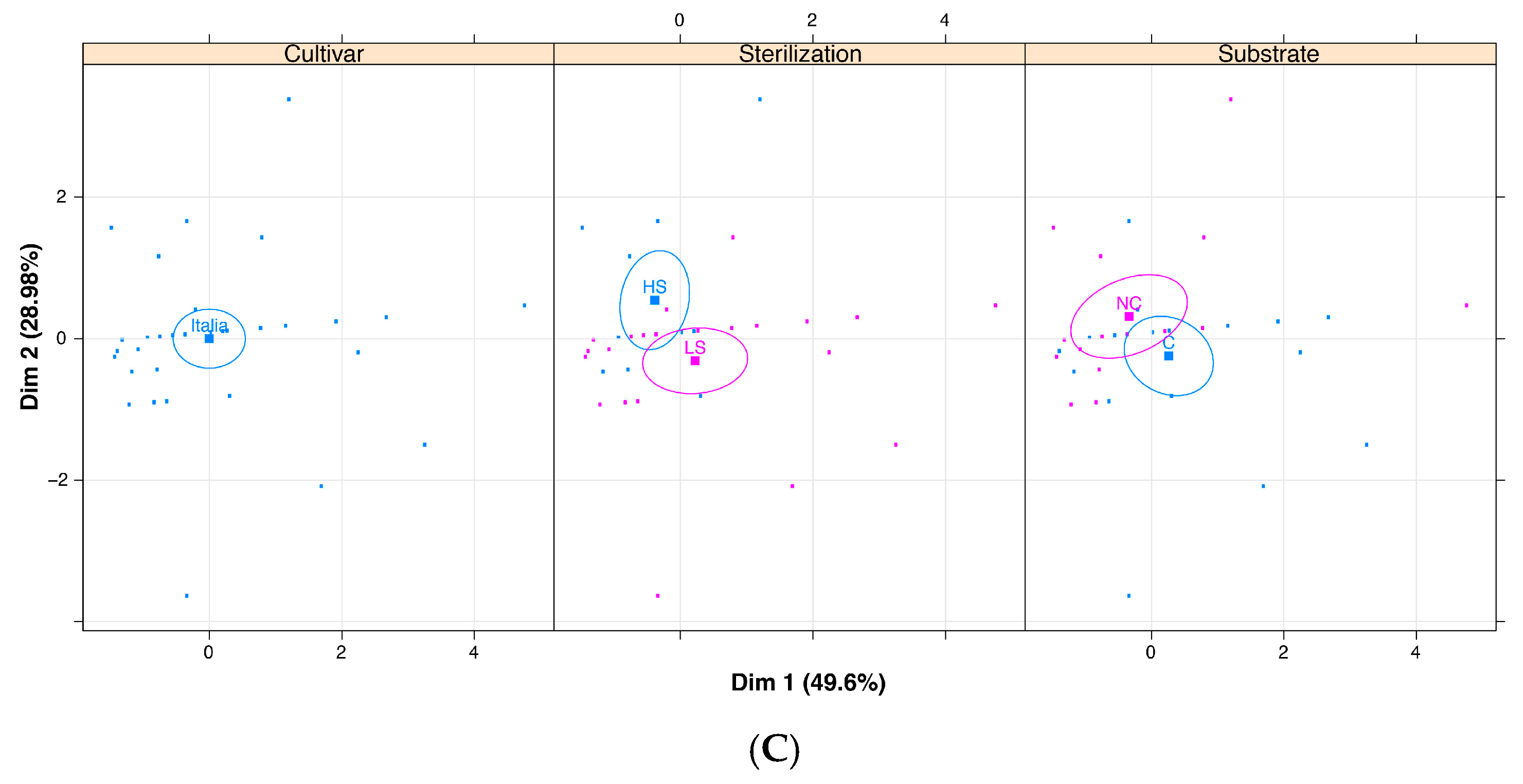
| Italia | Total (TC) | 14.73 | 9.18 | n.s. | 13.36 | 10.71 | n.s. |
| Friable (FC) | 12.30 | 8.25 | n.s. | 11.74 | 8.66 | n.s. | |
| Hard (HC) | 2.04 | 0.54 | n.s. | 1.54 | 1.16 | n.s. | |
| Embryogenic (EC) | 0.39 | 0.38 | n.s. | 0.07 | 0.89 | ** | |
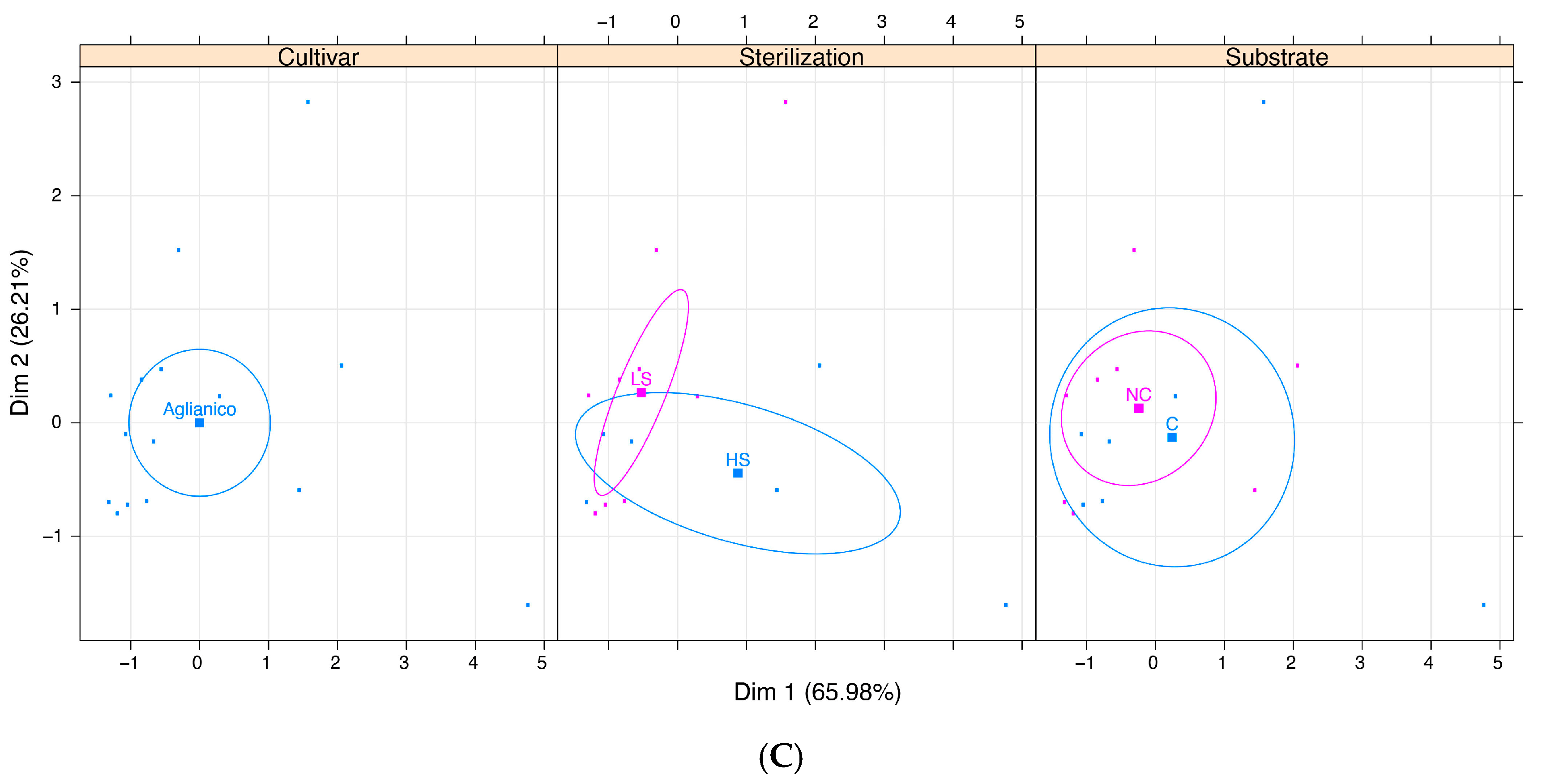
| Aglianico | Total (TC) | 20.90 | 15.80 | n.s. | 12.93 | 27.38 | n.s. |
| Friable (FC) | 11.25 | 9.82 | n.s. | 7.43 | 15.71 | n.s. | |
| Hard (HC) | 8.04 | 4.02 | n.s. | 3.21 | 10.71 | n.s. | |
| Embryogenic (EC) | 1.61 | 1.96 | n.s. | 2.29 | 0.95 | n.s. | |
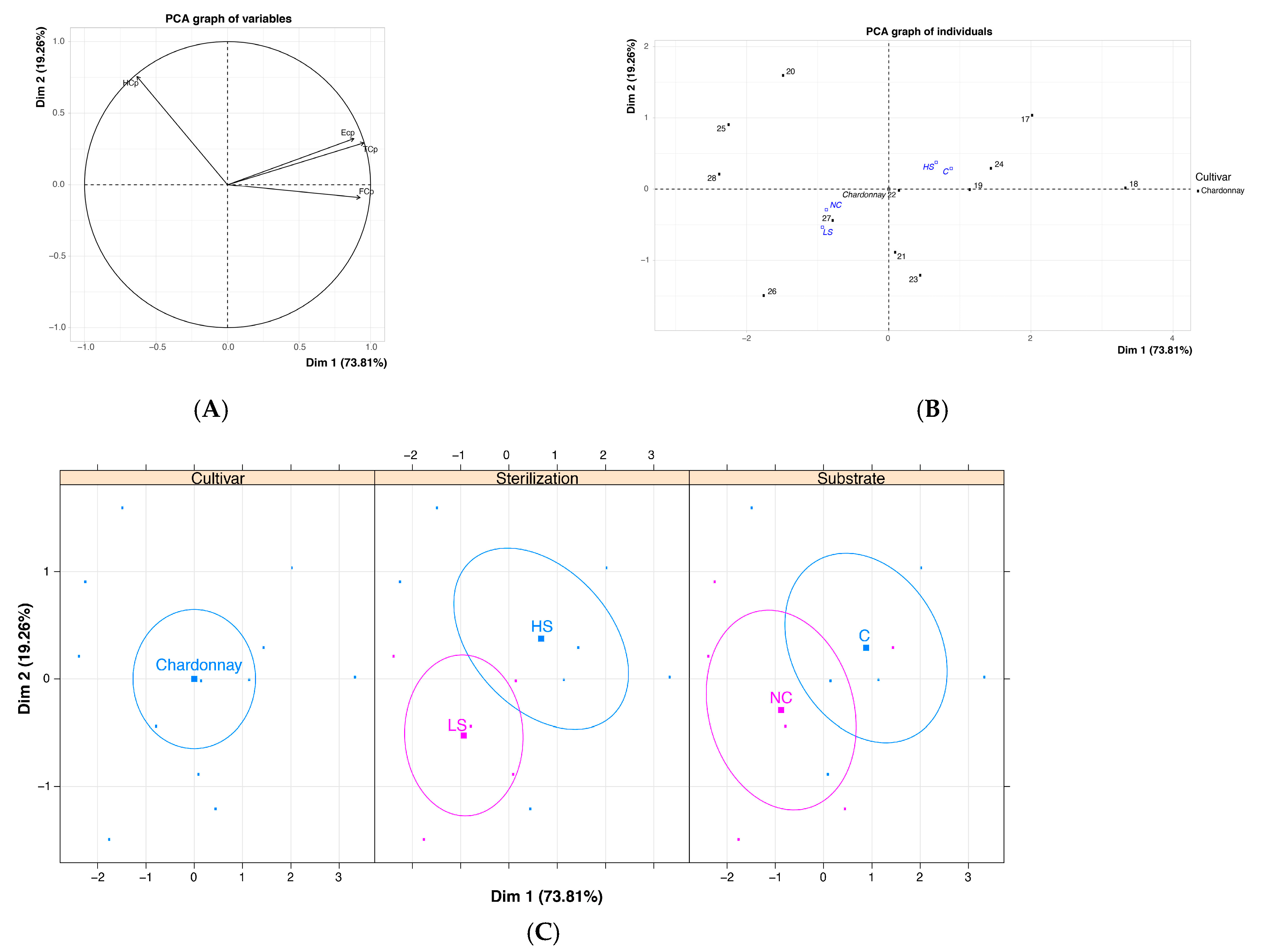
| Chardonnay | Total (TC) | 50.71 | 26.43 | * | 22.86 | 49.80 | * |
| Friable (FC) | 20.71 | 13.10 | n.s. | 9.14 | 22.45 | n.s. | |
| Hard (HC) | 4.05 | 5.48 | n.s. | 4.00 | 5.31 | n.s. | |
| Embryogenic (EC) | 25.95 | 7.86 | ** | 9.71 | 22.04 | n.s. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forleo, L.R.; D’Amico, M.; Basile, T.; Marsico, A.D.; Cardone, M.F.; Maggiolini, F.A.M.; Velasco, R.; Bergamini, C. Somatic Embryogenesis in Vitis for Genome Editing: Optimization of Protocols for Recalcitrant Genotypes. Horticulturae 2021, 7, 511. https://doi.org/10.3390/horticulturae7110511
Forleo LR, D’Amico M, Basile T, Marsico AD, Cardone MF, Maggiolini FAM, Velasco R, Bergamini C. Somatic Embryogenesis in Vitis for Genome Editing: Optimization of Protocols for Recalcitrant Genotypes. Horticulturae. 2021; 7(11):511. https://doi.org/10.3390/horticulturae7110511
Chicago/Turabian StyleForleo, Lucia Rosaria, Margherita D’Amico, Teodora Basile, Antonio Domenico Marsico, Maria Francesca Cardone, Flavia Angela Maria Maggiolini, Riccardo Velasco, and Carlo Bergamini. 2021. "Somatic Embryogenesis in Vitis for Genome Editing: Optimization of Protocols for Recalcitrant Genotypes" Horticulturae 7, no. 11: 511. https://doi.org/10.3390/horticulturae7110511
APA StyleForleo, L. R., D’Amico, M., Basile, T., Marsico, A. D., Cardone, M. F., Maggiolini, F. A. M., Velasco, R., & Bergamini, C. (2021). Somatic Embryogenesis in Vitis for Genome Editing: Optimization of Protocols for Recalcitrant Genotypes. Horticulturae, 7(11), 511. https://doi.org/10.3390/horticulturae7110511









