Abstract
Garlic is an important vegetable in terms of its economic value and also as a medicinal plant. In this study, chitosan (300 mM) and yeast extract (8 g/L) were used individually or in combination to improve the yields of garlic plants under drought conditions (i.e., 75% and 50% of the water they would normally receive from irrigation) for two seasons. Significant decreases in numbers of leaves per plant and plant height, plant dry weight, relative water content, and chlorophyll a and b concentrations were found in stressed garlic plants in both seasons. The greatest reductions in these characters were recorded in plants that received only 50% of the normal irrigation in both seasons. Levels of hydrogen peroxide, products of lipid peroxidation such as malondialdehyde, and superoxide, as well as percentages of electrolyte leakage, were elevated considerably and were signals of oxidative damage. The application of the yeast extract (8 g/L) or chitosan (300 mM) individually or in combination led to a remarkable increase in the most studied characters of the stressed garlic plants. The combination of yeast extract (8 g/L) plus chitosan (300 mM) led to increase plant height (44%), ascorbic acid levels (30.2%), and relative water content (36.8%), as well as the chlorophyll a (50.7%) and b concentrations (79%), regulated the proline content and levels of antioxidant enzymes in stressed garlic plants that received 75% of the normal irrigation, and this decreased the signs of oxidative stress (i.e., percentage of electrolyte leakage and levels of malondialdehyde, hydrogen peroxide, and superoxide).
1. Introduction
Garlic (Allium sativum L.) is an important vegetable crop in Egypt, where the annual production during the 2018 season was 286,213 tons obtained from 315.85 ha [1]. Garlic is the second most important species of the Allium genus and has several constituents including phenolic compounds, saponins, organosulfur compounds, and polysaccharides [2]. Garlic bulbs also contain numerous bioactive compounds, such as alliin, allicin, diallyl disulfide, and S-allylcysteine [3]. Shang et al. [3] found that these valuable bioactive compounds are very important and play significant roles as antioxidant, antimicrobial, anti-inflammatory, and anticancer compounds. Furthermore, the volatile oil of garlic can be used as a herbicide and insecticide to improve yield production [4,5]. During germination and development plants are exposed to many stresses, such as biotic [6,7,8,9] and abiotic stresses [10,11,12,13,14,15].
Drought is a very detrimental abiotic factor that obstructs the growth and decrease the yield of many plants. It causes a decrease in morphological features such as leaf number, leaf area, and plant height [16,17,18,19]. Physiological features such as relative water content (RWC) and chlorophyll concentrations [20,21,22] are also significantly reduced. Drought has negative effects on biochemical characters such as enzyme activity, the production of hydrogen peroxide and superoxide, and lipid peroxidation [23,24,25]; and decreases the yield [22,23,24,25,26,27]. Under drought conditions, biochemical and physiological features such as proline content, levels of malondialdehyde (MDA), percentages of electrolyte leakage (EL%), levels of superoxide and hydrogen peroxide, and enzyme activities were adversely affected in plants [27]. A decrease in chlorophyll and the level of photosynthesis is a very important signal in drought stress [28,29]. It is associated with a decrease in carbon dioxide uptake, closed stomatal pores, and a reduction in the activity of enzymes of the Calvin cycle pathway [30]. Reactive oxygen species (ROS), EL%, and levels of MDA are important indicators of various stress factors [31,32]. In drought, ROS, especially superoxide and hydrogen peroxide, have accumulated in numerous species [28,29]. The extreme occurrence of these parameters could be due to damage to membranes in many plants, such as sugar beets and barley. Oxidative damage can be controlled with the up-regulation of antioxidant components, which enhances plant tolerance of stress conditions and can scavenge ROS [33]. This mechanism depends on nonenzymatic and enzymatic compounds such as ascorbic acid, catalase, and peroxidase, which decrease the damage to membranes, proteins, and DNA [18].
Chitosan is an important polysaccharide and plays a pivotal role in human life because of its biological activity and its safety in agricultural processes [27]. Application of chitosan can increase leaf numbers, plant height, and chlorophyll content during stress (predominantly drought) by enhancing the nutrient status and antioxidant system of plants [34,35]. The foliar application of chitosan led to increased yields, nutrient uptakes, and chlorophyll concentrations [36]. Ahmed [37] reported that chitosan (4 and 6 mL L−1) led to improve productivity and storability of garlic plants. Additionally, Bistgani et al. [38] reported that chitosan at 400 µL L−1 led to improved dry weights of thyme plants that were stressed by drought.
Yeast is a natural, safe source of biofertilizer and can improve growth characters and plant yields. It contains many essential components, such as cytokines, riboflavin, thiamine, pyridoxine, and vitamins B1, B2, and B12, as well as other nutrients [39]. The valuable components of yeast, such as cytokinins, can stimulate cell division and elongation, as well as the synthesis of proteins and nucleic acids, and can increase mineral nutrients [40,41]. Application of yeast in drought conditions led to increased vegetative and yield characters of wheat plants, such as grain yields and 100-grain weights [26]. Abdelaal et al. [29] found that application of yeast led to improved plant fresh and dry weights, plant heights, and chlorophyll concentrations, as well as yields of wheat under water stress. Moreover, the application of yeast helped calendula plants to tolerate salt stress by improving their morphological, physiological, and anatomical features [14]. In a study by Haider et al. [42], yeast treatment at 6g/L resulted in maximum spike lengths, spike numbers, total phenols, prolines, and carbohydrates in wheat under drought conditions. Additionally, yeast application led to enhanced plant growth and differentiation and resulted in a remarkable increase in the numbers of stems per plant, plant height, leaf area, and chlorophyll content in potatoes [43]. Shalaby and El-Ramady [44] found that yeast extract led to improve yield, components and storability of garlic. Also, Ali [45] reported that application of yeast extract led to increase yield plant−1 and total yield of garlic plants.
Little information is available on the effect of chitosan and yeast on the physiological, morphological, and biochemical characters of garlic plants in drought conditions. Hence, the aim of our study is to assess the impact of chitosan and yeast individually or in combination as an environmentally friendly strategy for improving the yield of garlic plants associated with their biochemical, morphological, and physiological characters in drought conditions.
2. Materials and Methods
2.1. Experimental Site, Plant Materials, and Cultural Practices
Two field trials were conducted at a private farm in the Gharbia governorate during 2019/2020 and 2020/2021 to study the influence of chitosan (300 mM) and yeast extract (8 g/L) applied in a foliar spray on the vegetative, physiological, biochemical, and yield characters of garlic plants (Allium sativum L.) cv. Sids40 in drought conditions. The physiobiochemical studies were carried out at the EPCRS Excellence Center, Faculty of Agriculture, Kafrelsheikh University, Egypt. The experimental unit area was 14 m2 and consisted of six rows, and the planting date was 25 September during both seasons. The experiment was planned in a complete randomized block design with four replicates and each replicate contain 20 plants. During soil preparation, 48 m3 ha−1 of organic manure, 110 kg P2O5 ha−1, and 150 kg sulphur ha−1 were added to and mixed with the soil. Garlic cloves of uniform size were sown on both sides of each row 7 cm apart. Fertilization (240 kg nitrogen and 135 kg potassium ha‒1) was done three times, the first time at 30 days from planting, the second time at 60 days from planting, and the third time at 90 days from planting. Chitosan was purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA), chitosan solution was prepared by dissolving 0.3 g in 0.1 N HCl and diluting with distilled water with pH adjusted at 6.5 by 0.1 NaOH. Yeast extract was prepared by inoculating 8 g of active dry yeast with 1 L of nutrient broth and incubated for 48 h. The foliar spray application of yeast extract and chitosan was done twice, the first time at 40 days from planting and the second time at 80 days from planting. The fertilization rates and other cultural practices were carried out as recommended by the Egyptian Ministry of Agriculture. Experimental soil was taken to study the physical and chemical characters according to AOAC [46] as follow: electrical conductivity, 1.7 dS m−1; available nitrogen, 32.6 ppm; available potassium, 284 ppm; available phosphorus, 10.8 ppm; sand, 17.4%; silt, 34.6%; clay, 46.9%.
The foliar spray application of yeast extract and chitosan (1000 L ha−1) was done twice with an apparatus from the Jining Bafang Mining Machinery Co., Ltd. (Jining Yanzhou, China). The treatments were as follows:
- The plants (control) were irrigated eight times to simulate normal conditions of rainfall (100% irrigation).
- The plants were irrigated six times to simulate 75%, or moderate, drought.
- The plants received 75% irrigation and were sprayed with yeast 8 g/L.
- The plants received 75% irrigation and were sprayed with chitosan 300 mM.
- Some plants received 75% irrigation and were sprayed with yeast 8 g/L plus chitosan 300 mM.
- The plants received four irrigations to simulate 50%, or severe, drought.
- The plants received 50% irrigation and were sprayed with yeast 8 g/L.
- The plants received 50% irrigation and were sprayed with chitosan 300 mM.
- The plants received 50% irrigation and were sprayed with yeast 8 g/L plus chitosan 300 mM.
The harvest dates were 16 April 2020, and 19 April 2021 after 200 days from sowing.
2.2. Morphological Characters
The samples were taken for morphological studies at 150 days from transplanting; Plant height (cm), leaves number plant−1, and dry weight of plant (g) were recorded.
2.3. Physiological and Biochemical Studies
Physiological and biochemical studies were recorded in the fifth leaf at 150 days from sowing as follow:
2.3.1. Determination of Chlorophyll A, B Concentration and RWC
According to Lichtenthaler [47], samples of garlic fresh leaves were kept in solution of 80% acetone and 95% ethanol in the refrigerator. The chlorophylls concentrations were measured in extract at 663, 645 and 470 nm using a spectrophotometer. Relative water content (RWC%) was calculated as follows: RWC= (FW − DW)/(TW − DW) × 100, where FW is fresh weight, DW is dry weight and TW is turgid weight [48].
2.3.2. Determination of Proline Content
Garlic leaves (0.5 g) were placed in 3% sulphosalicylic acid and centrifuged for 20 min at 3000× g. 2 mL supernatant from extract was added to 2 mL ninhydrin reagent and 2 mL of glacial acetic acid. Proline was determined as mg g−1 FW at 520 nm using a spectrophotometer [49].
2.3.3. Assay of Electrolyte Leakage (EL%)
Ten discs of garlic leaves were placed in 25 mL deionized water and shaken for 20 h, then initial electrical conductivity was recorded. The discs were heated in a water bath at 80 °C for 1 h and shaken at 21 °C, then the final conductivity was determined. EL% was calculated as follow: initial/final conductivity × 100 [50].
2.3.4. Determination of Ascorbic Acid (AsA)
Garlic fresh leaves (500 mg) were taken to determine AsA, the samples was extracted in 10 mL trichloroacetic acid 6% (TCA) and centrifuged for 20 min at 1000× g, then 4 mL of the extract was mixed with 2 mL of dinitrophenyl hydrazine, then 1 drop of thiourea was added to the mixture and boiled for 15 min. The mixture was cooled to room temperature, 5 mL of H2SO4 80% were added to the mixture. AsA was determined in supernatant by a spectrophotometer at 530 nm as mg g−1 FW [51].
2.3.5. Assay of H2O2, O2− and MDA
For detecting O2−, garlic leaf samples were extracted in 50 mM phosphate buffer (pH 7.5) at a ratio of 1:8 (w/v) and centrifuged twice at 18,000× g. for 20 min. The reaction mixture for detecting O2− consisted of 4 mM epinephrine as an electron acceptor in 100 mM Tris-HCl buffer (pH 7.8) in the presence or absence of 2100 U/mL CuZn-SOD [52]. Absorbance was measured at 480 nm by employing Asys Expert 96 microplate spectrophotometer (Shanghai, China) supported by Kim software.
The H2O2 were assayed according to the method described by Yu et al. [53]. Samples of garlic leaf were extracted by homogenizing 0.5 g of garlic leaf with 3 mL of 50 mM K-phosphate buffer (pH 6.5) at 4 °C. The samples were centrifuged for 15 min at 11,500× g. 3 mL of supernatant was mixed with 1 mL of 0.1% TiCl4 in 20% H2SO4 (v/v), then the mixture was centrifuged at room temperature for 12 min at 11,500× g. The absorption of the supernatant was measured spectrophotometrically at 410 nm to determine the H2O2 content and expressed as arbitrary units (nmol g−1 fresh weight).
The lipid peroxidation was measured as malondialdehyde (MDA), a decomposed product of the peroxidized polyunsaturated fatty acid component of the membrane lipid, using thiobarbituric acid (TBA) as the reactive material following the method of Heath and Packer [54]. Garlic fresh leaves (500 mg) were homogenized in 3 mL 5% (w/v) trichloroacetic acid (TCA) and the homogenate was centrifuged at 11,500× g for 10 min. 1 mL supernatant was mixed with 4 mL of TBA reagent (0.5% of TBA in 20% TCA). The reaction mixture was heated at 95 °C for 30 min in a water bath and then quickly cooled in an ice bath and centrifuged at 11,500× g for 15 min. The absorbance of the supernatant was measured at 532 nm and was corrected for non-specific absorbance at 600 nm. The concentration of MDA was calculated as μ mol g−1 fresh weight.
2.3.6. Determination of Enzymes Activity
Garlic fresh leaves (500 mg) were homogenized and centrifuged (12,000× g) for 20 min at 4 °C, then the supernatant was used to measure the activities of total soluble enzyme using spectrophotometer. Activity of catalase (CAT) was determined at 240 nm in the supernatant based on the consumption rate of H2O2 as µmol min−1 mg protein−1 [55]. The reaction mixture contained 50 mM K-phosphate buffer (pH 7.0), 15 mM hydrogen peroxide and enzyme solution in a final volume of 700 µL. The reaction was initiated with enzyme extract and the activity was calculated using the extinction coefficient of 39.4 M−1 cm−1.
SOD activity was determined as µmol min−1 mg protein−1 at 560 nm. The activity was assayed based on the competition between SOD and NBT for the production of superoxide from xanthine and xanthine oxidase interaction following Spitz and Oberley [56].
POX activity was measured as µmol min−1 mg protein−1 as described by Castillo et al. [57]. The reaction mixture contained 10 mM phosphate buffer at pH 6.1, 12 mM H2O2, 96 mM guaiacol and enzyme extract. The blank contained a complete reaction mixture without H2O2. Absorbance was recorded after 1 min at 470 nm and the activity was measured using the extinction coefficient of 26.6 mM−1 cm−1.
2.4. Yield Characteristics
At harvest date (200 days), the plants were harvested for each replicate to determine total yield ha−1 while, total cured yield (ton ha−1) was calculated after curing for 7 days. A random sample of twenty bulbs was taken from each replicate to determine bulb diameter (cm).
2.5. Statistical Analysis
The obtained data were statistically analyzed using ANOVA procedures using the MSTAT-C statistical software package [58]. Duncan’s test was used to compare the means between treatments [59] when the difference was deemed significant (p ≤ 0.05).
3. Results
3.1. Effect of Yeast Extract and Chitosan on Plant Height, Number of Leaves per Plant, and Dry Weight of Plant for Garlic Plants in Drought Conditions
Drought stress significantly (p ≤ 0.05) decreased the number of leaves per plant, plant height, and plant dry weight of the garlic plants compared with the control plants that received normal irrigation during both seasons (Figure 1). These decreases were more pronounced in stressed garlic plants that received 50% of the normal irrigation. The application of yeast extract or chitosan individually or in combination caused a remarkable increase in the dry weight and height of plants and in the number of leaves per plant of stressed garlic plants that received 75% or 50% of the normal irrigation compared with stressed untreated garlic plants. The number of leaves per plant, plant height, and plant dry weight of garlic plants were greatly augmented by the application of yeast extract plus chitosan under drought conditions. The best results were obtained in the plants that received 75% irrigation followed by chitosan treatment during both seasons.
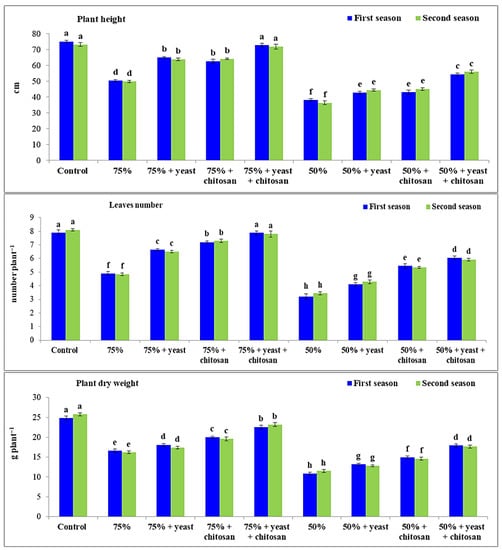
Figure 1.
Effect of yeast and chitosan on plant height, leaves number, plant dry weight of garlic plants under drought during 2019/2020 and 2020/2021 seasons. The letters on the columns show significant differences between the treatments according to ANOVA, Duncan’s multiple range test at 0.05 level. Data is the mean (±SE) of four replicates.
3.2. Effect of Yeast Extract or Chitosan on Concentrations of Chlorophyll A and B and on RWC in Stressed Garlic Plants
A significant decrease (p ≤ 0.05) in concentrations of chlorophyll and in the RWC was recorded in stressed garlic plants (75% and 50% of normal irrigation). The lowest concentrations of chlorophyll a and b and lowest RWC were observed in stressed plants that received 50% of normal irrigation compared with the control plants and the plants that received 75% of normal irrigation (Figure 2). Spraying the stressed plants (75% and 50% of normal irrigation) with yeast extract or chitosan caused a remarkable increase (p ≤ 0.05) in concentrations of chlorophyll a and b and in the RWC compared with stressed untreated garlic plants. The combination of yeast extract plus chitosan caused a remarkable increase in concentrations of chlorophyll a and b and in the RWC in the stressed garlic plants (75% of normal irrigation) without any significant differences compared with the control plants.
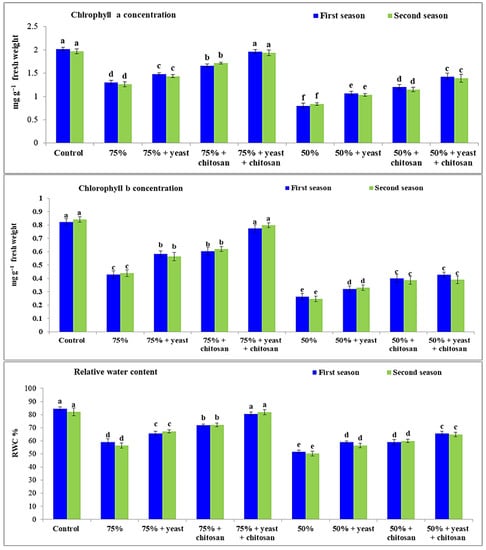
Figure 2.
Effect of yeast and chitosan on chlorophyll a, chlorophyll b and relative water content of garlic plants under drought during 2019/2020 and 2020/2021 seasons. The letters on the columns show significant differences between the treatments according to ANOVA, Duncan’s multiple range test at 0.05 level. Data is the mean (±SE) of four replicates.
3.3. Effect of Yeast Extract or Chitosan on Proline Levels, Percentage of Electrolyte Leakage and Concentration of Ascorbic Acid in Garlic Plants in Drought Conditions
Figure 3 shows that the proline content and EL% were considerably increased (p ≤ 0.05) in garlic plants in drought conditions (75% and 50% of normal irrigation) compared with the control plants. The garlic plants that received 50% of normal irrigation had high values for proline and EL% compared with the control plants and plants that received 75% of normal irrigation. Similarly, ascorbic acid was considerably improved in stressed garlic plants in drought conditions, especially in plants that received 50% of normal irrigation in both seasons, compared with controls. The foliar application of yeast or chitosan or the combination of yeast plus chitosan led to a notable reduction in EL% in stressed garlic plants in both seasons. The application of yeast plus chitosan regulated the levels of proline in stressed garlic plants. The best results were observed with yeast plus chitosan in plants that had received 75% irrigation. Likewise, the maximum value of ascorbic acid was recorded with 50% irrigation plus yeast plus chitosan in both seasons.
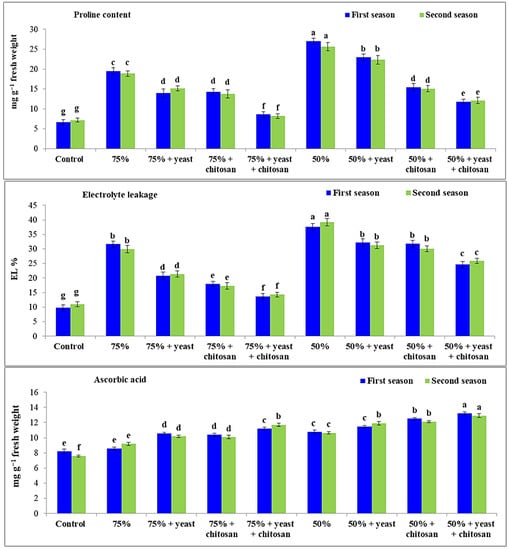
Figure 3.
Effect of yeast and chitosan on proline content, electrolyte leakage and ascorbic acid of garlic plants under drought during 2019/2020 and 2020/2021 seasons. The letters on the columns show significant differences between the treatments according to ANOVA, Duncan’s multiple range test at 0.05 level. Data is the mean (±SE) of four replicates.
3.4. Effect of Yeast Extract or Chitosan on Levels of Hydrogen Peroxide, Superoxide, and MDA of Garlic Plants in Drought Conditions
Hydrogen peroxide, superoxide, and MDA are very important components of garlic plants in drought conditions (Figure 4). Drought stress had a significant effect (p ≤ 0.05) on the content of each of these components. These studied characters were significantly augmented in garlic plants in drought conditions compared with control plants in both seasons. The highest levels of all three components were found in the plants with 50% of normal irrigation during both seasons, followed by plants with 75% of normal irrigation, compared with the controls. Superoxide, hydrogen peroxide, and MDA were decreased significantly in stressed garlic plants following the application of yeast or chitosan individually or in combination. The best effects were recorded in the stressed plants with 75% irrigation plus yeast plus chitosan, without any significant differences compared with the controls.
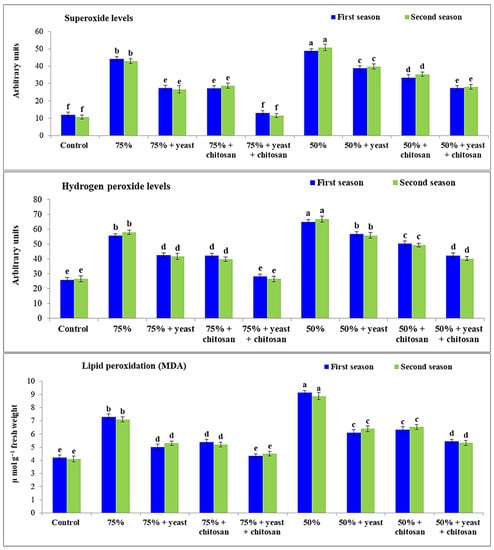
Figure 4.
Effect of yeast and chitosan on hydrogen peroxide level, superoxide level and lipid peroxidation of garlic under drought during 2019/2020 and 2020/2021 seasons. The letters on the columns show significant differences between the treatments according to ANOVA, Duncan’s multiple range test at 0.05 level. Data is the mean (±SE) of four replicates.
3.5. Effect of Yeast Extract or Chitosan on Catalase, Peroxidase, and Superoxide Dismutase Activity of Garlic in Drought Conditions
Drought stress induced the up-regulation of enzyme activity in garlic plants during both seasons; catalase, superoxide dismutase, and peroxidase activity significantly increased (p ≤ 0.05) in garlic plants that received two drought treatments compared with controls (Figure 5). The maximum activities of the three substances catalase, peroxidase, and superoxide dismutase were observed in the stressed garlic plants that received 50% of normal irrigation during both seasons, followed by plants that received 75% of normal irrigation, compared with the controls. However, yeast or chitosan applied individually or in combination was effective in regulating the catalase, superoxide dismutase, and peroxidase activity for both the 75% and 50% irrigations. Of all treatments, the greatest results of catalase and peroxidase were observed during both seasons for the 75% irrigation plus yeast plus chitosan, without any significant differences with controls.
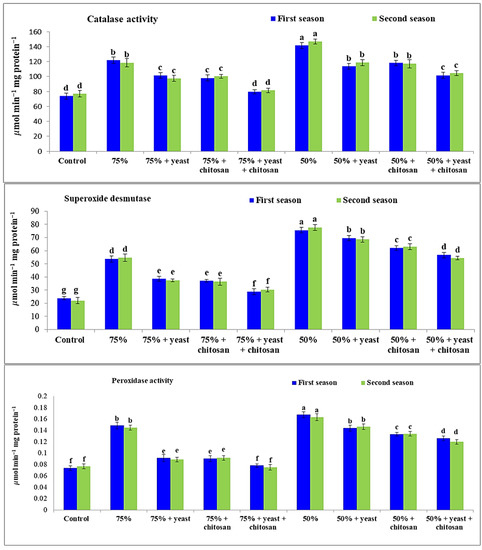
Figure 5.
Effect of yeast and chitosan on catalase (CAT), superoxide dismutase (SOD) and peroxidase activity (POX) of garlic under drought during 2019/2020 and 2020/2021 seasons. The letters on the columns show significant differences between the treatments according to ANOVA, Duncan’s multiple range test at 0.05 level. Data is the mean (±SE) of four replicates.
3.6. Effect of Yeast Extract or Chitosan on Bulb Diameter, Total Yield, and Total Cured Yield of Garlic Plants in Drought Conditions
Bulb diameter (cm), total yield (ton ha−1), and total cured yield (ton ha−1) were decreased significantly (p ≤ 0.05) in garlic plants that received 75% or 50% of normal irrigation during both seasons compared with controls (Figure 6). The lowest values of bulb diameter, total yield, and total cured yield were recorded in the stressed garlic plants that received 50% of normal irrigation. A significant increase was noted in these yield characters when plants received foliar treatment with yeast or chitosan separately or in combination compared with stressed untreated plants during both seasons. The treatment 75% irrigation plus yeast plus chitosan had the best values for bulb diameter, total yield, and total cured yield in comparison with other treatments and without any significant differences with controls.
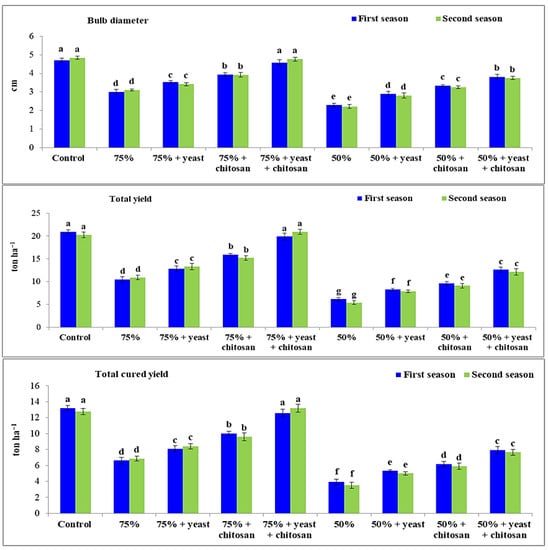
Figure 6.
Effect of yeast and chitosan on bulb diameter (cm), total yield (ton ha−1) and total cured yield (ton ha−1) of garlic under drought during 2019/2020 and 2020/2021 seasons. The letters on the columns show significant differences between the treatments according to ANOVA, Duncan’s multiple range test at 0.05 level. Data is the mean (±SE) of four replicates.
4. Discussion
It is well recognized that drought is a main factor that can harm plant production worldwide [26]. The stressed garlic plants in our study (subjected to 75% and 50% of normal irrigation) displayed a remarkable reduction in number of leaves, plant height, and plant dry weight during both seasons (Figure 1). The deleterious impact of drought on garlic plants could be due to the diminution in water absorption from soil to leaves, increased dehydration and reduced viscosity in the cells, and decreased cell division, all of which can negatively affect vegetative growth characters, especially plant height, number of leaves, and plant dry weight. The plants could adapt to drought conditions by producing fewer leaves and stomata and staying smaller in size as well as increasing the concentration of stress hormone such as ABA and salicylic acid [60]. A similar effect of drought or a water deficit was also seen in other plants such as maize [29], barley [27,28], and faba beans [33]. The application of yeast extract or chitosan individually or in combination considerably augmented the plant dry weight, plant height, and number of leaves in garlic plants in drought conditions. This increase may have been due to the synergistic role of yeast and chitosan in stimulating growth and increasing leaf numbers, root length, and plant dry weight compared with untreated plants in drought conditions. This could also be due to the fact that yeast is a biofertilizer and an essential source of many active compounds, such as vitamins, amino acids, and hormones, which induce plant growth [42,43]. These findings are in harmony with those recorded by El-Shawa et al. [14] and Abdelaal et al. [26]. Also, chitosan is a strong inducer of many secondary metabolites such as phenolic compounds in plants under stress [27,38,61]. Jasmonic acid biosynthesis plays a pivotal role in the regulation of water uptake in stressed plants. Chitosan is an anti-transpiration agent and can affect stomatal movement [62].
In the treatments in our study, concentrations of chlorophyll a and b were considerably reduced in garlic plants in both seasons (Figure 2). The reduction in chlorophylls could have been because drought injures chlorophyll pigment and causes destruction of light-harvesting protein complexes, decreases carbon dioxide fixation, and reduces NADP+ production through the Calvin cycle pathway. Drought causes oxidative damage to lipids, proteins, and pigments in chloroplasts [63,64]. Our findings agreed with those of Shinde and Thakur [65], who found that drought significantly decreased chlorophyll a and b concentrations in chickpea plants [65] and barley plants [23,27,28]. Also, Gedam et al. [66] reported that, membrane stability index (MSI), RWC, total chlorophyll content and antioxidant enzyme activity as well as bulb yield were negatively affected in onion plants under drought stress.
In the current research, RWC was considerably decreased in stressed garlic plants in both seasons, and this reduction might be attributed to the detrimental impact on water absorption, conductivity, and availability in the plants. These results are in agreement with the findings of previous studies on cotton [67], Zea mays L. [68], and Pisum sativum L. [69]. The application of yeast and chitosan overcame the negative influences of drought and improved chlorophyll concentrations because yeast is a rich source of many vital components such as amino acids, which increase the chlorophyll content in garlic in drought conditions. Moreover, the positive impact of chitosan may have been due to the improvement in chloroplast numbers and chlorophyll synthesis because of an increase in potassium and nitrogen, which are essential for growth and good yields [70]. Farouk and Amany [63] and Khan et al. [71] found that chitosan can mitigate the negative effects of drought and improve chlorophyll concentrations and total carbohydrate and photosynthesis processes in maize and cowpea plants. Our findings showed a remarkable increase in EL% and levels of proline and ascorbic acid in stressed garlic plants in drought conditions compared with controls (Figure 3). This increase could be attributed to the oxidative stress experienced by plant cells in drought, which negatively affects plasma membranes and permeability. The resulting increase in proline, EL%, and ascorbic acid signals that oxidative damage is occurring. The MDA, hydrogen peroxide, and superoxide dismutase were significantly augmented in stressed garlic plants, as well, and this also signaled that oxidative damage was occurring in plants affected by drought compared with controls (Figure 4). These high levels were seen in several plants in numerous conditions of abiotic stress [32,72,73] and biotic stress [74,75,76]. These results are in harmony with those recorded by Hafez et al. [27], who found that the MDA levels, EL%, and ROS increased considerably in drought-stressed barley plants because of damage to plasma membranes and the cytoplasm. Abdelaal et al. [23] reported that the levels of superoxide dismutase and hydrogen peroxide, EL%, and MDA levels were considerably elevated in barley plants as a response to drought. Interestingly enough, yeast extract and chitosan treatments helped garlic plants to recover from drought stress and led to the regulation of the proline content, increased the ascorbic acids, and reduced the EL% and superoxide dismutase, hydrogen peroxide, and MDA levels compared with stressed untreated garlic plants. The helpful effect of chitosan on stressed plants could be due to its role in increasing and regulating proline as an osmolyte and very importantly, in stabilizing the plasma membrane and protein levels and scavenging of ROS under stress [23,77,78]. Chitosan is also a significant regulator of osmosis in drought stress, so the application of chitosan led to increased membrane stability and decreased lipid peroxidation in many plants [79,80]. Proline plays a central role in regulating the function of mitochondria, protecting the chloroplasts against oxidative damage, and activating the gene expression that helps plants to recover from stresses. Proline application significantly increased onion growth characters compared to untreated plants, this effect may be due to improve cell membrane stability and RWC as well as photosynthetic efficiency [81]. Also, Srmida et al. [81] stated that application of proline could mitigate drought effect by increasing sugar content and via improving plant self-defense system of onion plants.
The valuable impact of chitosan could be attributed to its role in enhancing membrane stability and decreasing the levels of superoxide dismutase, hydrogen peroxide, and MDA because of the existence of specific chitosan-like amino groups that react with ROS and produce nontoxic radicals [82]. The activities of antioxidant enzymes such as catalase, superoxide dismutase, and peroxidase were considerably augmented in drought-stressed garlic plants compared with controls in both seasons (Figure 5). These antioxidant enzymes play an important role in stressed plants to help them grow well and mitigate the oxidative damage that can occur. Because of the increase in ROS levels, these findings were recorded in many plants with different stresses [8,11,28,33]. The application of yeast or chitosan individually or in combination led to adjustments in the catalase, superoxide dismutase, and peroxidase activity, and this protected cells in drought stress compared with stressed untreated garlic plants. The best result was achieved with 75% irrigation plus yeast plus chitosan. This useful effect of chitosan could be due to the fact that chitosan decreases the transpiration rate and stimulates stomatal closure, as well as regulates the antioxidant enzymes, consequently mitigating the damaging impact of drought [83]. Also, chitosan can improve the production of important amino acids, such as aspartic acid, proline, serine, threonine, lysine, and phenylalanine, in drought [84]. Similarly, the use of yeast extract helped stressed garlic plants to recover their enzyme activity during both seasons compared with stressed untreated garlic plants. This adjustment with yeast treatments was recorded in many plants in stressful conditions [26,29,41,42]. This impact of yeast might be attributed to its role as a biostimulant and its ability to increase the hormonal activity in plants [85] and act as a nutritional increment factor, which can increase the growth and development of plants [86]. Bulb diameter, total yield, and total cured yield were considerably reduced in stressed garlic plants (Figure 6). This decrease could be due to the adverse effect of drought on morphophysiological features such as plant height, plant fresh weight, RWC, number of leaves, and chlorophyll content. These results are in agreement with the results recorded in several plants [23,30]. The application of yeast or chitosan or the combination of the two considerably increased the bulb diameter, total yield, and total cured yield in the stressed garlic plants. The effect of the yeast in this outcome could be explained by the production of many important compounds, such as amino acids, alkaloids, vitamins, and enzymes, as well as essential elements that increase the photosynthetic rate. Also, this result might have been due to the supportive effect of chitosan in inducing gene overexpression, which is involved in photosynthesis and protein and hormone metabolism, consequently improving the yield. These results are in agreement with those of Landi et al. [87] for strawberries, El-Shawa et al. [14] for calendula, Abdelaal et al. [26] for wheat, and Pongprayoon et al. [34] for rice plants. In general, the utilization of yeast extract and chitosan for increasing the yield production of garlic plants in drought has multiple advantages because these natural compounds are nontoxic, inexpensive, and environmentally friendly. The application of yeast plus chitosan significantly increased the vegetative growth and bulb yield characters, as well as improved the physiobiochemical characters of garlic in drought conditions.
5. Conclusions
Generally, we revealed that the application of yeast extract (8 g/L) plus chitosan (300 mM) individually or in combination significantly increased the growth and bulb yield of garlic plants exposed to drought conditions (75% or 50% of normal irrigation). These treatments alleviated the adverse impacts of drought, increased the number of leaves per plant, plant height, plant dry weight, chlorophyll a and b concentrations, and RWC; decreased the oxidative stress signals such as EL% and levels of superoxide, MDA, and hydrogen peroxide; as well as adjusted the production of proline, ascorbic acid, and antioxidant enzymes such as peroxidase, catalase, and superoxide dismutase. Our findings revealed that yeast extract plus chitosan could be used as an inexpensive and nontoxic technique that is safe for the environment compared with synthetic compounds for improving the yield production of garlic plants in conditions of drought.
Author Contributions
Conceptualization, K.A., K.A.A., Y.H. and S.A.A.; methodology, K.A.A., K.A.A., Y.H. and S.A.A.; software, K.A., G.N., T.W., K.A.A., Y.H.; validation, K.A.; formal analysis, K.A., K.A.A., Y.H.; investigation, K.A., G.N., T.W.; resources, K.A., G.N., T.W. and S.A.A.; data curation, K.A., K.A.A., Y.H. and S.A.A.; writing—original draft preparation, K.A., Y.H. and S.A.A.; writing—review and editing, K.A., G.N., T.W., K.A.A., Y.H. and S.A.A.; visualization, K.A., G.N., T.W. and S.A.A. supervision, K.A., G.N., T.W. and S.A.A.; funding acquisition, K.A., K.A.A., Y.H., S.A., T.K.A. and S.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Researchers Supporting Project number (RSP-2021/241), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to Researchers Supporting Project number (RSP-2021/241), King Saud University, Riyadh, Saudi Arabia. Also many thanks to all members of Plant Pathology and Biotechnology Lab. and EPCRS Excellence Centre (Certified according to ISO/17025, ISO/9001, ISO/14001 and OHSAS/18001), Dept. of Agric. Botany, Fac. of Agric.; Kafrelsheikh University, Kafr-Elsheikh, Egypt.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Retrieved December 2020 from the FAOSTAT on the World Wide; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 25 August 2021).
- Abdelrahman, M.; Hirata, S.; Mukae, T.; Yamada, T.; Sawada, Y.; El-Syaed, M.; Yutaka, Y.; Sato, M.; Hirai, M.; Shigyo, M. Comprehensive Metabolite Profiling in Genetic Resources of Garlic (Allium sativum L.) Collected from Different Geographical Regions. Molecules 2021, 26, 1415. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Li, H.B. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Ismail, M.S.M.; Ghallab, M.M.A.; Soliman, M.F.M.; AboGhalia, A.H. Acaricidal activities of some essential and fixed oils on the two-spotted spider mite, Tetranychus urticae. Egypt. Acad. J. Biol. Sci. 2011, 3, 41–48. [Google Scholar] [CrossRef]
- Sharaby, A.; Montasser, S.A.; Mahmoud, Y.A.; Ibrahim, S.A. Natural plant essential oils for controlling the grasshopper (Heteracris littoralis) and their pathological effects on the alimentary canal. Ecol. Balk. 2012, 4, 39–52. [Google Scholar]
- Omara, R.I.; El-Kot, G.A.; Fadel, F.M.; Abdelaal, K.A.; Saleh, E.M. Efficacy of Certain Bioagents on Patho-Physiological Characters of Wheat Plants under Wheat Leaf Rust Stress. Physiol. Mol. Plant Pathol. 2019, 106, 102–108. [Google Scholar] [CrossRef]
- Hafez, Y.M.; Abdelaal, K.A. Investigation of susceptibility and resistance mechanisms of some Egyptian wheat cultivars (Triticum aestivum L.) inoculated with Blumeria graminis f.sp. tritici using certain biochemical, molecular characterization and SEM. J. Plant Prot. Pathol. Mansoura Univ. 2015, 6, 431–454. [Google Scholar] [CrossRef][Green Version]
- Abdelaal, K.A.; Hafez, Y.M.; Badr, M.M.; Youseef, W.A.; Esmaeil, S.M. Biochemical, histological and molecular changes in susceptible and resistant wheat cultivars inoculated with stripe rust fungus Puccinia striiformis f. sp. tritici. Egypt J. Biol. Pest. Control 2019, 24, 421–429. [Google Scholar]
- Abdelaal, K.A.A.; Essawy, M.; Quraytam, A.; Abdallah, F.; Mostafa, H.; Shoueir, K.; Fouad, H.; Fahmy, A.S.H.; Hafez, Y.M. Toxicity of Essential Oils Nanoemulsion against Aphis Craccivora and Their Inhibitory Activity on Insect Enzymes. Processes 2021, 9, 624. [Google Scholar] [CrossRef]
- El-Flaah, R.F.; El-Said, R.A.R.; Nassar, M.A.; Hassan, M.; Abdelaal, K.A.A. Effect of rhizobium, nano silica and ascorbic acid on morpho-physiological characters and gene expression of POD and PPO in faba bean (Vicia faba L.) under salinity stress conditions. Fresenius Environ. Bull. 2021, 30, 5751–5764. [Google Scholar]
- Abdelaal, K.A.A.; EL-Maghraby, L.M.; Elansary, H.; Hafez, Y.M.; Ibrahim, E.I.; El-Banna, M.; El-Esawi, M.; Elkelish, A. Treatment of Sweet Pepper with Stress Tolerance-Inducing Compounds Alleviates Salinity Stress Oxidative Damage by Mediating the Physio-Biochemical Activities and Antioxidant Systems. Agronomy 2020, 10, 26. [Google Scholar] [CrossRef]
- El-Banna, M.F.; Abdelaal, K.A.A. Response of Strawberry Plants Grown in the Hydroponic System to Pretreatment with H2O2 Before Exposure to Salinity Stress. J. Plant Prod. Mansoura Univ. 2018, 9, 989–1001. [Google Scholar] [CrossRef]
- Zhou, X.; Condori-Apfata, J.A.; Liu, X.; Condori-Pacsi, S.J.; Valencia, M.V.; Zhang, C. Transcriptomic Changes Induced by Drought Stress in Hardneck Garlic during the Bolting/Bulbing Stage. Agronomy 2021, 11, 246. [Google Scholar] [CrossRef]
- El-Shawa, G.M.R.; Rashwan, E.M.; Abdelaal, K.A.A. Mitigating salt stress effects by exogenous application of proline and yeast extract on morphophysiological, biochemical and anatomical characters of calendula plants. Sci. J. Flowers Ornam. Plants 2020, 7, 461–482. [Google Scholar] [CrossRef]
- Hasan, M.K.; El Sabagh, A.; Sikdar, M.S.I.; Alam, M.J.; Ratnasekera, D.; Barutcular, C.; Abdelaal, K.A.A.; Islam, M.S. Comparative adaptable agronomic traits of Blackgram and mungbean for saline lands. Plant Arch. 2017, 17, 589–593. [Google Scholar]
- ALKahtani, M.D.F.; Attia, K.A.; Hafez, Y.M.; Khan, N.; Eid, A.M.; Ali, M.A.M.; Abdelaal, K.A.A. Chlorophyll Fluorescence Parameters and Antioxidant Defense System Can Display Salt Tolerance of Salt Acclimated Sweet Pepper Plants Treated with Chitosan and Plant Growth Promoting Rhizobacteria. Agronomy 2020, 10, 1180. [Google Scholar] [CrossRef]
- Hafez, Y.; Elkohby, W.; Mazrou, Y.S.A.; Ghazy, M.; Elgamal, A.; Abdelaal, K.A. Alleviating the detrimental impacts of salt stress on morpho-hpysiological and yield characters of rice plants (Oryza sativa L.) using actosol, Nano-Zn and Nano-Si. Fresenius Environ. Bull. 2020, 29, 6882–6897. [Google Scholar]
- Abdelaal, K.A.A.; AlKahtani, M.D.F.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The pivotal role of plant growth promoting bacteria in alleviating the adverse effects of drought and facilitating sustainable agriculture. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Marostica, T.F.; Cazarolli1, L.H.; Moura, G.S.; Luz, V.C.D.; Guimarães, E.A.C.M.; Cargnelutti, D. Does Allium sativum L. tolerate water deficit? Sci. Elec. Arch. 2019, 12, 43–51. [Google Scholar] [CrossRef][Green Version]
- Ragab, A.Y.; Geries, L.S.M.; Abdelaal, K.A.A.; Hanna, S.A. Growth and productivity of onion plant (Allium cepa L.) as affected by transplanting method and NPK fertilization. Fresenius Environ. Bull. 2019, 28, 7777–7786. [Google Scholar]
- AlKahtani, M.D.F.; Hafez, Y.M.; Attia, K.; Rashwan, E.; Husnain, L.A.; AlGwaiz, H.I.M.; Abdelaal, K.A.A. Evaluation of Silicon and Proline Application on the Oxidative Machinery in Drought-Stressed Sugar Beet. Antioxidants 2021, 10, 398. [Google Scholar] [CrossRef]
- Rashwan, E.; Alsohim, A.S.; El-Gammaal, A.; Hafez, Y.; Abdelaal, K.A.A. Foliar application of nano zink-oxide can alleviate the harmful effects of water deficit on some flax cultivars under drought conditions. Fresenius Environ. Bull. 2020, 29, 8889–8904. [Google Scholar]
- Abdelaal, K.A.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.S.E.; Abu-Elsaoud, A.M.; Hafez, Y.M. Exogenous Application of Proline and Salicylic Acid can Mitigate the Injurious Impacts of Drought Stress on Barley Plants Associated with Physiological and Histological Characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2020, 10, 17672. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.A.A.; Rashed, S.H.; Ragab, A.; Hossian, A.; El Sabagh, A. Yield and quality of two sugar beet (Beta vulgaris L. ssp. vulgaris var. altissima Doll) cultivars are influenced by foliar application of salicylic Acid, irrigation timing, and planting density. Acta Agric. Slov. 2020, 115, 239–248. [Google Scholar] [CrossRef]
- Abdelaal, K.; Elafry, M.; Abdel-Latif, I.; Elshamy, R.; Hassan, M.; Hafez, Y. Pivotal role of yeast and ascorbic acid in improvement the morpho-physiological characters of two wheat cultivars under water deficit stress in calcareous soil. Fresenius Environ. Bull. 2021, 30, 2554–2565. [Google Scholar]
- Hafez, Y.M.; Attia, K.A.; Alamery, S.; Ghazy, A.; Al-Dosse, A.; Ibrahim, E.; Rashwan, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K.A.A. Beneficial Effects of Biochar and Chitosan on Antioxidative Capacity, Osmolytes Accumulation, and Anatomical Characters of Water-Stressed Barley Plants. Agronomy 2020, 10, 630. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Hafez, Y.M.; El-Afry, M.M.; Tantawy, D.S.; Alshaal, T. Effect of some osmoregulators on photosynthesis, lipid peroxidation, antioxidative capacity and productivity of barley (Hordeum vulgare L.) under water deficit stress. Environ. Sci. Pollut. Res. 2018, 25, 30199–30211. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Hafez, Y.M.; El Sabagh, A.; Saneok, H. Ameliorative effects of Abscisic acid and yeast on morpho-physiological and yield characteristics of maize plant (Zea mays L.) under water deficit conditions. Fresenius Environ. Bull. 2017, 26, 7372–7383. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Omara, R.I.; Abdelaal, K.A.A. Biochemical, histopathological and genetic analysis associated with leaf rust infection in wheat plants (Triticum aestivum L). Physiol. Mol. Plant. Pathol. 2018, 104, 48–57. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Mazrou, Y.S.A.; Hafez, Y.M. Silicon Foliar Application Mitigates Salt Stress in Sweet Pepper Plants by Enhancing Water Status, Photosynthesis, Antioxidant Enzyme Activity and Fruit Yield. Plants 2020, 9, 733. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; El-Afry, M.; Metwaly, M.; Zidan, M.; Rashwan, E. Salt tolerance activation in faba bean plants using proline and salicylic acid associated with physio-biochemical and yield characters improvement. Fresenius Environ. Bull. 2021, 30, 3175–3186. [Google Scholar]
- Pongprayoon, W.; Roytrakul, S.; Pichayagkura, R.; Chadchawan, S. The role of hydrogen peroxide in chitosan-induced resistance to osmotic stress in rice (Oryza sativa L.). Plant Growth Regul. 2013, 70, 159–173. [Google Scholar] [CrossRef]
- Monirul, I.M.; Humayun, K.M.; Mamun, A.N.K.; Monirul, I.; Pronabananda, D. Studies on yield and yield attributes in tomato and chilli using foliar application of oligo-chitosan. GSC Biol. Pharm. Sci. 2018, 3, 20–28. [Google Scholar]
- Ahmed, K.B.M.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and its oligosaccharides, a promising option for sustainable crop production—A review. Carbohydr. Polym. 2020, 227, 115331. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Luo, X.; Tu, R. Application of bioactive coatings based on chitosan for soybean seed protection. Int. J. Carbohydr. Chem. 2012, 2012, 104565. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Siadat, S.A.; Bakhshandeh, A.; Pirbalouti, A.G.; Hashemi, M. Morpho-physiological and phytochemical traits of (Thymus daenensis Celak.) in response to deficit irrigation and chitosan application. Acta Physiol. Plant. 2017, 39, 231. [Google Scholar] [CrossRef]
- Barnett, J.A.; Payne, R.W.; Yarrow, D. (Eds.) Yeasts: Characterisation and Identification, 3rd ed.; Cambridge University Press: Cambridge, MA, USA, 1990. [Google Scholar] [CrossRef]
- Matter, F.M.A.; Abou-Sreea, A.I.B. Influence of application methods of bio-fertilization on morhological growth characters, seed yield and chemical composition of fenugreek plants. Egypt. J. Hort. 2016, 43, 19–33. [Google Scholar]
- Nagodowithana, W.T. Yeast Technology; Van Nostrsnd Reinhold: New York, NY, USA, 1991; 273p. [Google Scholar]
- Haider, I.; Raza, M.; Sammar, A.; Iqbal, R.; Ahmad, S.; Aslam, M.U.; Israr, M.; Riaz, U.; Sarfraz, M.; Abbas, N.; et al. Alleviating the Drought Stress in Wheat (Triticum aestivum L.) by Foliar Application of Amino Acid and Yeast. Pak. J. Agric. Res. 2021, 34, 239–246. [Google Scholar] [CrossRef]
- Sarhan, T.; Abdullah, O.K. Effect of Azotobacter inoculation. Dry bread yeast suspension varying levels of urea on growth of potato Cv. Desiree. In Proceedings of the Tropentag World Food System—A Contribution from Europe, Zurich, Switzerland, 14–16 September 2010. [Google Scholar]
- Shalaby, T.A.; El-Ramady, H. Effect of foliar application of bio-stimulants on growth, yield, components, and storability of garlic (Allium sativum L.). Aust. J. Crop Sci. 2014, 8, 271–275. [Google Scholar]
- Ali, M.A.M. Effect of some Bio-stimulants on Growth, Yield and Bulb Quality of Garlic Grown in Newly Reclaimed Soil, New Valley-Egypt. J. Plant Prod. Mansoura Univ. 2017, 8, 1285–1294. [Google Scholar] [CrossRef][Green Version]
- Association of Official Analytical Chemists (A.O.A.C.). Official Methods of Analysis, 26th ed.; A.O.A.C. International: Washington, DC, USA, 2005. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Sanchez, F.J.; de Andrés, E.F.; Tenorio, J.L.; Ayerbe, L. Growth of epicotyls, turgor maintenance and osmotic adjustment in pea plants (Pisum sativum L.) subjected to water stress. Field Crop. Res. 2004, 86, 81–90. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Szalai, G.; Janda, T.; Padi, E.; Szigeti, Z. Role of light in post-chilling symptoms in maize. J. Plant Physiol. 1996, 148, 378–383. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Yesbergenova, Z.; Yang, G.; Oron, E.; Soffer, D.; Fluhr, R.; Sagi, M. The plant Mo-hydroxylases aldehyde oxidase and xanthine dehy-drogenase have distinct reactive oxygen species signatures and are induced by drought and abscisic acid. Plant J. 2005, 42, 862–876. [Google Scholar] [CrossRef]
- Yu, C.W.; Murphy, T.M.; Lin, C.H. Hydrogen peroxide-induces chilling tolerance in mung beans mediated through ABA independent glutathione accumulation. Funct. Plant Biol. 2003, 30, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef]
- Spitz, D.R.; Oberley, L.W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989, 179, 8–18. [Google Scholar] [CrossRef]
- Castillo, F.I.; Penel, I.; Greppin, H. Peroxidase release induced by ozone in sedum album leaves. Plant Physiol. 1984, 74, 846–851. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; Wiley Inter Science: New York, NY, USA, 1984; 690p. [Google Scholar]
- Duncan, B.D. Multiple ranges and multiple F-test. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Liu, F.; Christian, R.; Shahanzari, J.A.; Andersen, M.N.; Jacobsen, E.E. ABA regulated stomata control and photosynthetic water use efficiency of potato (Solanum tuberosum L.) during progressive soil drying. Plant Sci. 2005, 168, 831–836. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef] [PubMed]
- Abu-Muriefah, S.S. Effect of chitosan on common bean (Phaseolus vulgaris L.) plants grown under water stress conditions. Int. Res. J. Agric. Sci. Soil Sci. 2013, 3, 192–199. [Google Scholar]
- Farouk, S.; Amany, A.R. Improving growth and yield of cowpea by foliar application of chitosan under water stress. Egypt. J. Biol. 2012, 14, 14–16. [Google Scholar] [CrossRef]
- Lai, Q.; Zhi-yi, B.; Zhu-Jun, Z.; Qiong-Qiu, Q.; Bi-Zeng, M. Effects of osmotic stress on antioxidant enzymes activities in leaf discs of PSAG12-IPT modified gerbera. J. Zhejiang Univ. Sci. 2007, 8, 458–464. [Google Scholar] [CrossRef]
- Shinde, B.P.; Thakur, J. Influence of Arbuscular mycorrhizal fungi on chlorophyll, proteins, proline and total carbohydrates content of the pea plant under water stress condition. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 809–821. [Google Scholar]
- Gedam, P.A.; Thangasamy, A.; Shirsa, D.V.; Ghosh, S.; Bhagat, K.P.; Sogam, O.A.; Gupta, A.J.; Mahajan, V.; Soumia, P.S.; Salunkhe, V.N.; et al. Screening of Onion (Allium cepa L.) Genotypes for Drought Tolerance Using Physiological and Yield Based Indices Through Multivariate Analysis. Front. Plant Sci. 2021, 12, 600371. [Google Scholar] [CrossRef]
- Massacci, A.; Nabiev, S.M.; Pietrosanti, L.; Nematov, S.K.; Chernikova, T.N.; Thor, K.; Leipner, J. Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gas-exchange analysis and chlorophyll fluorescence imaging. Plant Physiol. Biochem. 2008, 46, 189–195. [Google Scholar] [CrossRef]
- Nayyar, H.; Gupta, D. Differential sensitivity of C3 and C4 plants to water deficit stress: Association with oxidative stress and antioxidants. Environ. Exp. Bot. 2006, 58, 106–113. [Google Scholar] [CrossRef]
- AL-Quraan, N.A.; Al-Ajlouni, Z.I.; Qawasma, N.F. Physiological and Biochemical Characterization of the GABA Shunt Pathway in Pea (Pisum sativum L.) Seedlings under Drought Stress. Horticulturae 2021, 7, 125. [Google Scholar] [CrossRef]
- Possingham, J.V. Plastid replication and development in the life cycle of higher plants. Annu. Rev. Plant Physiol. 1980, 31, 113–129. [Google Scholar] [CrossRef]
- Khan, W.M.; Prithiviraj, B.; Smiyh, D.L. Effect of foliar application of chitin oligosaccharides on photosynthesis of maize and soybean. Photosynthetica 2002, 40, 87. [Google Scholar] [CrossRef]
- Gupta, A.; Medina-Rico, A.; Delgado-Cano, A. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Ghaffaria, H.; Tadayona, M.R.; Bahadora, M.; Razmjoo, J. Investigation of the proline role in controlling traits related to sugar and root yield of sugar beet under water deficit conditions. Agric. Water Manag. 2021, 243, 106448. [Google Scholar] [CrossRef]
- Omar, A.; Zayed, B.; Abdel Salam, A.; Hafez, Y.M.; Abdelaal, K.A.A. Folic acid as foliar application can improve growth and yield characters of rice plants under irrigation with drainage water. Fresenius Environ. Bull. 2020, 29, 9420–9428. [Google Scholar]
- Shahin, A.; Esmaeil, R.A.; Badr, M.; Abdelaal, K.A.A.; Hassan, F.A.S.; Hafez, Y.M. Phenotypic characterization of race-specific and slow rusting resistance to stem rust disease in promising wheat genotypes. Fresenius Environ. Bull. 2021, 30, 6223–6236. [Google Scholar]
- Esmail, S.M.; Omara, R.I.; Abdelaal, K.A.; Hafez, M. Histological and biochemical aspects of compatible and incompatible wheat-Puccinia striiformis interactions. Physiol. Mol. Plant Pathol. 2019, 106, 120–128. [Google Scholar] [CrossRef]
- Rocychoudhury, A.; Banerjee, A. Endogenous glycine betaine accumulation mediates abiotic stress tolerance in plants. Trop. Plant Res. 2016, 3, 105–111. [Google Scholar]
- Kheradmand, M.A.; Shahmoradzadeh, F.S.; Fatahi, E.; Raoofi, M.M. Effect of water stress on oil yield and some characteristics of Brassica napus. Int. Res. J. Appl. Basic Sci. 2014, 8, 1447–1453. [Google Scholar]
- Yang, F.; Hu, J.; Li, J.; Wu, X.; Qian, Y. Chitosan enhances leaf membrane stability and antioxidant enzyme activities in apple seedlings under drought stress. Plant Growth Regul. 2009, 58, 131–136. [Google Scholar] [CrossRef]
- Jiao, Z.; Li, Y.; Li, J.; Xu, X.; Li, H.; Lu, D.; Wang, J. Effects of exogenous chitosan on physiological characteristics of potato seedlings under drought stress and rehydration. Potato Res. 2012, 55, 293–301. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Rady, M.O.A.; Marey, R.A.; Abd El-Mageed, T.A. Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Sci. Hortic. 2020, 272, 109580. [Google Scholar] [CrossRef]
- Sun, T.; Xie, W.M.; Xu, P.X. Superoxide anion scavenging activity of graft chitosan derivatives. Carbohydr. Polym. 2004, 58, 379–382. [Google Scholar] [CrossRef]
- Bittelli, M.; Flury, M.; Campbell, G.S.; Nichols, E.J. Reduction of transpiration through foliar application of chitosan. Agric. For. Meteorol. 2001, 107, 167–175. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, X.; Merewitz, E.; Peng, Y.; Ma, X.; Yan, Y. Metabolic pathways regulated by chitosan contributing to drought resistance in white clover. J. Proteome Res. 2017, 16, 3039–3052. [Google Scholar] [CrossRef]
- Su, Y.; Xia, S.; Zhong, R.; Wang, L. Phytohormonal quantification based on biological principles. Horm. Metab. Signal. Plants 2017, 13, 431–470. [Google Scholar]
- Vasconcelos, A.C.F.D.; Chaves, L.H.G. Biostimulants and their role in improving plant growth under abiotic stresses. In Biostimulants in Plant Science; Intech Open: London, UK, 2019. [Google Scholar] [CrossRef]
- Landi, L.; De Miccoli, R.M.; Pollastro, S.; Feliziani, E.; Faretra, F.; Romanazzi, G. Global transcriptome analysis and identification of differentially expressed genes in strawberry after preharvest application of Benzothiadiazole and chitosan. Front. Plant Sci. 2017, 8, 235. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).