Cryopreservation of Hazelnut (Corylus avellana L.) Axillary Buds from In Vitro Shoots Using the Droplet Vitrification Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Explant Preparation for Cryopreservation and Loading Phase
2.3. Explant Dehydration, Thawing and Unloading
2.4. Cold Pre-Treatment of Mother Plant
2.5. Rooting Induction of Recovered Explants
2.6. Data Collection and Statistical Analysis
3. Results
3.1. Effect of Type and Length of Plant Vitrification Solution Treatment
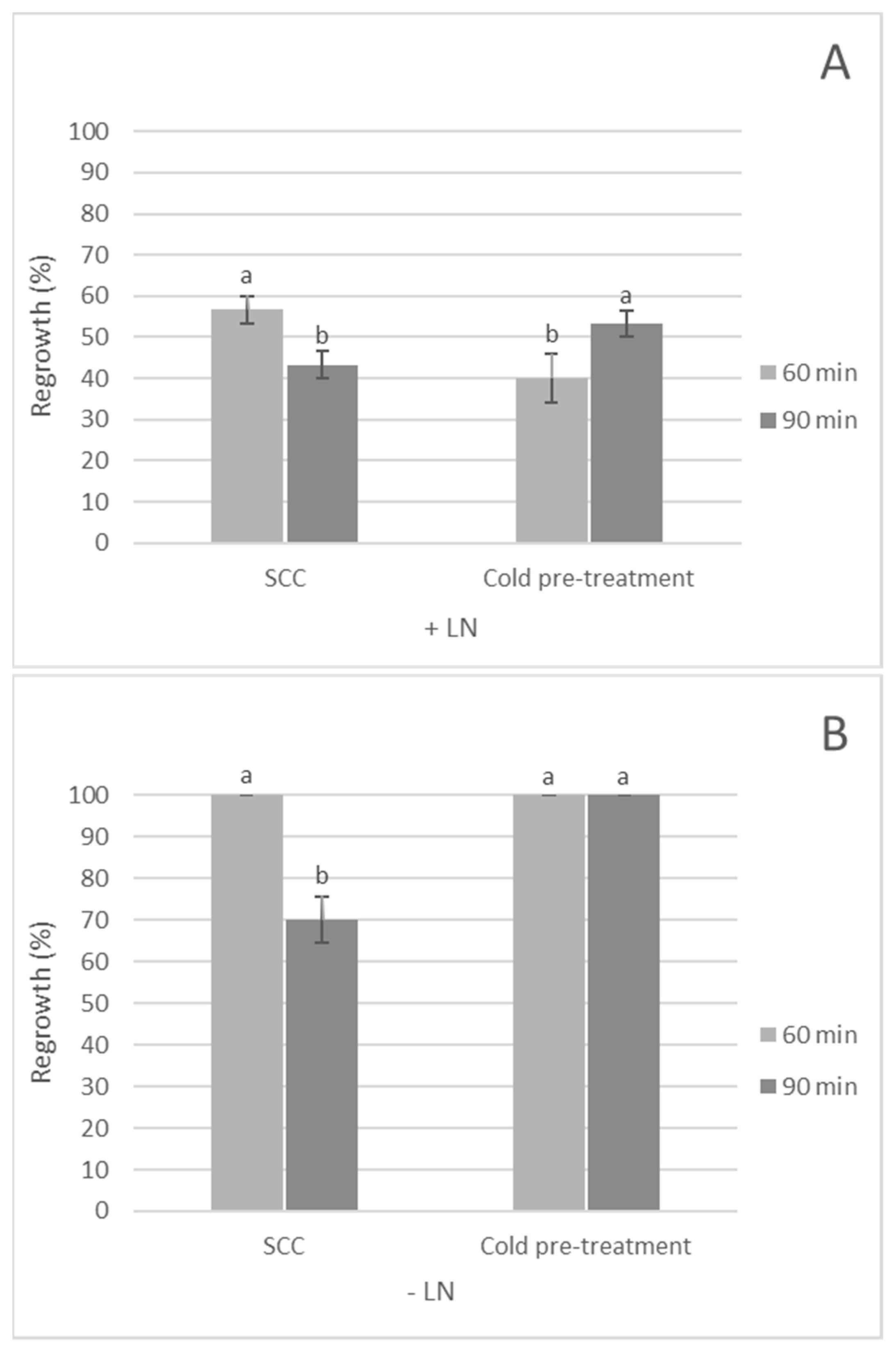
3.2. Effect of Cold Pre-Treatment of Mother Plant
3.3. Rooting of Recovered Explants
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DMSO | dimethyl sulfoxide |
| DV | droplet vitrification |
| LN | liquid nitrogen |
| PVS2 | plant vitrification solution 2 |
| PVS3 | plant vitrification solution 3 |
References
- Pence, V.C.; Ballesteros, D.; Walters, C.; Reed, B.M.; Philpott, M.; Dixon, K.W.; Pritchard, H.W.; Culley, T.M.; Vanhove, A.C. Cryobiotechnologies: Tools for expanding long‒term ex situ conservation to all plant species. Biol. Conserv. 2020, 250, 108736. [Google Scholar] [CrossRef]
- González‒Arnao, M.T.; Martizez‒Montero, M.E.; Cruz‒Cruz, C.A.; Engelmann, F. Advances in cryogenic techniques for the long term preservation of plant biodiversity. In Biotechnology and Biodiversity; Ahuja, M., Ramawat, K., Eds.; Springer: New York, NY, USA, 2014; pp. 129–170. [Google Scholar]
- Panis, B. Sixty years of plant cryopreservation: From freezing hardy mulberry twigs to establishing reference crop collections for future generations. Acta Hortic. 2019, 1234, 1–7. [Google Scholar] [CrossRef]
- Panis, B.; Van den Houwe, I.; Swennen, R.; Rhee, J.; Roux, N. Securing plant genetic resources for perpetuity through cryopreservation. Indian J. Plant Genet. Resour. 2016, 29, 300–302. [Google Scholar] [CrossRef]
- Reed, B.M. Plant cryopreservation: A continuing requirement for food and ecosystem security. In Vitro Cell. Dev. Biol. Plant 2017, 53, 285–288. [Google Scholar] [CrossRef]
- Dereuddre, J.; Scottez, C.; Arnaud, Y.; Duron, M. Resistance of alginate-coated axillary shoot tips of pear tree (Pyrus communis L. cv Beurré Hardy) in vitro plantlets to dehydration and subsequent freezing in liquid nitrogen: Effects of previous cold hardiness. C. R. de l’Académie Sci. 1990, 310, 317–323. [Google Scholar]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.M. Cryopreservation—Practical considerations. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 3–13. [Google Scholar]
- Sakai, A.; Hirai, D.; Niino, T. Development of PVS‒based vitrification and encapsulation‒vitrification protocols. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 421–426. [Google Scholar]
- Benelli, C.; DE Carlo, A.; Engelmann, F. Recent advances in the cryopreservation of shoot-derived germplasm of economically important fruit trees of Actinidia, Diospyros, Malus, Olea, Prunus, Pyrus and Vitis. Biotechnol. Adv. 2013, 31, 175–185. [Google Scholar] [CrossRef]
- DE Carlo, A.; Benelli, C.; Lambardi, M. Development of a shoot-tip vitrification protocol and comparison with encapsulation-based procedures for plum (Prunus domestica L.) cryopreservation. CryoLetters 2002, 21, 215–222. [Google Scholar]
- Engelmann, F. In vitro conservation methods. Biotechnology and Plant Genetic Resources. In Biotechnology and Plant Genetic Resources; Callow, J.A., Ford‒Loyd, B.V., Newbury, H.J., Eds.; CAB International: Oxford, UK, 1997; pp. 119–161. [Google Scholar]
- Engelmann, F. Plant cryopreservation: Progress and prospects. Vitr. Cell. Dev. Biol. Anim. 2004, 40, 427–433. [Google Scholar] [CrossRef]
- Sakai, A.; Engelmann, F. Vitrification, encapsulation‒vitrification and droplet‒vitrification. CryoLetters 2007, 28, 151–172. [Google Scholar]
- Wang, M.-R.; Lambardi, M.; Engelmann, F.; Pathirana, R.; Panis, B.; Volk, G.M.; Wang, Q.-C. Advances in cryopreservation of in vitro-derived propagules: Technologies and explant sources. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 144, 7–20. [Google Scholar] [CrossRef]
- Mathew, L.; McLachlan, A.; Jibran, R.; Burritt, D.J.; Pathirana, R. Cold, antioxidant and osmotic pre-treatments maintain the structural integrity of meristematic cells and improve plant regeneration in cryopreserved kiwifruit shoot tips. Protoplasma 2018, 255, 1065–1077. [Google Scholar] [CrossRef]
- Nishizawa, S.; Sakai, A.; Amano, Y.; Matsuzawa, T. Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci. 1993, 91, 67–73. [Google Scholar] [CrossRef]
- Reed, B.M.; Engelmann, F.; Dulloo, M.E.; Engels, J.M.M. Technical Guidelines for the Management of Field and In Vitro Germplasm Collections; Handbook for Genebanks No. 7; IPGRI/SGRP Bioversity International: Rome, Italy, 2004. [Google Scholar]
- Pennycooke, J.C.; Towill, L.E. Cryopreservation of shoot tips from in vitro plants of sweet potato [Ipomoea batatas (L.) Lam.] by vitrification. Plant Cell Rep. 2000, 19, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Panis, B.; Piette, B.; Swennen, R. Droplet vitrification of apical meristems: A cryopreservation protocol applicable to all Mu-saceae. Plant Sci. 2005, 168, 45–55. [Google Scholar] [CrossRef]
- Panis, B.; Piette, B.; Andre, E.; Houwe, I.V.D.; Swennen, R. Droplet vitrification: The first generic cryopreservation protocol for organized plant tissues? Acta Hortic. 2011, 908, 157–162. [Google Scholar] [CrossRef]
- Condello, E.; Caboni, E.; Andre, E.; Piette, B.; Druart, R.; Swennen, R.; Panis, B. Cryopreservation of apple in vitro axillary buds using droplet-vitrification. CryoLetters 2011, 32, 175–185. [Google Scholar] [PubMed]
- Li, B.-Q.; Feng, C.-H.; Wang, M.-R.; Hu, L.-Y.; Volk, G.; Wang, Q.-C. Recovery patterns, histological observations and genetic integrity in Malus shoot tips cryopreserved using droplet-vitrification and encapsulation-dehydration procedures. J. Biotechnol. 2015, 214, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Ružić, D.; Vujović, T.; Cerović, R. Cryopreservation of cherry rootstock Gisela 5 (Prunus cerasus × Prunus canescens) shoot tips by droplet-vitrification technique. J. Hortic. Res. 2013, 21, 79–85. [Google Scholar] [CrossRef][Green Version]
- Vujović, T.; Sylvestre, I.; Ružić, D.; Engelmann, F. Droplet-vitrification of apical shoot tips of Rubus fruticosus L. and Prunus cerasifera Ehrh. Sci. Hortic. 2011, 130, 222–228. [Google Scholar] [CrossRef]
- Vujović, T.I.; Ružić, Đ.V.; Cerović, R.M. Cryopreservation of Serbian autochthonous Prunus spp. by droplet-vitrification. Biologia 2015, 70, 1359–1365. [Google Scholar] [CrossRef]
- Volk, G.M.; Bonnart, R.; Shepherd, A.; Yin, Z.; Lee, R.; Polek, M.; Krueger, R. Citrus cryopreservation: Viability of diverse taxa and histological observations. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 128, 327–334. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Shepherd, A.N.; Kretzschmar, A.A.; Volk, G.M. Cryopreservation of grapevine (Vitis spp.) shoot tips from growth chamber‒sourced plants and histological observations. Vitis 2019, 58, 71–78. [Google Scholar]
- Bi, W.-L.; Hao, X.-Y.; Cui, Z.-H.; Volk, G.M.; Wang, Q.-C. Droplet-vitrification cryopreservation of in vitro-grown shoot tips of grapevine (Vitis spp.). Vitr. Cell. Dev. Biol. Anim. 2018, 54, 590–599. [Google Scholar] [CrossRef]
- Pathirana, R.; McLachlan, A.; Hedderley, D.; Panis, B.; Carimi, F. Pre-treatment with salicylic acid improves plant regeneration after cryopreservation of grapevine (Vitis spp.) by droplet vitrification. Acta Physiol. Plant. 2016, 38, 12. [Google Scholar] [CrossRef]
- Silvestri, C.; Bacchetta, L.; Bellincontro, A.; Cristofori, V. Advances in cultivar choice, hazelnut orchard management, and nut storage to enhance product quality and safety: An overview. J. Sci. Food Agric. 2021, 101, 27–43. [Google Scholar] [CrossRef]
- FAO. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 17 September 2021).
- Bacchetta, L.; Rovira, M.; Tronci, C.; Aramini, M.; Drogoudi, P.; Silva, A.P.; Solar, A.; Avanzato, D.; Botta, R.; Valentini, N.; et al. A multidisciplinary approach to enhance the conservation and use of hazelnut Corylus avellana L. genetic resources. Genet. Resour. Crop. Evol. 2015, 62, 649–663. [Google Scholar] [CrossRef]
- Monastra, F.; Raparelli, E.; Fanigliulo, R. Clonal selection of “Tonda Gentile Romana”. Acta Hortic. 1997, 445, 39–43. [Google Scholar] [CrossRef]
- Bassil, N.; Mok, D.; Mok, M.; Rebhuhn, B. Micropropagation of the hazelnut, corylus avellana. Acta Hortic. 1992, 300, 137–140. [Google Scholar] [CrossRef]
- Yu, X.; Reed, B. A Micropropagation System for Hazelnuts (Corylus Species). HortScience 1995, 30, 120–123. [Google Scholar] [CrossRef]
- Nas, M.N.; Read, P.E. Micropropagation of hybrid hazelnut: Medium composition, physical state and iron source affect shoot morphogenesis, multiplication and explant vitality. Acta Hortic. 2001, 556, 251–258. [Google Scholar] [CrossRef]
- Nas, M.N.; Read, P.E. Improved Rooting and Acclimatization of Micropropagated Hazelnut Shoots. HortScience 2004, 39, 1688–1690. [Google Scholar] [CrossRef]
- Damiano, C.; Catenaro, E.; Giovinazzi, J.; Frattarelli, A.; Caboni, E. Micropropagation of hazelnut (corylus avellana L.). Acta Hortic. 2005, 686, 221–226. [Google Scholar] [CrossRef]
- Latawa, J.; Shukla, M.R.; Saxena, P.K. An efficient temporary immersion system for micropropagation of hybrid hazelnut. Botany 2016, 94, 1–8. [Google Scholar] [CrossRef]
- Sgueglia, A.; Gentile, A.; Frattarelli, A.; Urbinati, G.; Germanà, M.A.; Caboni, E. Micropropagation of Sicilian cultivars with an aim to preserve genetic diversity in hazelnut (Corylus avellana L.). Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2018, 153, 720–724. [Google Scholar] [CrossRef]
- Gonzalez‒Benito, M.E.; Perez, C. Cryopreservation of embryonic axes of two cultivars of hazelnut (Corylus avellana L). CryoLetters 1994, 15, 41–46. [Google Scholar]
- Reed, B.; Hummer, K. Long-term storage of hazelnut embryonic axes in liquid nitrogen. Acta Hortic. 2001, 556, 177–180. [Google Scholar] [CrossRef]
- Reed, B.M.; Normah, M.N.; Yu, X. Stratification is necessary for successful cryopreservation of axes of stored hazelnut seed. CryoLetters 1994, 15, 377–384. [Google Scholar]
- Agrawal, A.; Singh, S.; Malhotra, E.V.; Meena, D.P.S.; Tyagi, R.K. In vitro conservation and cryopreservation of clonally propagated horticultural species. In Conservation and Utilization of Horticultural Genetic Resources; Rajasekharan, P., Rao, V., Eds.; Springer: New York, NY, USA, 2019; pp. 529–578. [Google Scholar]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Höfer, M.; Hanke, M.-V. Cryopreservation of fruit germplasm. Vitr. Cell. Dev. Biol. Anim. 2017, 53, 372–381. [Google Scholar] [CrossRef]
- Lambardi, M.; Shaarawi, S. Importance of in vitro culture for developing cryopreservation strategies of woody plants. Acta Hortic. 2017, 1187, 177–188. [Google Scholar] [CrossRef]
- Reed, B.M. Implementing cryopreservation for long‒term germplasm preservation in vegetatively propagated species. In Bio-technology in Agriculture and Forestry 50: Cryopreservation of Plant Germplasm II, Towill, L.E., Bajaj, Y.P.S., Eds.; Springer: New York, NY, USA, 2002; pp. 22–33. [Google Scholar]
- Zhao, Y.; Wu, Y.; Chang, Y.; Reed, B.M. Cryopreservation of Fruit and Ornamental Trees. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Sgueglia, A.; Gentile, A.; Frattarelli, A.; Germanà, M.; Caboni, E. Cryopreservation of Italian cultivars of hazelnut. Acta Hortic. 2021, 1307, 159–162. [Google Scholar] [CrossRef]
- Gentile, A.; Frattarelli, A.; Nota, P.; Condello, E.; Caboni, E. The aromatic cytokinin meta-topolin promotes in vitro propagation, shoot quality and micrografting in Corylus colurna L. Plant Cell Tissue Organ Cult. 2017, 128, 693–703. [Google Scholar] [CrossRef]
- Driver, J.A.; Kuniyuki, A.H. In vitro propagation of Paradox walnut rootstock. HortScience 1984, 19, 507–509. [Google Scholar]
- McCown, B.H.; Lloyd, G. Woody plant medium (WPM)—A mineral nutrient formulation for microculture of woody plant species. HortScience 1981, 16, 453. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Chang, T.; Zhao, G. Ice Inhibition for Cryopreservation: Materials, Strategies, and Challenges. Adv. Sci. 2021, 8, 2002425. [Google Scholar] [CrossRef]
- Roque-Borda, C.; Kulus, D.; de Souza, A.V.; Kaviani, B.; Vicente, E. Cryopreservation of Agronomic Plant Germplasm Using Vitrification-Based Methods: An Overview of Selected Case Studies. Int. J. Mol. Sci. 2021, 22, 6157. [Google Scholar] [CrossRef]
- Halmagyi, A.; Deliu, C.; Isac, V. Cryopreservation of Malus cultivars: Comparison of two droplet protocols. Sci. Hortic. 2010, 124, 387–392. [Google Scholar] [CrossRef]
- Kim, H.-H.; Lee, Y.-G.; Shin, D.-J.; Ko, H.-C.; Gwag, J.-G.; Cho, E.-G.; Engelmann, F. Development of alternative plant vitrification solutions in droplet-vitrification procedures. CryoLetters 2009, 30, 320–334. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.; Hiti-Bandaralage, J.; Folgado, R.; Hayward, A.; Lahmeyer, S.; Folsom, J.; Mitter, N. Cryopreservation of Woody Crops: The Avocado Case. Plants 2021, 10, 934. [Google Scholar] [CrossRef] [PubMed]
- Kushnarenko, S.V.; Romadanova, N.V.; Reed, B.M. Cold acclimation improves regrowth of cryopreserved apple shoot tips. CryoLetters 2009, 30, 47–54. [Google Scholar] [PubMed]
- Niino, T.; Sakai, A. Cryopreservation of alginate-coated in vitro-grown shoot tips of apple, pear and mulberry. Plant Sci. 1992, 87, 199–206. [Google Scholar] [CrossRef]
- Wu, Y.; Engelmann, F.; Zhao, Y.; Zhou, M.; Chen, S. Cryopreservation of apple shoot tips: Importance of cryopreservation technique and of conditioning of donor plants. CryoLetters 1999, 20, 121–130. [Google Scholar]
- Paul, H.; Daigny, G.; Sangwan‒Norreel, B.S. Cryopreservation of apple (Malus × domestica Borkh.) shoot tips following encapuation‒dehydration or encapsulation‒vitrification. Plant Cell Rep. 2000, 19, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Pokorná, E.; Faltus, M.; Máchová, P.; Zámečník, J.; Fulín, M. Grey poplar explant acclimation to improve the dehydration tolerance and cryopreservation. Biol. Plant. 2020, 64, 119–128. [Google Scholar]
- Ai, P.-F.; Lu, L.-P.; Song, J.-J. Cryopreservation of in vitro-grown shoot-tips of Rabdosia rubescens by encapsulation-dehydration and evaluation of their genetic stability. Plant Cell, Tissue Organ Cult. (PCTOC) 2012, 108, 381–387. [Google Scholar] [CrossRef]
- Kulus, D.; Zalewska, M. In vitro plant recovery from alginate encapsulated Chrysanthemum × grandiflorum/Ramat./Kitam. shoot tips. Prop. Ornam. Plants 2014, 14, 3–12. [Google Scholar]
- Kulus, D.; Abratowska, A.; Mikuła, A. Morphogenetic response of shoot tips to cryopreservation by encapsulation‒dehydration in a solid mutant and periclinal chimeras of Chrysanthemum × grandiflorum /Ramat./Kitam. Acta Physiol. Plant. 2018, 40, 18. [Google Scholar] [CrossRef]
- Hao, Y.-J.; You, C.-X.; Deng, X.-X. Effects of cryopreservation on developmental competency, cytological and molecular stability of citrus callus. CryoLetters 2002, 23, 27–35. [Google Scholar]

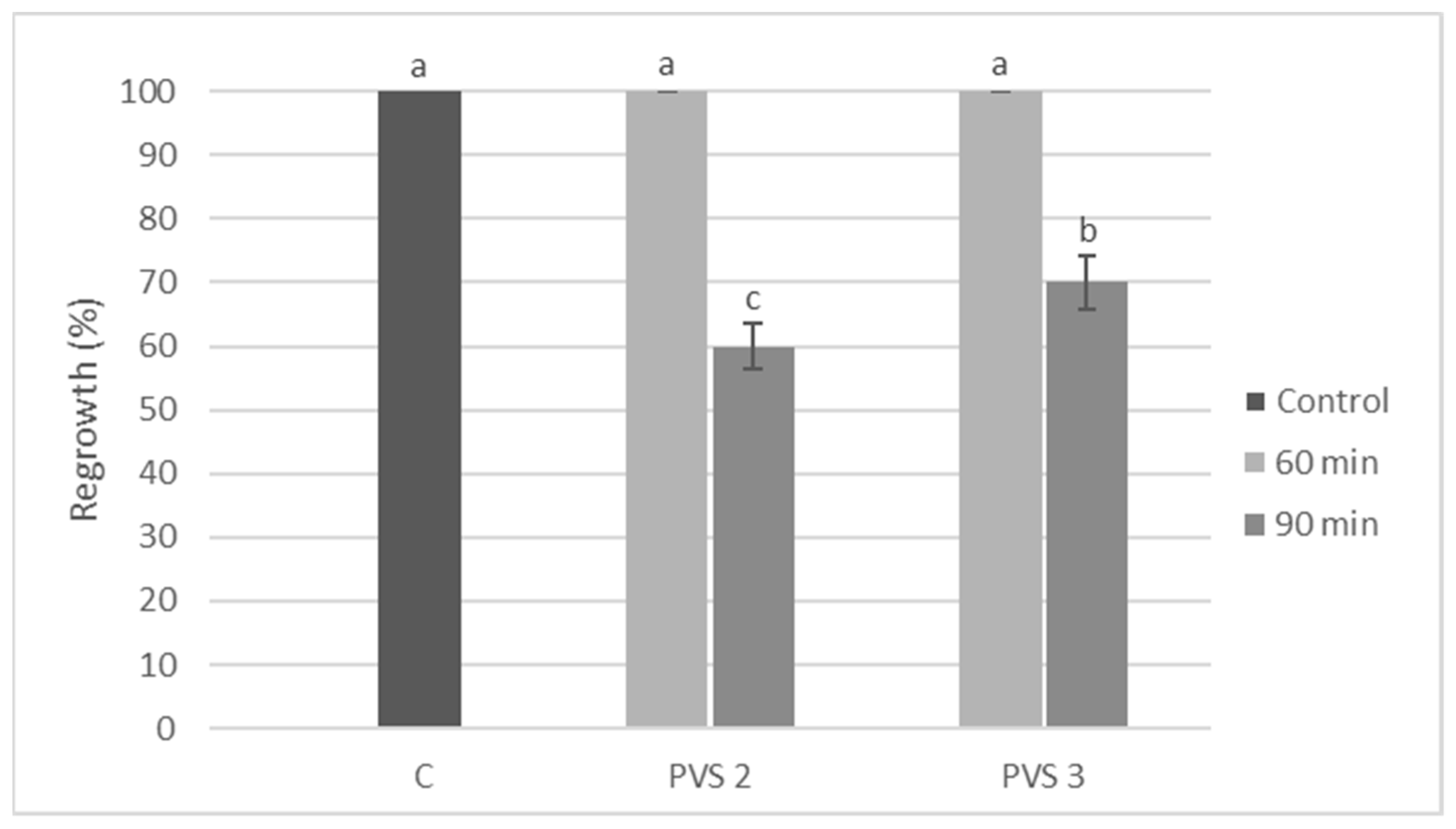


| Type of Vitrification Solution | Timing of Application (min) | Regrowth (%) |
|---|---|---|
| PVS2 | 60 | 41.5 ± 1.5 b |
| PVS2 | 90 | 35.6 ± 2.2 c |
| PVS3 | 60 | 56.7 ± 3.3 a |
| PVS3 | 90 | 43.3 ± 3.3 b |
| Cryopreservation | Rooting (%) | N. of Roots/Shoot |
|---|---|---|
| +LN | 78 a | 5.9 ± 0.5 a |
| SSC | 80 a | 6.1 ± 0.4 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgueglia, A.; Frattarelli, A.; Gentile, A.; Urbinati, G.; Lucioli, S.; Germanà, M.A.; Caboni, E. Cryopreservation of Hazelnut (Corylus avellana L.) Axillary Buds from In Vitro Shoots Using the Droplet Vitrification Method. Horticulturae 2021, 7, 494. https://doi.org/10.3390/horticulturae7110494
Sgueglia A, Frattarelli A, Gentile A, Urbinati G, Lucioli S, Germanà MA, Caboni E. Cryopreservation of Hazelnut (Corylus avellana L.) Axillary Buds from In Vitro Shoots Using the Droplet Vitrification Method. Horticulturae. 2021; 7(11):494. https://doi.org/10.3390/horticulturae7110494
Chicago/Turabian StyleSgueglia, Alessandra, Andrea Frattarelli, Adele Gentile, Gaia Urbinati, Simona Lucioli, Maria Antonietta Germanà, and Emilia Caboni. 2021. "Cryopreservation of Hazelnut (Corylus avellana L.) Axillary Buds from In Vitro Shoots Using the Droplet Vitrification Method" Horticulturae 7, no. 11: 494. https://doi.org/10.3390/horticulturae7110494
APA StyleSgueglia, A., Frattarelli, A., Gentile, A., Urbinati, G., Lucioli, S., Germanà, M. A., & Caboni, E. (2021). Cryopreservation of Hazelnut (Corylus avellana L.) Axillary Buds from In Vitro Shoots Using the Droplet Vitrification Method. Horticulturae, 7(11), 494. https://doi.org/10.3390/horticulturae7110494





