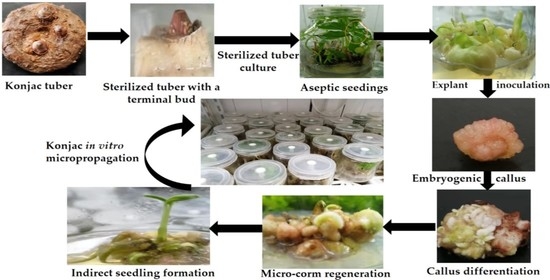
Somatic Embryogenesis and Indirect In Vitro Plant Regeneration in Amorphophallus konjac K. Koch by One-Step Seedling Formation
Abstract
:1. Introduction
2. Materials and Methods
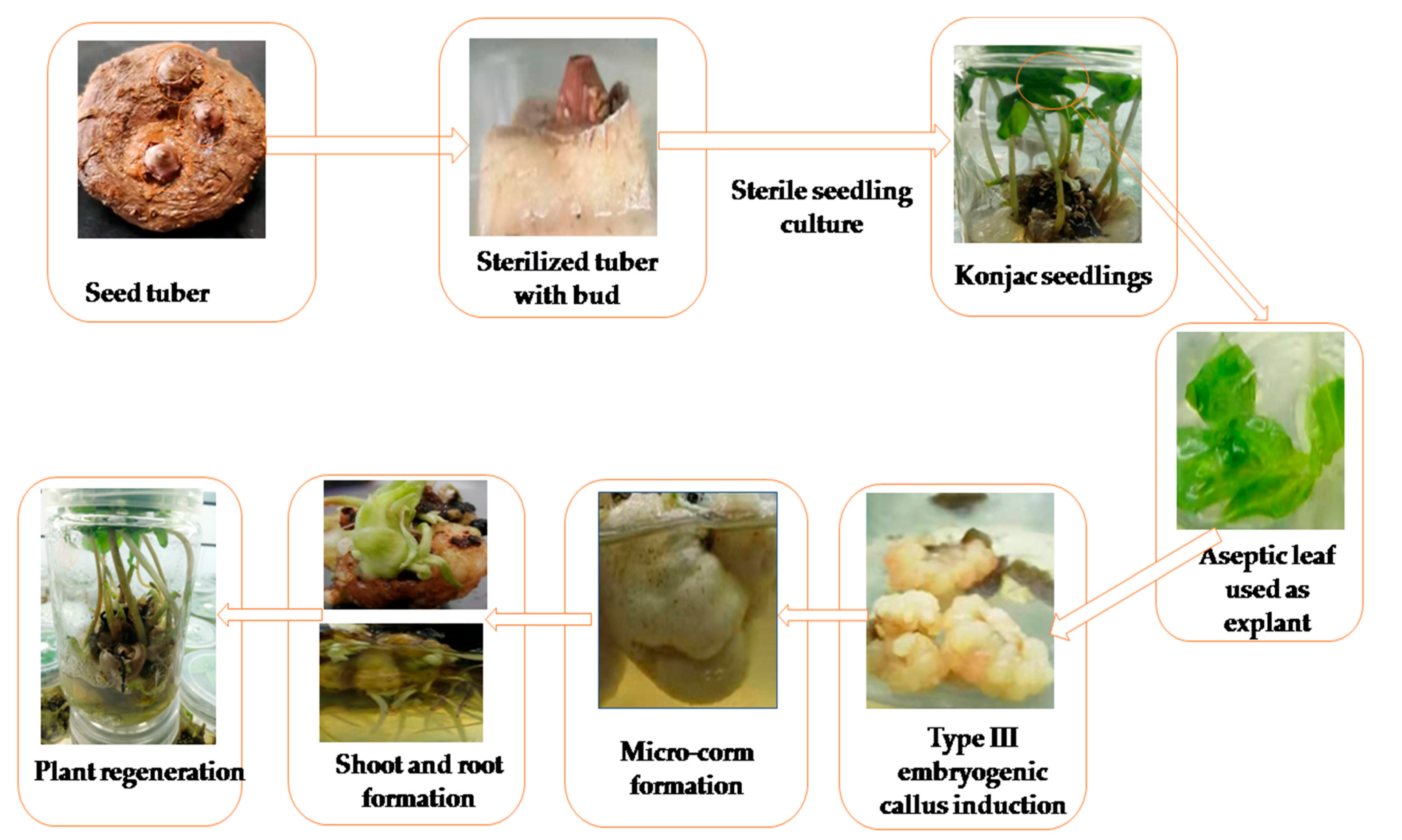
2.1. Plant Material and Sterile Seedlings Preparation
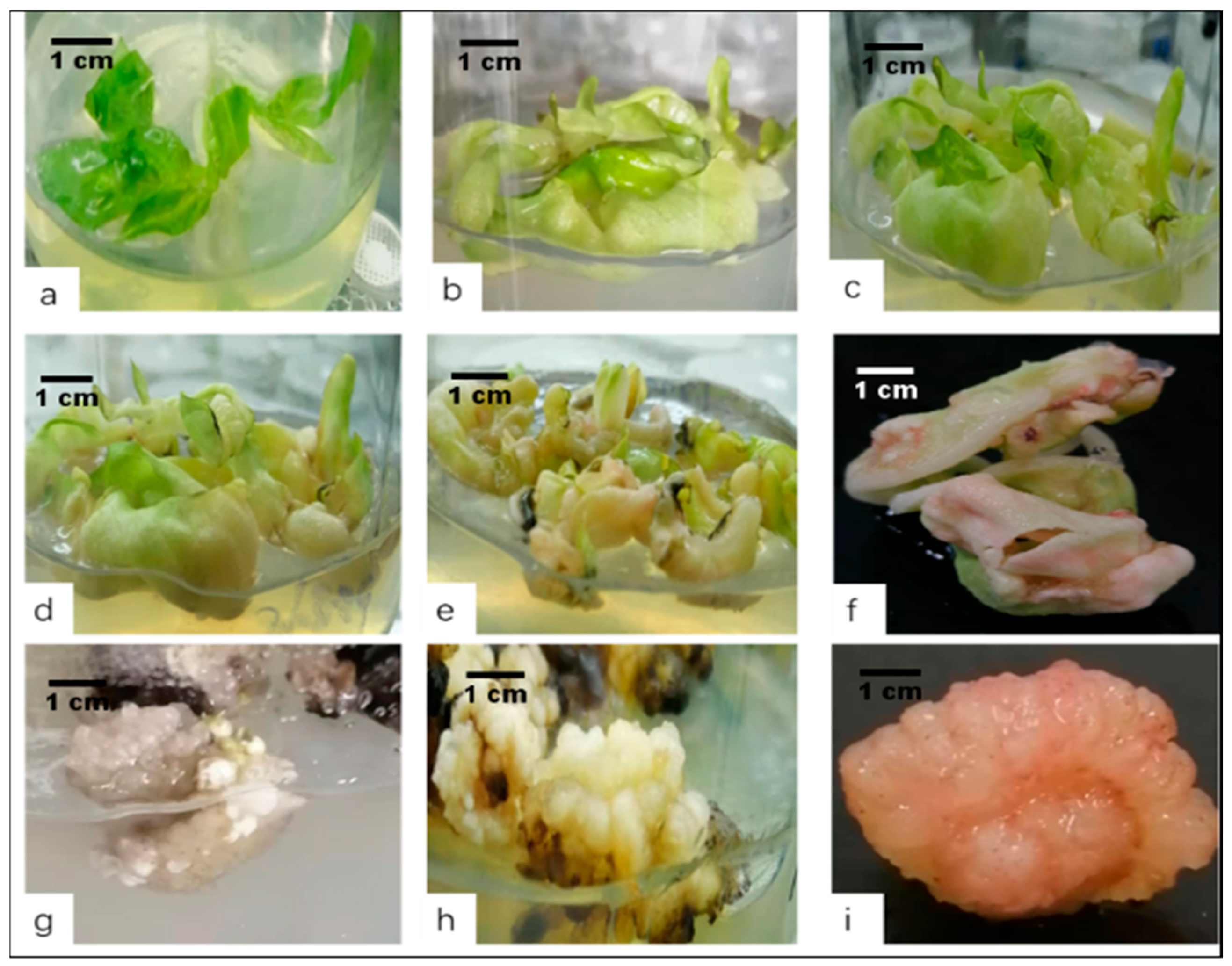
2.2. Explant Inoculation and Embryogenic Callus Induction
2.3. Adventitious Shoot Induction from the Callus
2.4. One-Step Seedling Formation System
2.5. Statistical Analysis
3. Results
3.1. Optimization of Surface Sterilization Treatments on Percentage of Aseptic Culture
3.2. Effect of PGRs on Embryogenic Callus Induction
3.3. Shoot Induction
3.4. One-Step Seedling Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| 6-BA | 6-benzyladenine |
| GA3 | gibberellic acid |
| IAA | indole-3-acetic acid |
| IBA | indole-3-butyric acid |
| Kn | kinetin |
| MS | Murashige and Skoog (1962) medium |
| NAA | α-naphthaleneacetic acid |
| PGRs | plant growth regulators |
References
- Hetterscheid, W.; Ittenbach, S. Everything you always wanted to know about Amorphophallus, but were afraid to stick your nose into. Aroideana 1996, 19, 7–131. [Google Scholar]
- Cescutti, P.; Campa, C.; Delben, F.; Rizzo, R. Structure of the oligomers obtained by enzymatic hydrolysis of the glucomannan produced by the plant Amorphophallus konjac. Carbohydr. Res. 2002, 337, 2505–2511. [Google Scholar] [CrossRef]
- Fang, W.; Wu, P. Variations of Konjac glucomannan (KGM) from Amorphophallus konjac and its refined powder in China. Food Hydrocoll. 2004, 18, 167–170. [Google Scholar] [CrossRef]
- Yuan, Z.; Cheng, J.; Lan, G.; Lu, F. A cellulose/Konjac glucomannan–based macroporous antibacterial wound dressing with synergistic and complementary effects for accelerated wound healing. Cellulose 2021, 28, 1–19. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Yan, D. The effects of konjac oligosaccharides on decreasing blood lipid. Chin. J. Bio-Chem. Pharm. 2002, 23, 181–182. [Google Scholar]
- Chen, X.; Liu, H.; Xia, N.; Shang, J.; Tran, V.; Guo, K. Preparation and Properties of Oriented Cotton Stalk Board with Konjac Glucomannan-Chitosan-Polyvinyl Alcohol Blend Adhesive. Bioresources 2015, 10, 3736–3748. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.-T.; Chen, H.-L. Effects of Konjac Glucomannan on Putative Risk Factors for Colon Carcinogenesis in Rats Fed a High-Fat Diet. J. Agric. Food Chem. 2011, 59, 989–994. [Google Scholar] [CrossRef]
- Gang, X.; Cailian, W.; Mei, S.; Qiufang, C. Shoot tips culture and plant regeneration in Amorphophallus konjac in vitro. Shengwu Jishu Biotechnol. 1994, 4, 19–21. [Google Scholar]
- Liu, R.; Long, Y.; Liu, Y.; Wen, Z. A tissue culture and plant regeneration system of Amorphophallus konjac in Guizhou. Guizhou Agric. Sci. 2012, 7, 42–47. [Google Scholar]
- Thach, B.D.; Linh, N.K.; Duy, T.T.B.; Giang, T.T.L.; Uyen, N.P.A.; Suong, N.K.; Van Du, N. Preliminary selection and in vitro propagation of amorphophallus species with high content of gluco-mannan distributed in vietnam. Eur. J. Adv. Res. Biol. Life Sci. 2016, 4, 7. [Google Scholar]
- Hu, J.B.; Liu, J.; Yan, H.B.; Xie, C.H. Histological observations of morphogenesis in petiole derived callus of Amorphophallus rivieri Durieu in vitro. Plant Cell Rep. 2005, 24, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Vibha, J.B.; Choudhary, K.; Singh, M.; Rathore, M.S.; Shekhawat, N.S. An efficient somatic embryogenesis system for velvet bean [Mucuna pruriens (L.) DC.]: A source of anti Parkinson’s drug. Plant Cell Tissue Organ Cult. 2009, 99, 319–325. [Google Scholar] [CrossRef]
- Von Arnold, S.; Sabala, I.; Bozhkov, P.; Dyachok, J.; Filonova, L. Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult. 2002, 69, 233–249. [Google Scholar] [CrossRef]
- Mikuła, A.; Rybczyński, J.J. Somatic embryogenesis of Gentiana genus I. The effect of the preculture treatment and primary explant origin on somatic embryogenesis of Gentiana cruciata (L.), G. pannonica (Scop.), and G. tibetica (King). Acta Physiol. Plant. 2001, 23, 15–25. [Google Scholar] [CrossRef]
- Gourguillon, L.; Gondet, L.; Lobstein, A. Effects of explant type, culture media and growth regulators for callus induction of a potential bioactive halophyte: Armeria maritima (Plumbaginaceae). Planta Med. 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Yan, M.-M.; Xu, C.; Kim, C.-H.; Um, Y.-C.; Bah, A.A.; Guo, D.-P. Effects of explant type, culture media and growth regulators on callus induction and plant regeneration of Chinese jiaotou (Allium chinense). Sci. Hortic. 2009, 123, 124–128. [Google Scholar] [CrossRef]
- Horstman, A.; Bemer, M.; Boutilier, K. A transcriptional view on somatic embryogenesis. Regeneration 2017, 4, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Guo, X.; Hu, X. Multi-factor orthogonal test for callus induction of konjac. Hubei Agric. Sci. 2016, 55, 3479–3481. [Google Scholar]
- Qin, T.; Li, X.; Zhang, J.; Huang, Y. Establishment of in vitro regeneration system of konjac flower. Hubei Agric. Sci. 2015, 54, 6054–6057. (In Chinese) [Google Scholar]
- Hu, X. Research on Callus Induction from Coleoptile Explants of Amorphophallus. Agric. Sci. Technol. 2014, 15. (In Chinese) [Google Scholar]
- Márquez-López, R.E.; Pérez-Hernández, C.; Ku-González, Á.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M. Localization and transport of indole-3-acetic acid during somatic embryogenesis in Coffea canephora. Protoplasma 2018, 255, 695–708. [Google Scholar] [CrossRef]
- Ahmad, F.I.; Johan, N.S.; Wagiran, A. Effect of 2,4-D on Embryogenic Callus Induction of Malaysian indica Rice (Oryza sativa L.) Cultivars MR123 and MR127. J. Teknol. 2013, 64, 64. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Liu, J.; Yan, H.; Xie, C. Different types of calluses and differentiation ability of Konjac. J. Huazhong Agric. Univ. 2004, 23, 654–658. (In Chinese) [Google Scholar]
- Kumar, K.; Singh, D.; Saroj, P. Callus induction, somatic embryogenesis, in vitro plantlet development and ex vitro transplantation of two date palm (Phoenix dactylifera L.) cultivars. Int. J. Chem. Stud. 2020, 8, 758–763. [Google Scholar] [CrossRef]
- Botini, N.; Almeida, F.A.; Cruz, K.Z.C.M.; Reis, R.S.; Vale, E.M.; Garcia, A.B.; Santa-Catarina, C.; Silveira, V. Stage-specific protein regulation during somatic embryo development of Carica papaya L. ‘Golden’. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140561. [Google Scholar] [CrossRef] [PubMed]
- Baharan, E.; Pour Mohammadi, P. Induction of direct somatic embryogenesis and callogenesis in date palm (Phoenix dactylifera L.) using leaf explants. Biotechnology 2018, 99, 197–203. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, W.; Sun, M. Characterization and expression pattern analysis of DcNAC gene in somatic embryos of Dendrobium candidum Wall Ex Lindl. Plant Cell Tissue Organ Cult. 2011, 107, 151–159. [Google Scholar] [CrossRef]


| Sterilization Methods | Sterilization Agents and Washing Time |
|---|---|
| A | 75% alcohol for 15 s + 20% NaOCl for 15 min + sterile water 3 times |
| B | 75% alcohol for 15 s + 20% H2O2 for 15 min + sterile water 3 times |
| C | 75% alcohol for 15 s + 0.1% HgCl2 for 15 min + sterile water 3 times |
| Sterilization Methods | Number of Explants Inoculated | Number of Explants Contaminated | Number of Explants Survived | Number of Explant Deaths | Contamination Rate (%) | Survival Rate (%) | Death Rate (%) |
|---|---|---|---|---|---|---|---|
| A | 50 | 19.67 ± 0.47 a | 20.33 ± 0.47 b | 10 ± 0.82 a | 39.33 ± 0.94 a | 40.67 ± 0.94 c | 20 ± 1.63 b |
| B | 50 | 10 ± 0.82 b | 29.67 ± 0.47 c | 10.33 ± 0.47 a | 20 ± 1.63 b | 59.33 ± 0.94 b | 20.67 ± 0.94 b |
| C | 50 | 0 ± 0.00 c | 41 ± 0.82 a | 9 ± 0.82 b | 0 ± 0.00 c | 82 ± 1.63 a | 18 ± 1.63 c |
| Treatments | 6-BA (mg/L) | NAA (mg/L) | 2,4-D (mg/L) | Induction Rate (%) | Type I Induction Rate (%) | Type II Induction Rate (%) | Type III Inductionrate (%) | Induction Browning Rate (%) |
|---|---|---|---|---|---|---|---|---|
| M1-1 | 0.1 | 0.1 | 0.5 | 67.63 ± 0.60 | 20.53 ± 0.21 | 45.50 ± 0.29 | 1.60 ± 0.16 | 32.37 ± 0.60 |
| M1-2 | 0.1 | 0.1 | 1.0 | 73.67 ± 0.94 | 29.00 ± 0.82 | 20.53 ± 0.42 | 24.50 ± 0.71 | 26.33 ± 0.94 |
| M1-3 | 0.1 | 0.1 | 2.0 | 75.20 ± 0.59 | 24.50 ± 0.41 | 35.20 ± 0.28 | 15.50 ± 0.41 | 24.80 ± 0.59 |
| M1-4 | 0.5 | 0.5 | 0.5 | 85.67 ± 0.17 | 20.37 ± 0.33 | 34.97 ± 0.12 | 30.33 ± 0.24 | 14.33 ± 0.17 |
| M1-5 | 0.5 | 0.5 | 1.0 | 92.73 ± 1.52 | 16.00 ± 0.82 | 21.00 ± 0.82 | 55.73 ± 0.19 | 7.27 ± 1.52 |
| M1-6 | 0.5 | 0.5 | 2.0 | 90.27 ± 0.61 | 20.53 ± 0.37 | 45.73 ± 0.38 | 24.00 ± 0.82 | 9.73 ± 0.61 |
| M1-7 | 1.0 | 1.0 | 0.5 | 89.43 ± 1.23 | 15.17 ± 0.62 | 33.27 ± 0.52 | 41.00 ± 0.82 | 10.57 ± 1.23 |
| M1-8 | 1.0 | 1.0 | 1.0 | 86.37 ± 1.65 | 15.5 ± 0.71 | 45.2 ± 0.59 | 25.67 ± 0.47 | 13.63 ± 1.65 |
| M1-9 | 1.0 | 1.0 | 2.0 | 85.67 ± 0.95 | 17.6 ± 0.54 | 42.7 ± 0.57 | 25.37 ± 0.47 | 14.33 ± 0.95 |
| Treatment | 6-BA (mg/L) | IBA (mg/L) | KT (mg/L) | Number of Micro-Corms Induced | Induction Rate (%) | Browning Rate (%) |
|---|---|---|---|---|---|---|
| M2-1 | 0.1 | 0.1 | 0.1 | 7.20 ± 0.75 c | 18 ± 1.87 c | 82 ± 1.87 d |
| M2-2 | 0.1 | 0.5 | 0.2 | 5.2 ± 0.75 e | 13 ± 1.87 e | 87 ± 1.87 b |
| M2-3 | 0.1 | 1.0 | 0.5 | 6 ± 0.63 d | 15 ± 1.58 d | 85 ± 1.58 bc |
| M2-4 | 0.5 | 0.1 | 0.2 | 6.2 ± 0.4 d | 15.5 ± 1 d | 84.5 ± 1 c |
| M2-5 | 0.5 | 0.5 | 0.5 | 7 ± 0.63 c | 17.5 ± 1.58 c | 82.5 ± 1.58 d |
| M2-6 | 0.5 | 1.0 | 0.1 | 9.2 ± 0.75 b | 23 ± 1.87 b | 77 ± 1.87 e |
| M2-7 | 1.0 | 0.1 | 0.5 | 8.6 ± 0.49 b | 21.5 ± 1.22 b | 78.5 ± 1.22 e |
| M2-8 | 1.0 | 0.5 | 0.1 | 3.2 ± 0.74 f | 8 ± 1.87 f | 92 ± 1.87 a |
| M2-9 | 1.0 | 1.0 | 0.2 | 15.2 ± 0.75 a | 38 ± 1.87 a | 62 ± 1.87 f |
| Treatments | 6-BA (mg/L) | NAA (mg/L) | GA3 (mg/L) | Budding Time/Days | Seedling Time/Days | Number of Seedlings/Explants |
|---|---|---|---|---|---|---|
| M3-1 | 1.0 | 0.1 | 0.05 | 25 ± 1.90 e | 44 ± 1.79 d | 3.4 ± 0.49 e |
| M3-2 | 1.0 | 0.2 | 0.1 | 29.6 ± 1.85 b | 55.6 ± 1.74 b | 3.6 ± 0.49 e |
| M3-3 | 1.0 | 0.5 | 0.2 | 27.8 ± 1.33 c | 48 ± 1.67 c | 5.8 ± 0.75 b |
| M3-4 | 1.5 | 0.1 | 0.1 | 26.4 ± 1.62 de | 39.8 ± 1.72 e | 2.4 ± 1.02 f |
| M3-5 | 1.5 | 0.2 | 0.2 | 34.8 ± 1.60 a | 60.2 ± 1.94 a | 3.2 ± 0.75 e |
| M3-6 | 1.5 | 0.5 | 0.05 | 25.8 ± 1.72 e | 49.2 ± 1.47 c | 4.6 ± 1.02 d |
| M3-7 | 2.0 | 0.1 | 0.2 | 26.2 ± 1.94 de | 49.2 ± 1.47 c | 4.4 ± 1.02 d |
| M3-8 | 2.0 | 0.2 | 0.05 | 27.6 ± 1.85 c | 54.8 ± 1.83 bc | 6.2 ± 1.17 a |
| M3-9 | 2.0 | 0.5 | 0.1 | 22.2 ± 1.94 f | 44.4 ± 1.74 d | 5.2 ± 0.75 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Mohammadi, M.A.; Qin, Y.; Zhang, Z. Somatic Embryogenesis and Indirect In Vitro Plant Regeneration in Amorphophallus konjac K. Koch by One-Step Seedling Formation. Horticulturae 2021, 7, 497. https://doi.org/10.3390/horticulturae7110497
Li D, Mohammadi MA, Qin Y, Zhang Z. Somatic Embryogenesis and Indirect In Vitro Plant Regeneration in Amorphophallus konjac K. Koch by One-Step Seedling Formation. Horticulturae. 2021; 7(11):497. https://doi.org/10.3390/horticulturae7110497
Chicago/Turabian StyleLi, Dandan, Mohammad Aqa Mohammadi, Yuan Qin, and Zongshen Zhang. 2021. "Somatic Embryogenesis and Indirect In Vitro Plant Regeneration in Amorphophallus konjac K. Koch by One-Step Seedling Formation" Horticulturae 7, no. 11: 497. https://doi.org/10.3390/horticulturae7110497
APA StyleLi, D., Mohammadi, M. A., Qin, Y., & Zhang, Z. (2021). Somatic Embryogenesis and Indirect In Vitro Plant Regeneration in Amorphophallus konjac K. Koch by One-Step Seedling Formation. Horticulturae, 7(11), 497. https://doi.org/10.3390/horticulturae7110497