On the Biochemical and Physiological Responses of ‘Crimson Seedless’ Grapes Coated with an Edible Composite of Pectin, Polyphenylene Alcohol, and Salicylic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Coating Treatment Formation
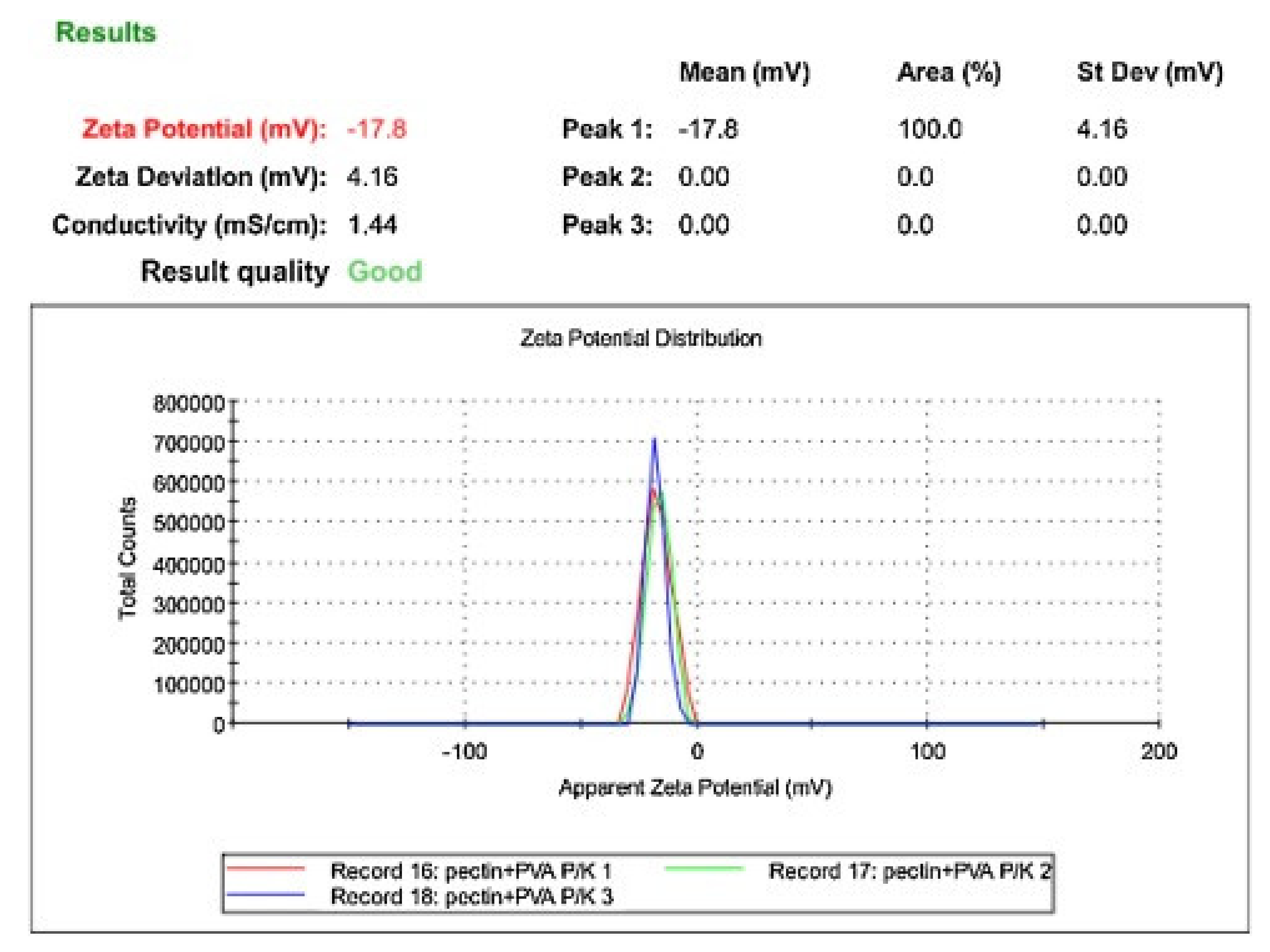
2.3. Scanning Electron Microscopy (SEM) and Zeta Potential Characterization of PE + PVA
2.4. Crimson Seedless Coating Treatment Protocol and Shelf Life
2.5. Rachis Browning (RB-Index), Berry Firmness (BF), and Separation Force (BSF)
2.6. Soluble Solids Content (SSC%), Total Acidity (TA%), SSC/TA-Ratio, and Ascorbic Acid Content (AA)
2.7. Cellular Metabolism Enzyme Activities
2.8. The Amount MDA Amount and EL%
2.9. O2− and H2O2 Production Rates, as Well as DPPH* (%)
2.10. Statistical Analysis
3. Results
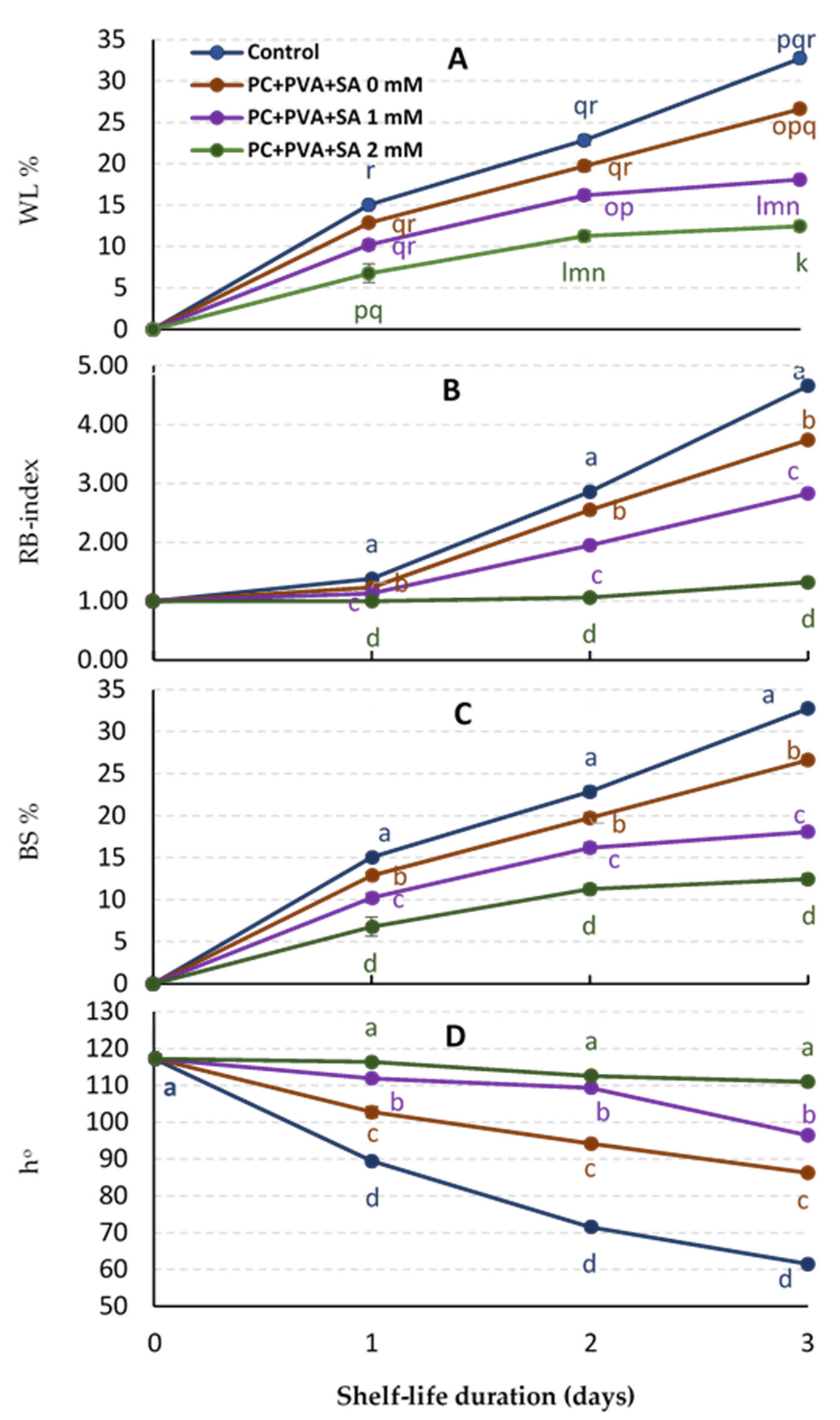
3.1. Weight loss (WL%), RB-Index, Berry Shattering (BS%), and Berry Color (ho)
3.2. BF, BRF, and AA
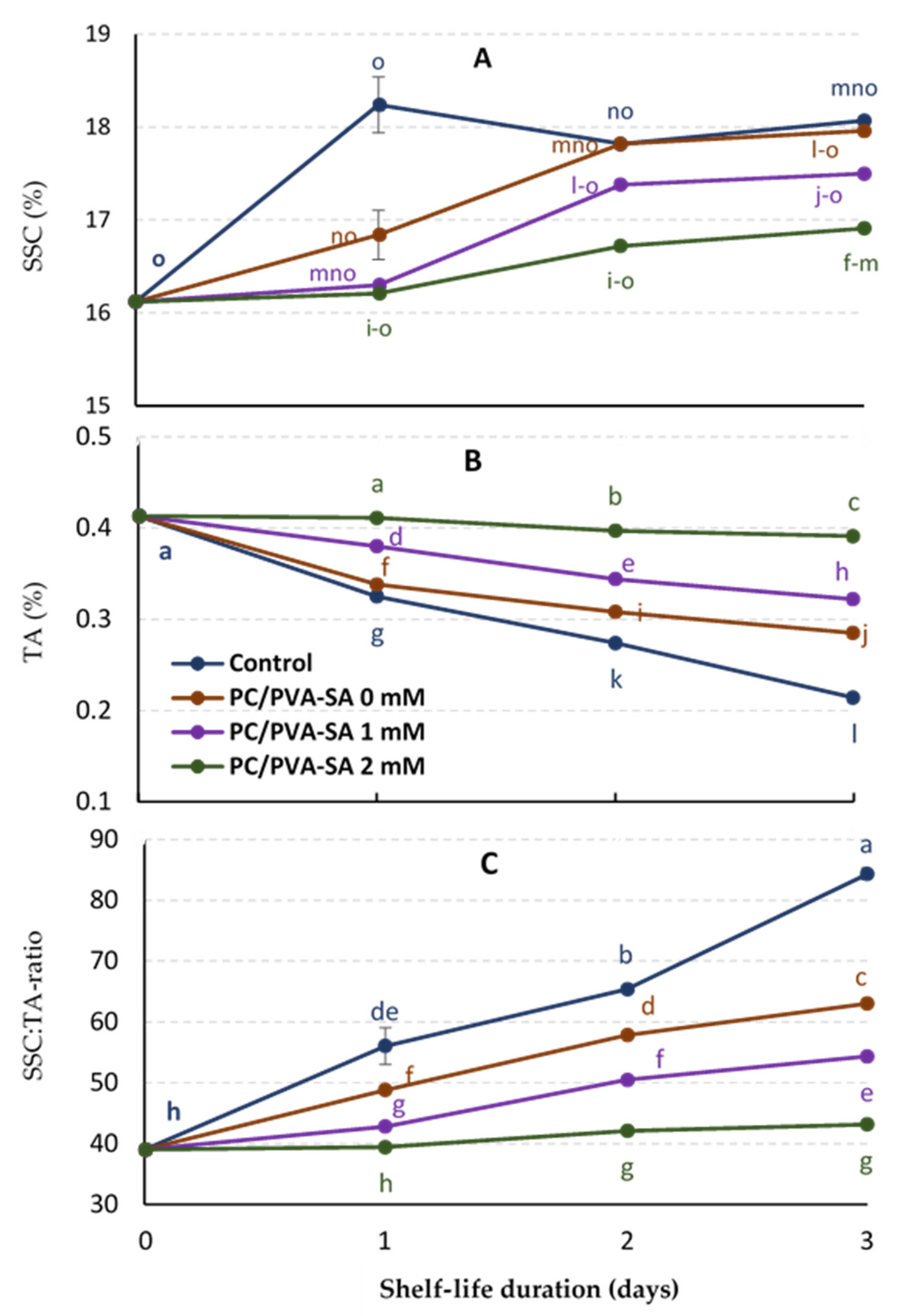
3.3. SSC%, TA%, and SSC: TA-Ratio
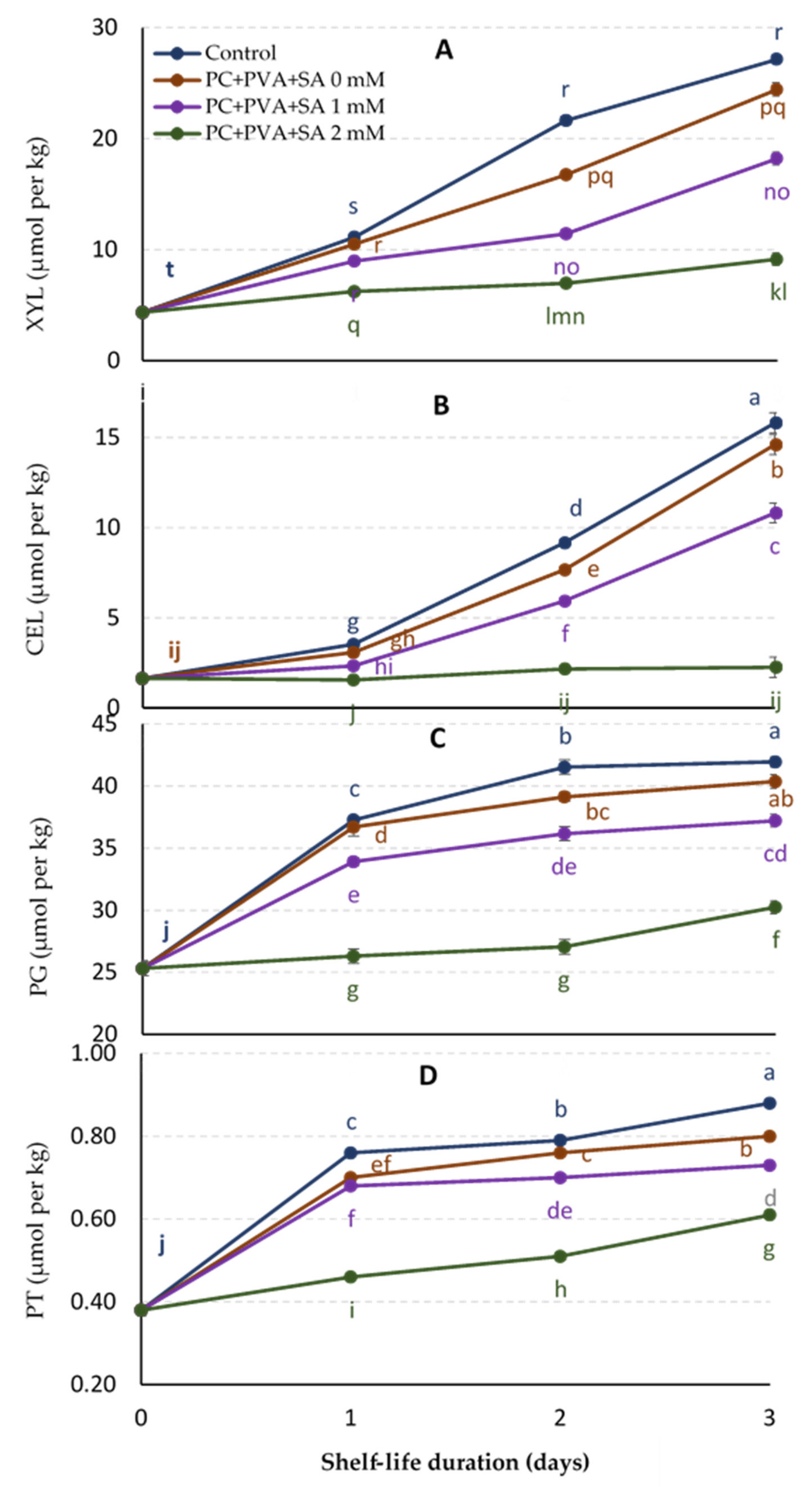
3.4. Cellular Metabolism Enzyme Activities (CMEAs)
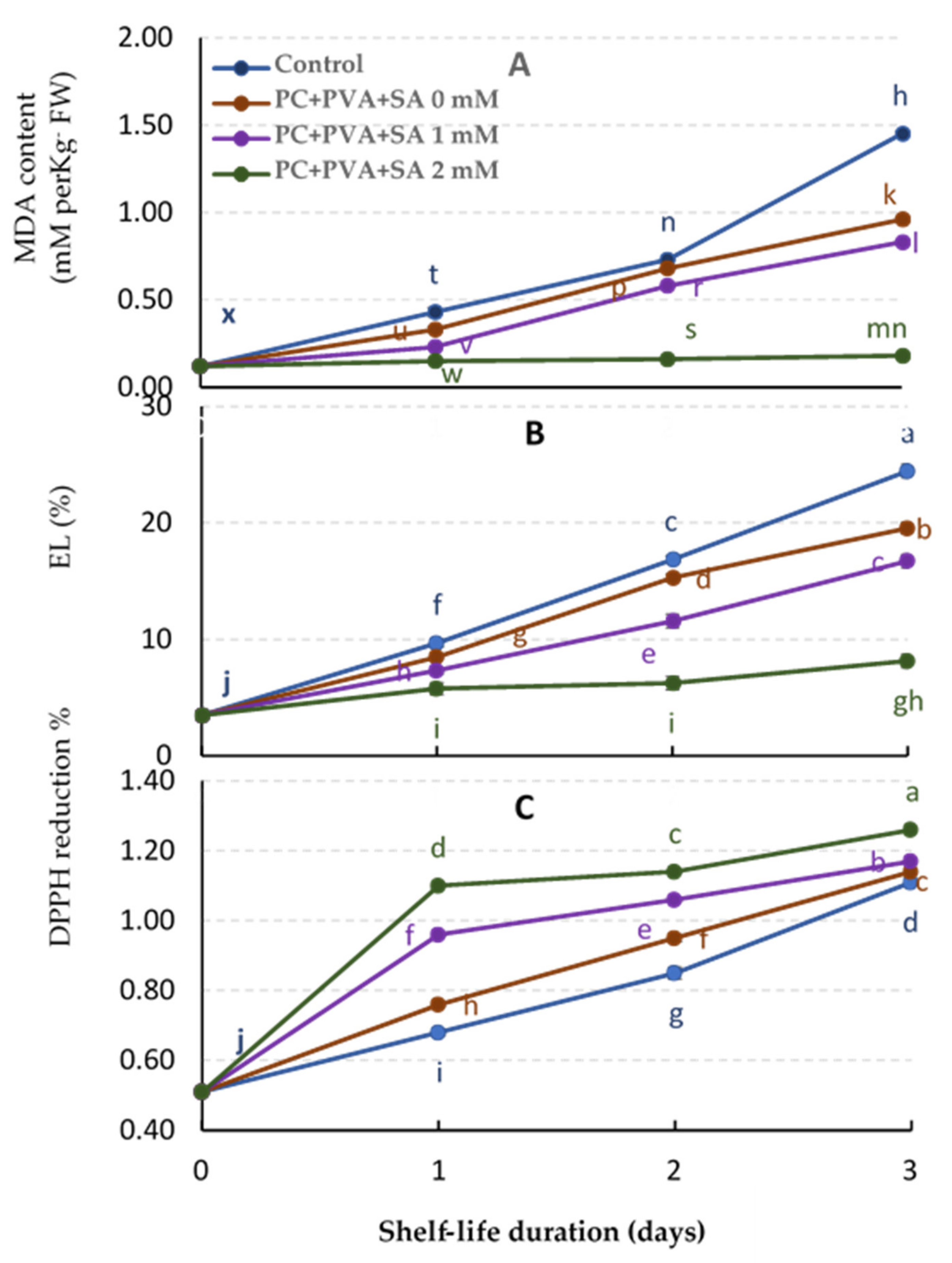
3.5. MDA, EL%, and DPPH% Reduction
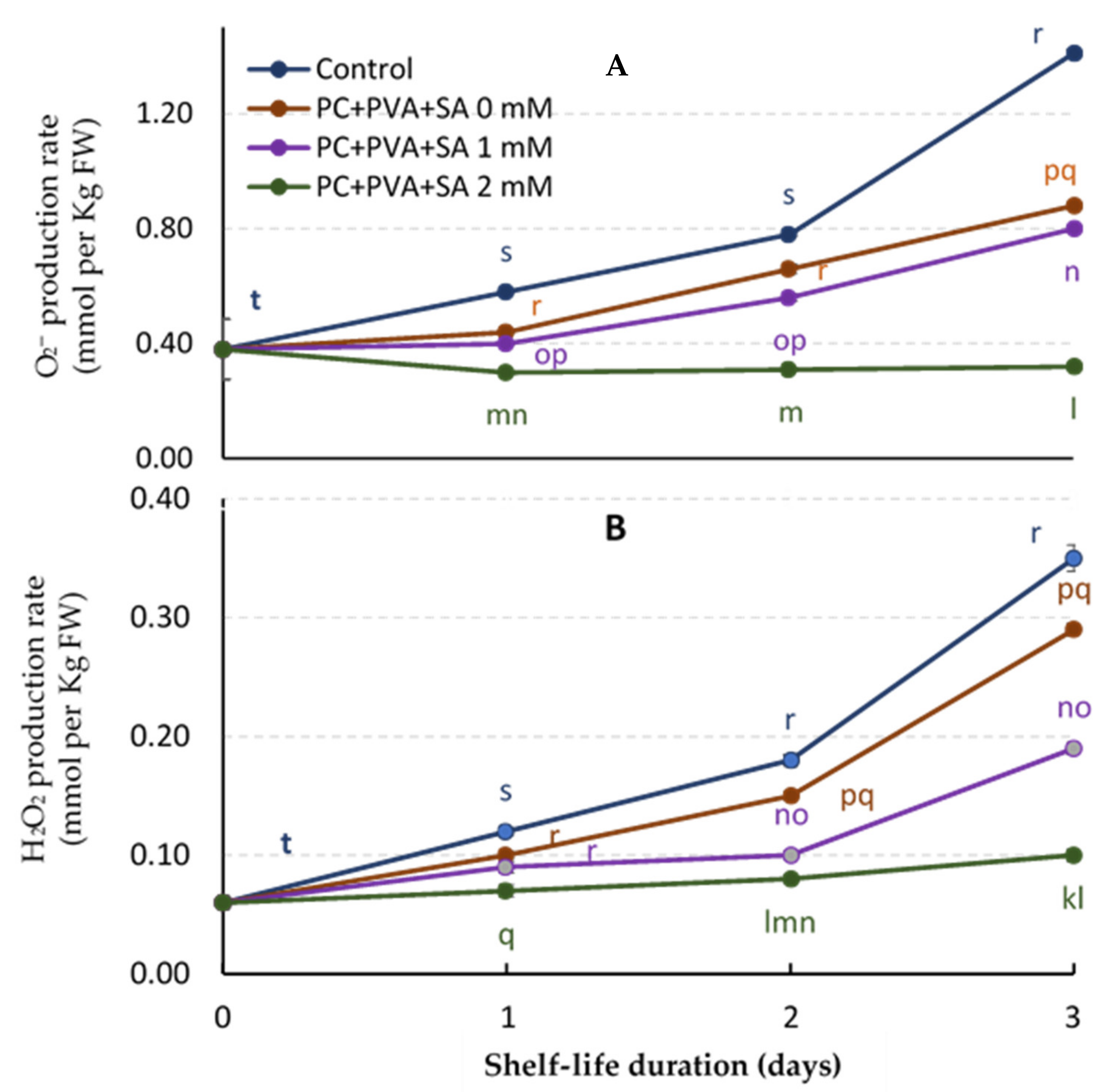
3.6. O2− and H2O2 Production Rate
3.7. Multivariate Analysis of Leaf Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fraige, K.; Pereira-Filho, E.R.; Carrilho, E. Fingerprinting of anthocyanins from grapes produced in Brazil using HPLC-DAD-MS and exploratory analysis by principal component analysis. Food Chem. 2014, 145, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Martinson, T.E.; Liu, R.H. Phytochemical profiles and antioxidant activities of wine grapes. Food Chem. 2009, 116, 332–339. [Google Scholar] [CrossRef]
- El-Gendy, R.S. Evaluation of flame seedless grapevines grafted on some rootstocks. J. Hortic. Sci. Ornam. Plants 2013, 5, 1–10. [Google Scholar]
- El-Hady, E.S.; Shaltout, A.D.; Desouky, I.M.; Haggag, L.F. Characteristics of some grape cultivars as affected by some grape rootstocks. Middle East J. Agric. Res. 2014, 3, 609–617. [Google Scholar]
- FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 31 December 2019).
- Lo’ay, A.A.; El-Boray, M.S. Improving fruit cluster quality attributes of ‘Flame Seedless’ grapes using preharvest application of ascorbic and salicylic acid. Sci. Hortic. 2018, 233, 339–348. [Google Scholar] [CrossRef]
- Atieno, L.; Owino, W.; Ateka, E.M.; Ambuko, J. Influence of Coating Application Methods on the Postharvest Quality of Cassava. Int. J. Food Sci. 2019, 2019, 2148914. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M. An Extensive Review of Natural Polymers Used as Coatings for Postharvest Shelf-Life Extension: Trends and Challenges. Polymers 2021, 13, 3271. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Nisperos-Carriedo, M.O.; Baker, R.A. Use of edible coatings to preserve quality of lightly (and slightly) processed products. Crit. Rev. Food Sci. Nutr. 1995, 35, 509–524. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in edible coatings for fresh fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Singh, C.; Sharma, H.K.; Sarkar, B.C. Influence of process conditions on the mass transfer during osmotic dehydration of coated pineapple samples. J. Food Process. Preserv. 2010, 34, 700–714. [Google Scholar] [CrossRef]
- Saini, C.S.; Sharma, H.K. Effect of pectin coating on colour and quality of dehydrated pineapple during storage. Asian J. Dairy Food Res. 2016, 35, 120–129. [Google Scholar] [CrossRef][Green Version]
- Jiang, Y.; Li, Y.; Jiang, E. Effects of chitosan on shelf life of cold-stored litchi fruit at ambient temperature. LWT—Food Sci. Technol. Int. 2005, 38, 757–761. [Google Scholar] [CrossRef]
- Qi, H.; Hu, W.; Jiang, A.; Tian, M.; Li, Y. Extending shelf-life of fresh-cut “Fuji” apples with chitosan-coatings. Innov. Food Sci. Emerg. Technol. 2011, 121, 62–66. [Google Scholar] [CrossRef]
- Chien, P.; Sep, F.; Yang, F. Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J. Food Eng. 2007, 78, 225–229. [Google Scholar] [CrossRef]
- Bourtoom, T. Edible films and coatings, characteristics and properties. Int. Food Res. J. 2008, 153, 215–218. [Google Scholar]
- Lo’ay, A.A.; Dawood, H.D. Active chitosan/PVA with ascorbic acid and berry quality of ‘Superior seedless’ grapes. Sci. Hortic. 2017, 224, 286–292. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; El-Khateeb, A.Y. Impact of chitosan/PVA with salicylic acid, cell wall degrading enzyme activities and berries shattering of ‘Thompson seedless’ grape vines during shelf life. Sci. Hortic. 2018, 238, 281–287. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Dawood, H.D. Minimize browning incidence of banana by postharvest active chitosan/PVA Combines with oxalic acid treatment to during shelf-life. Sci. Hortic. 2017, 226, 208–215. [Google Scholar] [CrossRef]
- Ciolacu, L.; Nicolau, A.I.; Hoorfar, J. Global Safety of Fresh Produce. A Handbook of Best Practice, Innovative Commercial Solutions and Case Studies; Woodhead Publishing Limite: Sawston, UK, 2014. [Google Scholar]
- Ferrari, C.C.; Sarantópouos, C.; Carmello-Guerreiro, S.; Hubinger, M.D. Effect of osmotic dehydration and pectin edible coatings on quality and shelf life of fresh-cut melon. Food Bioprocess Technol. 2013, 61, 80–91. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Leyva, J.M.; Ortega-Ramírez, L.A.; Carrazco-Lugo, D.K.; Pérez-Carlón, J.J.; Melgarejo-Flores, B.G.; González-Aguilar, G.A.; Miranda, M.R.A. Pectin–cinnamon leaf oil coatings add antioxidant and antibacterial properties to fresh-cut peach. Flavour Fragr. J. 2013, 28, 39–45. [Google Scholar] [CrossRef]
- Gonzalez, J.S.; Martínez, Y.N.; Castro, G.R.; Alvarez, V.A. Preparation and characterization of polyvinyl alcohol–pectin cryogels containing enrofloxacin and keratinase as potential transdermal delivery device. Adv. Mater. Lett. 2016, 7, 640–645. [Google Scholar] [CrossRef]
- Najafi, T.R.; Derakhshana, M.A.; Majidiab, R.F.; Amania, A. Preparation of an ascorbic acid /PVA-chitosan electrospun mat: A core/shell transdermal delivery system. RSC Adv. 2015, 5, 50462–50469. [Google Scholar] [CrossRef]
- Vasconcelos, C.L.; Bezerril, P.M.; dos Santos, D.E.S.; Dantas, T.N.C.; Pereira, M.R.; Fonseca, J.L.C. Effect of molecular weight and ionic strength on the formation of polyelectrolyte complexes based on poly (methacrylic acid) and chitosan. Biomacromolecules 2006, 7, 1245–1252. [Google Scholar] [CrossRef]
- Jin, L.; Bai, R. Mechanisms of lead adsorption on chitosan/PVA hydro gel beads. Langmuir 2002, 18, 9765–9770. [Google Scholar] [CrossRef]
- Amit, K.M.; Jayeeta, B.; Sanjay, K.; Uttam, C.B. Biosynthesis of silver nanoparticles: Elucidation of prospective mechanism and therapeutic potential. J. Colloid Interface Sci. 2014, 415, 39–47. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Taha, N.A. Evaluation rachis browning phenomena of ‘Superior Seedless’ vines grafted on different rootstocks during shelf life. Sci. Hortic. 2020, 261, 109040. [Google Scholar] [CrossRef]
- AOAC. Association of Official of Analytical Chemist, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for the determination of reducing suga. Anal. Chem. 1959, 31, 426–429. [Google Scholar] [CrossRef]
- Collmer, A.; Reid, J.L.; Mount, M.S. Assay methods for pectic enzymes. In Methods in Enzymology; Wood, W.A., Kellogg, S.T., Eds.; Academic Press: San Diego, CA, USA, 1988; Volume 161, pp. 329–335. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Iturbe-Ormaetxe, I.; Escuredo, P.R.; Arrese-Igor, C.; Becana, M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 1998, 116, 173–181. [Google Scholar] [CrossRef]
- Lo’ay, A.A. Biological indicators to minimize berry shatter during handling of “Thompson seedless” grapevines. World Appl. Sci. J. 2011, 12, 1107–1113. [Google Scholar]
- Yang, H.; Wu, F.; Cheng, J. Reduced chilling injury in cucumber by nitricoxide and the antioxidant response. Food Chem. 2011, 127, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Dong, J.; Zhang, M.; Xu, X.; Sun, L. Cold-induced endogenous nitric oxide generation plays a role in chilling tolerance of loquat fruit during postharvest storage. Postharvest Biol. Technol. 2012, 65, 5–12. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agr. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Çelikkol, I.; Türkben, C. Effects of postharvest applications on berry quality, microbial population and morphological (epicuticular wax) deterioration of ready-to-eat table grapes. J. Food Agri. Environ. 2012, 10, 213–220. [Google Scholar]
- Elboudwarej, A.F.; Shirazi, A.; Cameron, A.; Herner, R.C. Measurements of transpiration of different parts of grape clusters. In Proceedings of the 41st Annual Meeting of the American Society for Enology and Viticulture, Los Angeles, CA, USA, 29 June–2 July 1990. [Google Scholar]
- Zhang, M.; Li, C.L.; Huan, Y.J.; Tao, Q.; Wang, H.O. Preservation of fresh grapes at ice-temperature-high-humidity. Int. Agrophysics 2001, 15, 139–143. [Google Scholar]
- De Sousa, L.L.; de Andrade, S.C.A.; Athayde, A.J.A.A.; de Oliveira, C.E.V.; de Sales, C.V.; Madruga, M.S.; de Souza, E.L. Efficacy of Origanum vulgare L. and Rosmarinus officinalis L. essential oils in combination to control postharvest pathogenic Aspergilli and autochthonous mycoflora in Vitis labrusca L. (table grapes). Int. J. Food Microbiol. 2013, 165, 312–318. [Google Scholar] [CrossRef]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef]
- Rogiers, S.; Hatfield, J.; Jaudzems, V.; White, R.; Keller, M. Grape berry cv. Shiraz epicuticular wax and transpiration during ripening and preharvest weight loss. Am. J. Enol. Vitic 2004, 55, 121–127. [Google Scholar]
- Pereira, V.A., Jr.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time–Temperature Indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Shafiee, M.; Taghavi, T.S.; Babalar, M. Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid, and calcium dipping) improved postharvest fruit quality of strawberry. Sci. Hortic. 2010, 124, 40–45. [Google Scholar] [CrossRef]
- Srivastava, M.K.; Dwivedi, U.N. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 2000, 158, 87–96. [Google Scholar] [CrossRef]
- Tareen, M.J.; Abbasi, N.A.; Hafiz, I.A. Postharvest application of salicylic acid enhanced antioxidant enzyme activity and maintained quality of peach cv. ‘Flordaking’ fruit during storage. Sci. Hortic. 2012, 142, 221–228. [Google Scholar] [CrossRef]
- Abdel-Salam, M.M. Effect of Foliar Application of Salicylic Acid and Micronutrients on the Berries Quality of “Bez El Naka” Local Grape Cultivar. Middle East J. Appl. Sci. 2016, 6, 178–188. [Google Scholar]
- Bermstein, Z.; Lustig, I. A new method of firmness measurement of grape berries and other juicy fruits. Vitis 1981, 20, 15–21. [Google Scholar]
- Pasanphan, W.; Buettner, G.R.; Chirachanchai, S. Chitosan gallate as a novel potential polysaccharide antioxidant: An EPR study. Carbohydr. Res. 2010, 345, 132–140. [Google Scholar] [CrossRef]
- Champa, W.A.H.; Gill, M.I.S.; Arora, N.K. Preharvest salicylic acid treatments to improve quality and postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless. J. Food Sci. Technol. 2015, 52, 3607–3616. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Elgammal, R.E.; Alhaithloul, H.A.S.; Alghanem, S.M.; Fikry, M.; Abdein, M.A.; Hikal, D.M. Enhance Fruit Ripening Uniformity and Accelerate the Rutab Stage by Using ATP in ‘Zaghloul’ Dates during the Shelf Life. Foods 2021, 10, 2641. [Google Scholar] [CrossRef]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Wei, J.; Ma, F.; Shi, S.; Qi, X.; Zhu, X.; Yuan, J. Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biol. Technol. 2010, 56, 147–154. [Google Scholar] [CrossRef]
- Bu, J.; Yu, Y.; Aisikaer, G.; Ying, T. Postharvest UV-C irradiation inhibits the production of ethylene and the activity of cell wall-degrading enzymes during softening of tomato (Lycopersicon esculentum L.) fruit. Postharvest Biol. Technol. 2013, 86, 337–345. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, Y.; Li, C.; Wei, M.; Lv, J.; Meng, K. Inhibitory effects of sodium silicate on the fungal growth and secretion of cell wall-degrading enzymes by Trichothecium roseum. J. Phytopathol. 2017, 165, 620–625. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Y.; Lin, H.; Lin, M.; Li, H.; Yuan, F.; Chen, Y.; Xiao, J. Effects of paper containing 1-MCP postharvest treatment on the disassembly of cell wall polysaccharides and softening in Younai plum fruit during storage. Food Chem. 2018, 264, 1–8. [Google Scholar] [CrossRef]
- Abu-Sarra, A.F.; Abu-Goukh, A.A. Changes in pectinesterase, polygalacturonase and cellulase activity during mango fruit ripening. J. Hortic. Sci. 1992, 67, 561–568. [Google Scholar] [CrossRef]
- Giongo, L.; Poncetta, P.; Loretti, P.; Costa, F. Texture profiling of blueberries (Vaccinium spp.) during fruit development, ripening and storage. Postharvest Biol. Technol. 2013, 76, 34–39. [Google Scholar] [CrossRef]
- Babalar, M.; Asghari, M.; Talaei, A.; Khosroshahi, A. Effect of pre-and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit. Food Chem. 2007, 105, 449–453. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Zhang, S.; Ferguson, I. The role of salicylic acid in postharvest ripening of kiwifruit. Postharvest Biol. Technol. 2003, 28, 67–74. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Hodges, D.M.; Lester, G.E.; Muneo, K.D.; Toivonen, P.M. Oxidative stress: Importance for postharvest quality. HortScience 2004, 39, 924–929. [Google Scholar] [CrossRef]
- McCollum, T.; Bowman, K.; Castle, W. Effects of rootstock on fruit quality and postharvest behavior of ‘Marsh’ grapefruit. Proc. Fla. State Hort. Soc. 2002, 115, 44–46. [Google Scholar]
- Cai, H.; Yuan, X.; Pan, J.; Li, H.; Wu, Z.; Wang, Y. Biochemical and proteomic analysis of grape berries (Vitis labruscana) during cold storage upon postharvest salicylic acid treatment. J. Agric. Food Chem. 2014, 62, 10118–10125. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Z.; Lv, X.; Cheng, N.; Peng, B.; Cao, W. Effect of 24-epibrassinolide on chilling injury of peach fruit in relation to phenolic and proline metabolisms. Postharvest Biol. Technol. 2016, 111, 390–397. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Jannatizadeh, A.; Sheikh-Assadi, M.; Malakzadeh, P. Alleviation of postharvest chilling injury in anthurium cut flowers by salicylic acid treatment. Sci. Hort. 2016, 202, 70–76. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Dawood, H.D. Chilling injury, fruit color maturity stages, and antioxidant enzyme activities of lemon ‘baladi CV’ fruits under cold storage stress. Sci. Hortic. 2019, 257, 108676. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Dawood, H.D. Tolerance of ‘Baladi’ mandarin fruits to cold storage by postharvest pectin/PVA blend with ascorbic acid treatment. Sci. Hortic. 2019, 256, 108637. [Google Scholar] [CrossRef]
- Promyou, S.; Ketsa, S. Cultivar difference in sensitivity to chilling injury of anthurium flowers (Anthurium andraeanum) during low temperature storage. Acta Hortic. 2014, 1025, 179–186. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]



| WL% | RB-Index | Hue | BS% | BF | BSF | SSC% | TA% | SSC: TA | XYL | CEL | PG | PT | MDA | IL% | DPPH* | H2O2 | O2− | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WL% | * 1.0000 | |||||||||||||||||
| RB-Index | 0.8988 | 1.0000 | ||||||||||||||||
| Hue | −0.5777 | −0.6235 | 1.0000 | |||||||||||||||
| BS% | 0.8961 | 0.9838 | −0.6130 | 1.0000 | ||||||||||||||
| BF | −0.9525 | −0.8805 | 0.6583 | −0.8774 | 1.0000 | |||||||||||||
| BSF | −0.9348 | −0.9311 | 0.6676 | −0.9010 | 0.9286 | 1.0000 | ||||||||||||
| SSC% | 0.8304 | 0.7213 | −0.5380 | 0.7135 | −0.8682 | −0.8217 | 1.0000 | |||||||||||
| TA% | −0.9459 | −0.9227 | 0.6669 | −0.9073 | 0.9524 | 0.9824 | −0.8346 | 1.0000 | ||||||||||
| SSC: TA | 0.9230 | 0.9312 | −0.6819 | 0.9184 | −0.9206 | −0.9779 | 0.8315 | −0.9832 | 1.0000 | |||||||||
| XYL | 0.9529 | 0.9675 | −0.6346 | 0.9541 | −0.9425 | −0.9565 | 0.7900 | −0.9608 | 0.9407 | 1.0000 | ||||||||
| CEL | 0.8868 | 0.9888 | −0.5981 | 0.9685 | −0.8802 | −0.9153 | 0.7197 | −0.9057 | 0.8991 | 0.9632 | 1.0000 | |||||||
| PG | 0.9177 | 0.8011 | −0.5854 | 0.7900 | −0.9377 | −0.8955 | 0.8400 | −0.9367 | 0.8737 | 0.8983 | 0.8037 | 1.0000 | ||||||
| PT | 0.9440 | 0.7799 | −0.5484 | 0.7742 | −0.9404 | −0.8829 | 0.8391 | −0.9155 | 0.8596 | 0.8817 | 0.7779 | 0.9769 | 1.0000 | |||||
| MDA | 0.9164 | 0.9820 | −0.6304 | 0.9699 | −0.9102 | −0.9492 | 0.7685 | −0.9537 | 0.9576 | 0.9589 | 0.9722 | 0.8486 | 0.8330 | 1.0000 | ||||
| IL% | 0.9644 | 0.9699 | −0.6087 | 0.9658 | −0.9419 | −0.9487 | 0.8030 | −0.9588 | 0.9443 | 0.9845 | 0.9615 | 0.8938 | 0.8898 | 0.9761 | 1.0000 | |||
| DPPH* | 0.6632 | 0.4800 | −0.1151 | 0.5142 | −0.5270 | −0.4012 | 0.4074 | −0.4103 | 0.3863 | 0.5293 | 0.4853 | 0.4525 | 0.5712 | 0.4690 | 0.5705 | 1.0000 | ||
| H2O2 | 0.9094 | 0.9731 | −0.6079 | 0.9564 | −0.8668 | −0.9490 | 0.7237 | −0.9163 | 0.9315 | 0.9542 | 0.9639 | 0.7943 | 0.7940 | 0.9603 | 0.9541 | 0.4938 | 1.0000 | |
| O2− | 0.8209 | 0.9311 | −0.7148 | 0.9177 | −0.8669 | −0.9055 | 0.6818 | −0.9002 | 0.9251 | 0.8884 | 0.9098 | 0.7694 | 0.7448 | 0.9494 | 0.8938 | 0.3217 | 0.9075 | 1.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo’ay, A.A.; Rabie, M.M.; Alhaithloul, H.A.S.; Alghanem, S.M.S.; Ibrahim, A.M.; Abdein, M.A.; Abdelgawad, Z.A. On the Biochemical and Physiological Responses of ‘Crimson Seedless’ Grapes Coated with an Edible Composite of Pectin, Polyphenylene Alcohol, and Salicylic Acid. Horticulturae 2021, 7, 498. https://doi.org/10.3390/horticulturae7110498
Lo’ay AA, Rabie MM, Alhaithloul HAS, Alghanem SMS, Ibrahim AM, Abdein MA, Abdelgawad ZA. On the Biochemical and Physiological Responses of ‘Crimson Seedless’ Grapes Coated with an Edible Composite of Pectin, Polyphenylene Alcohol, and Salicylic Acid. Horticulturae. 2021; 7(11):498. https://doi.org/10.3390/horticulturae7110498
Chicago/Turabian StyleLo’ay, A. A., M. M. Rabie, Haifa A. S. Alhaithloul, Suliman M. S. Alghanem, Aly M. Ibrahim, Mohamed A. Abdein, and Zinab A. Abdelgawad. 2021. "On the Biochemical and Physiological Responses of ‘Crimson Seedless’ Grapes Coated with an Edible Composite of Pectin, Polyphenylene Alcohol, and Salicylic Acid" Horticulturae 7, no. 11: 498. https://doi.org/10.3390/horticulturae7110498
APA StyleLo’ay, A. A., Rabie, M. M., Alhaithloul, H. A. S., Alghanem, S. M. S., Ibrahim, A. M., Abdein, M. A., & Abdelgawad, Z. A. (2021). On the Biochemical and Physiological Responses of ‘Crimson Seedless’ Grapes Coated with an Edible Composite of Pectin, Polyphenylene Alcohol, and Salicylic Acid. Horticulturae, 7(11), 498. https://doi.org/10.3390/horticulturae7110498








