In Vitro Adventitious Regeneration of Artemisia annua L. Influencing Artemisinin Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Culture Media and Conditions for the Regeneration Induction
2.4. Shoot Elongation, Multiplication and Root Development
2.5. Acclimatization and Field Cultivation
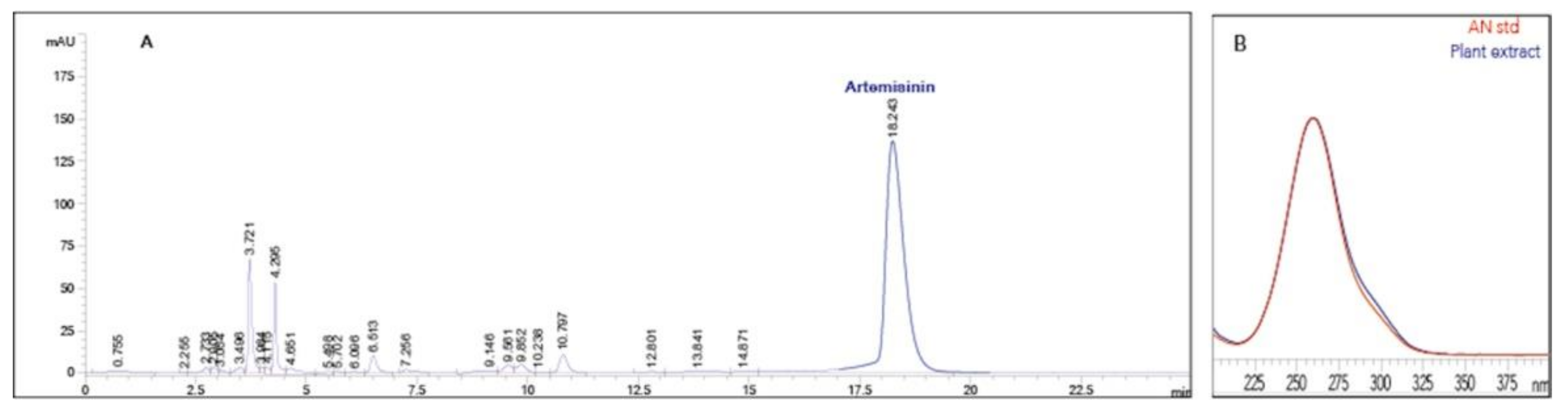
2.6. Artemisinin, Dihydroartemisinic Acid and Artemisinic Acid Extraction and Analysis
2.7. Statistical Analysis
3. Results
3.1. Effects of Plant Growth Regulators on the Induction of the Regeneration Process
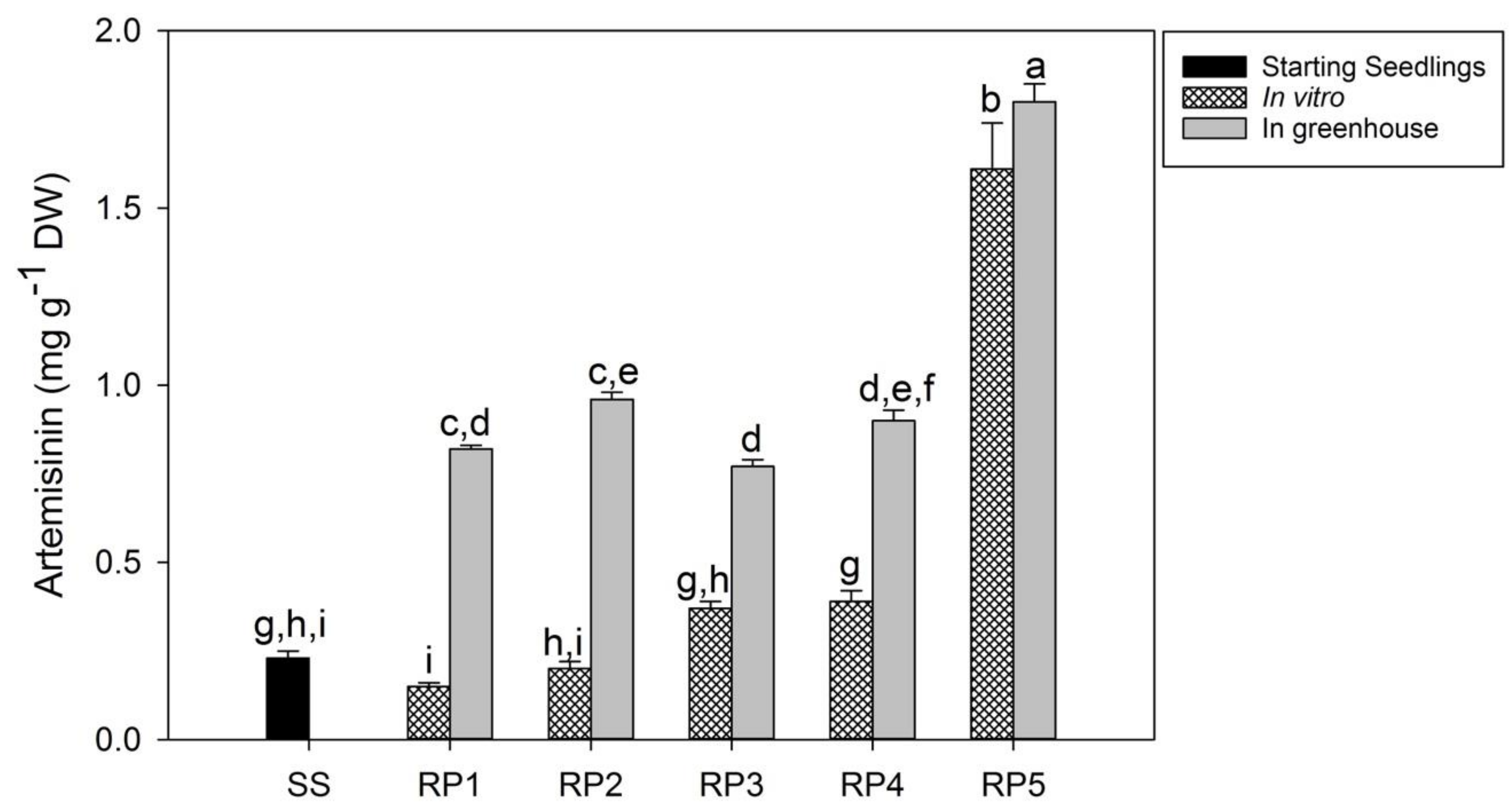
3.2. Micropropagation of Regenerants, Root Development and Acclimatization
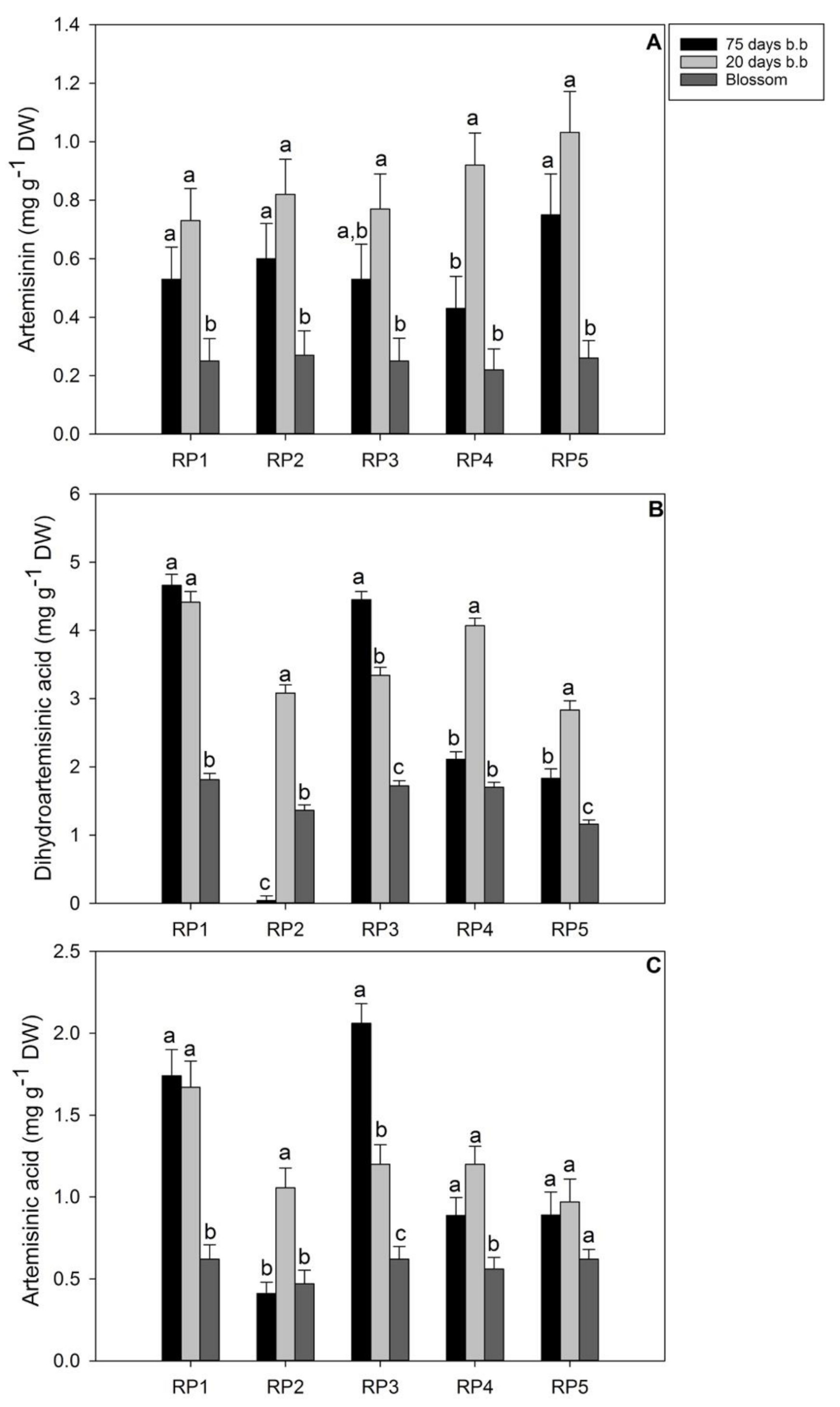
3.3. Evaluation of Artemisinin and Precursors in Seedlings and RPs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, J.F.S.; Simon, J.E.; Janick, J. Artemisia annua: Botany, Horticulture, Pharmacology. Hortic. Rev. 1996, 19, 319–371. [Google Scholar] [CrossRef]
- WHO. Antimalarial Drug Combination Therapy: Report of a WHO Technical Consultation; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Delabays, N.; Simonnet, X.; Gaudin, M. The genetics of artemisinin content in Artemisia annua L. and the breeding of high yielding cultivars. Curr. Med. Chem. 2001, 8, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Shen, Q.; Yan, T.; Fu, X. Transgenic approach to increase artemisinin content in Artemisia annua L. Plant Cell Rep. 2014, 33, 605–615. [Google Scholar] [CrossRef]
- Di Sansebastiano, G.P.; Rizzello, F.; Durante, M.; Caretto, S.; Nisi, R.; De Paolis, A.; Faraco, M.; Montefusco, A.; Piro, G.; Mita, G. Subcellular compartmentalization in protoplasts from Artemisia annua cell cultures: Engineering attempts using a modified SNARE protein. J. Biotechnol. 2015, 202, 146–152. [Google Scholar] [CrossRef] [PubMed]
- De Paolis, A.; Caretto, S.; Quarta, A.; Di Sansebastiano, G.P.; Sbrocca, I.; Mita, G.; Frugis, G. Genome-Wide Identification of WRKY Genes in Artemisia annua: Characterization of a Putative Ortholog of AtWRKY40. Plants 2020, 9, 1669. [Google Scholar] [CrossRef] [PubMed]
- Wetzstein, H.Y.; Porter, J.A.; Janick, J.; Ferreira, J.F.S.; Mutui, T.M. Selection and Clonal Propagation of High Artemisinin Genotypes of Artemisia annua. Front. Plant Sci. 2018, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Wetzstein, H.Y.; Janick, J.; Ferreira, J.F.S. Germplasm Release of Four High-artemisinin Clones of Artemisia annua L. Hortscience 2019, 54, 2081–2082. [Google Scholar] [CrossRef]
- Caretto, S.; Quarta, A.; Durante, M.; Nisi, R.; De Paolis, A.; Blando, F.; Mita, G. Methyl jasmonate and miconazole differently affect arteminisin production and gene expression in Artemisia annua suspension cultures. Plant Biol. 2011, 13, 51–58. [Google Scholar] [CrossRef]
- Durante, M.; Caretto, S.; Quarta, A.; De Paolis, A.; Nisi, R.; Mita, G. beta-Cyclodextrins enhance artemisinin production in Artemisia annua suspension cell cultures. Appl. Microbiol. Biotechnol. 2011, 90, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Saxena, P.; Alam, P.; Wajid, S.; Abdin, M.Z. Modulation of artemisinin biosynthesis by elicitors, inhibitor, and precursor in hairy root cultures of Artemisia annua L. J. Plant Interact. 2014, 9, 811–824. [Google Scholar] [CrossRef]
- Paddon, C.J.; Keasling, J.D. Semi-synthetic artemisinin: A model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 2014, 12, 355–367. [Google Scholar] [CrossRef]
- Weathers, P.J.; Elfawal, M.A.; Towler, M.J.; Acquaah-Mensah, G.K.; Rich, S.M. Pharmacokinetics of artemisinin delivered by oral consumption of Artemisia annua dried leaves in healthy vs. Plasmodium chabaudi-infected mice. J. Ethnopharmacol. 2014, 153, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.F.S.; Benedito, V.A.; Sandhu, D.; Marchese, J.A.; Liu, S. Seasonal and Differential Sesquiterpene Accumulation in Artemisia annua Suggest Selection Based on Both Artemisinin and Dihydroartemisinic Acid may Increase Artemisinin in planta. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Larkin, P.J.; Scowcroft, W.R. Somaclonal variation—A novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 1981, 60, 197–214. [Google Scholar] [CrossRef]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech 2016, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Vergauwe, A.; Cammaert, R.; Vandenberghe, D.; Genetello, C.; Inze, D.; Van Montagu, M.; Van den Eeckhout, E. Agrobacterium tumefaciens-mediated transformation of Artemisia annua L. and regeneration of transgenic plants. Plant Cell Rep. 1996, 15, 929–933. [Google Scholar] [CrossRef]
- Chen, D.; Ye, H.; Li, G. Expression of a chimeric farnesyl diphosphate synthase gene in Artemisia annua L. transgenic plants via Agrobacterium tumefaciens-mediated transformation. Plant Sci. 2000, 155, 179–185. [Google Scholar] [CrossRef]
- Han, J.L.; Wang, H.; Ye, H.C.; Liu, Y.; Li, Z.Q.; Zhang, Y.; Zhang, Y.S.; Yan, F.; Li, G.F. High efficiency of genetic transformation and regeneration of Artemisia annua L. via Agrobacterium tumefaciens-mediated procedure. Plant Sci. 2005, 168, 73–80. [Google Scholar] [CrossRef]
- Lualon, W.; De-Eknamkul, W.; Tanaka, H.; Shoyama, Y.; Putalun, W. Artemisinin production by shoot regeneration of Artemisia annua L. using thidiazuron. Z. Naturforsch. C J. Biosci. 2008, 63, 96–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lei, C.Y.; Wang, H.; Liu, B.Y.; Ye, H.C. Effects of silver nitrate on shoot regeneration of Artemisia annua L. Plant Biotechnol. 2014, 31, 71–75. [Google Scholar] [CrossRef][Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 51, 473–497. [Google Scholar] [CrossRef]
- Smith, T.C.; Weathers, P.J.; Cheetham, R.D. Effects of gibberellic acid on hairy root cultures of Artemisia annua: Growth and artemisinin production. Vitr. Cell. Dev. Biol.-Plant 1997, 33, 75–79. [Google Scholar] [CrossRef]
- Liu, C.-Z.; Guo, C.; Wang, Y.; Ouyang, F. Factors influencing artemisinin production from shoot cultures of Artemisia annua L. World J. Microbiol. Biotechnol. 2003, 19, 535–538. [Google Scholar] [CrossRef]
- Almaarri, K.; Xie, D.Y. In vitro direct organogenesis and micropropagation of Artemisia annua. J. Biotechnol. Veg. 2010, 26, 327–337. [Google Scholar]
- Hailu, T.; Abera, B.; Mariam, G. In vitro Mass Propagation of Artemisia (Artemisia annua L.) cv: Anamed. Plant Tissue Cult. Biotechnol. 2014, 23, 165–176. [Google Scholar] [CrossRef]
- Preece, J.E.; Sutter, E.G. Acclimatization of micropropagated plants to the greenhouse and field. In Micropropagation: Technology and Application; Debergh, P.C., Zimmerman, R.H., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 71–93. [Google Scholar] [CrossRef]
- Caretto, S.; Paradiso, A.; D’Amico, L.; De Gara, L. Ascorbate and glutathione metabolism in two sunflower cell lines of differing α-tocopherol biosynthetic capability. Plant Physiol. Biochem. 2002, 40, 509–513. [Google Scholar] [CrossRef]
- Covello, P.S.; Teoh, K.H.; Polichuk, D.R.; Reed, D.W.; Nowak, G. Functional genomics and the biosynthesis of artemisinin. Phytochemistry 2007, 68, 1864–1871. [Google Scholar] [CrossRef]
- Teoh, K.H.; Polichuk, D.R.; Reed, D.W.; Covello, P.S. Molecular cloning of an aldehyde dehydrogenase implicated in artemisinin biosynthesis in Artemisia annua. Botany 2009, 87, 635–642. [Google Scholar] [CrossRef]
- Us-Camas, R.Y.; Rivera-Solís, G.; Duarte-Aké, F.; De-la-Peña, C. In vitro culture: An epigenetic challenge for plants. Plant Cell Tissue Organ Cult. (PCTOC) 2014, 118, 187–201. [Google Scholar] [CrossRef]
- Sanchez-Muñoz, R.; Moyano, E.; Khojasteh, A.; Bonfill, M.; Cusido, R.M.; Palazon, J. Genomic methylation in plant cell cultures: A barrier to the development of commercial long-term biofactories. Eng. Life Sci. 2019, 19, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Mohan Jain, S. Tissue culture-derived variation in crop improvement. Euphytica 2001, 118, 153–166. [Google Scholar] [CrossRef]
- Khan, S.A.; Rahman, L.U.; Shanker, K.; Singh, M. Agrobacterium tumefaciens-mediated transgenic plant and somaclone production through direct and indirect regeneration from leaves in Stevia rebaudiana with their glycoside profile. Protoplasma 2014, 251, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Woerdenbag, H.J.; Lüers, J.F.J.; van Uden, W.; Pras, N.; Malingré, T.M.; Alfermann, A.W. Production of the new antimalarial drug artemisinin in shoot cultures of Artemisia annua L. Plant Cell Tissue Organ Cult. 1993, 32, 247–257. [Google Scholar] [CrossRef]

| Medium | Plant Growth Regulators (μM) | Shoot Producing Explants (%) | Regenerated Shoots/Explant (Mean) |
|---|---|---|---|
| RM1 | BA 4.4 + IBA 0.35 | 91.8 | 4.9 ± 2.6 |
| RM2 | TDZ 4.5 + IBA 0.35 | 4 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blando, F.; Rizzello, F.; Durante, M.; De Paolis, A.; Caretto, S.; Mita, G. In Vitro Adventitious Regeneration of Artemisia annua L. Influencing Artemisinin Metabolism. Horticulturae 2021, 7, 438. https://doi.org/10.3390/horticulturae7110438
Blando F, Rizzello F, Durante M, De Paolis A, Caretto S, Mita G. In Vitro Adventitious Regeneration of Artemisia annua L. Influencing Artemisinin Metabolism. Horticulturae. 2021; 7(11):438. https://doi.org/10.3390/horticulturae7110438
Chicago/Turabian StyleBlando, Federica, Francesca Rizzello, Miriana Durante, Angelo De Paolis, Sofia Caretto, and Giovanni Mita. 2021. "In Vitro Adventitious Regeneration of Artemisia annua L. Influencing Artemisinin Metabolism" Horticulturae 7, no. 11: 438. https://doi.org/10.3390/horticulturae7110438
APA StyleBlando, F., Rizzello, F., Durante, M., De Paolis, A., Caretto, S., & Mita, G. (2021). In Vitro Adventitious Regeneration of Artemisia annua L. Influencing Artemisinin Metabolism. Horticulturae, 7(11), 438. https://doi.org/10.3390/horticulturae7110438








