Mycorrhizal Compatibility and Germination-Promoting Activity of Tulasnella Species in Two Species of Orchid (Cymbidium mannii and Epidendrum radicans)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolation and Tulasnella Identification
2.2. Seed Harvest
2.3. Symbiotic Germination
2.4. Assessment of the Fungal Capacity to Promote Seed Germination
2.5. Statistical Analysis
3. Results
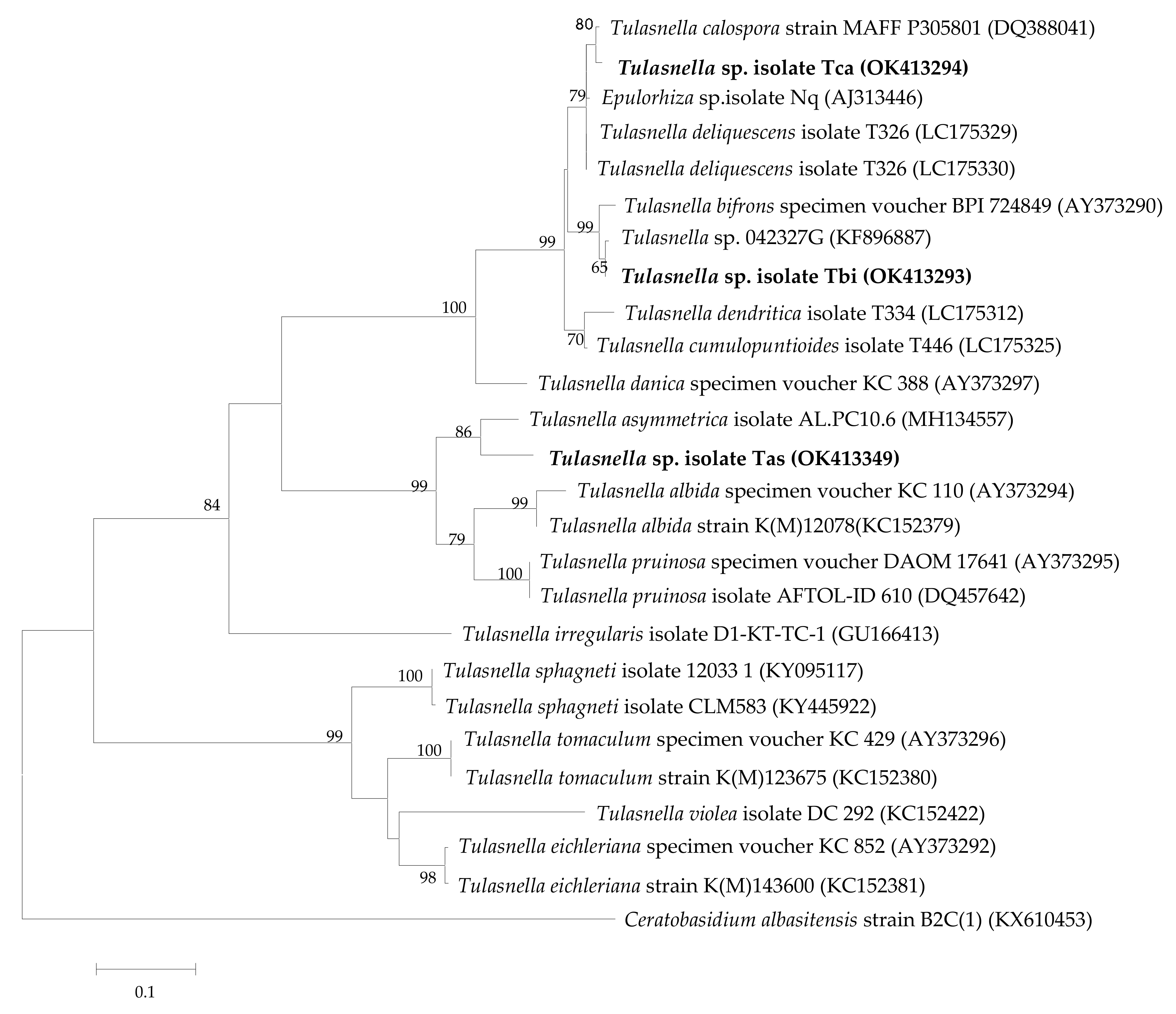
3.1. Tulasnella Identification and Phylogenetic Analyses
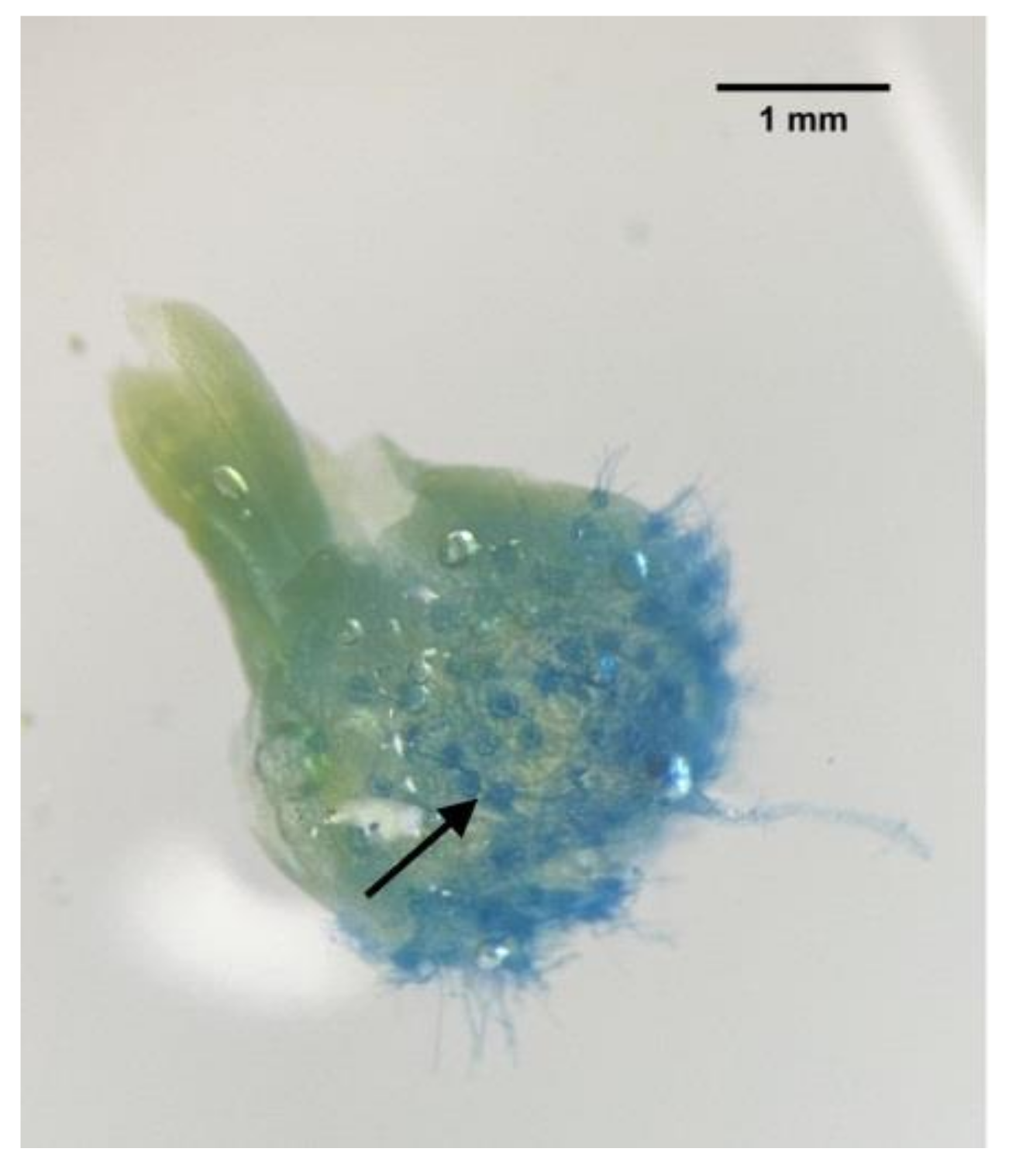
3.2. Colonization of Mycorrhizal Fungi
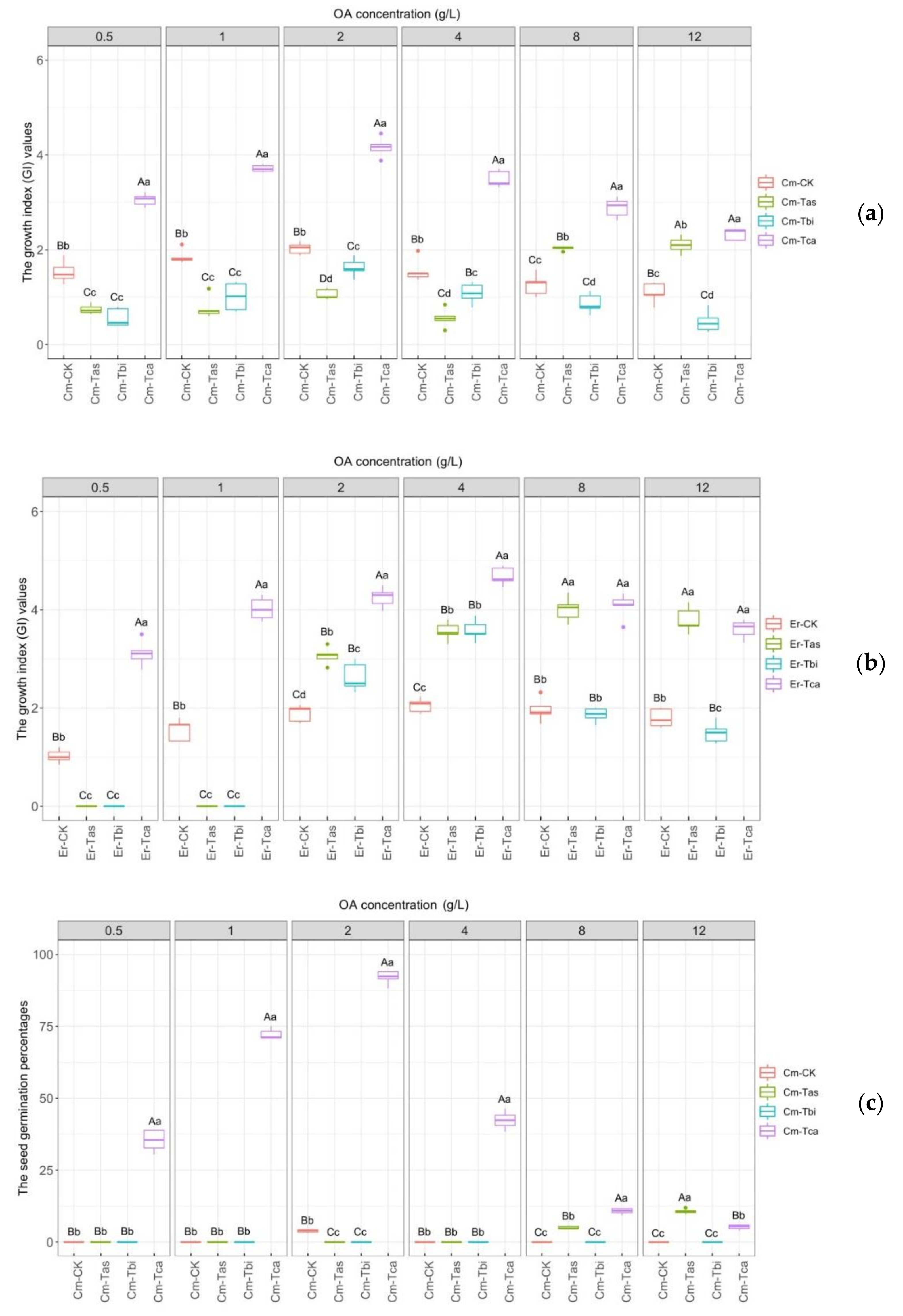
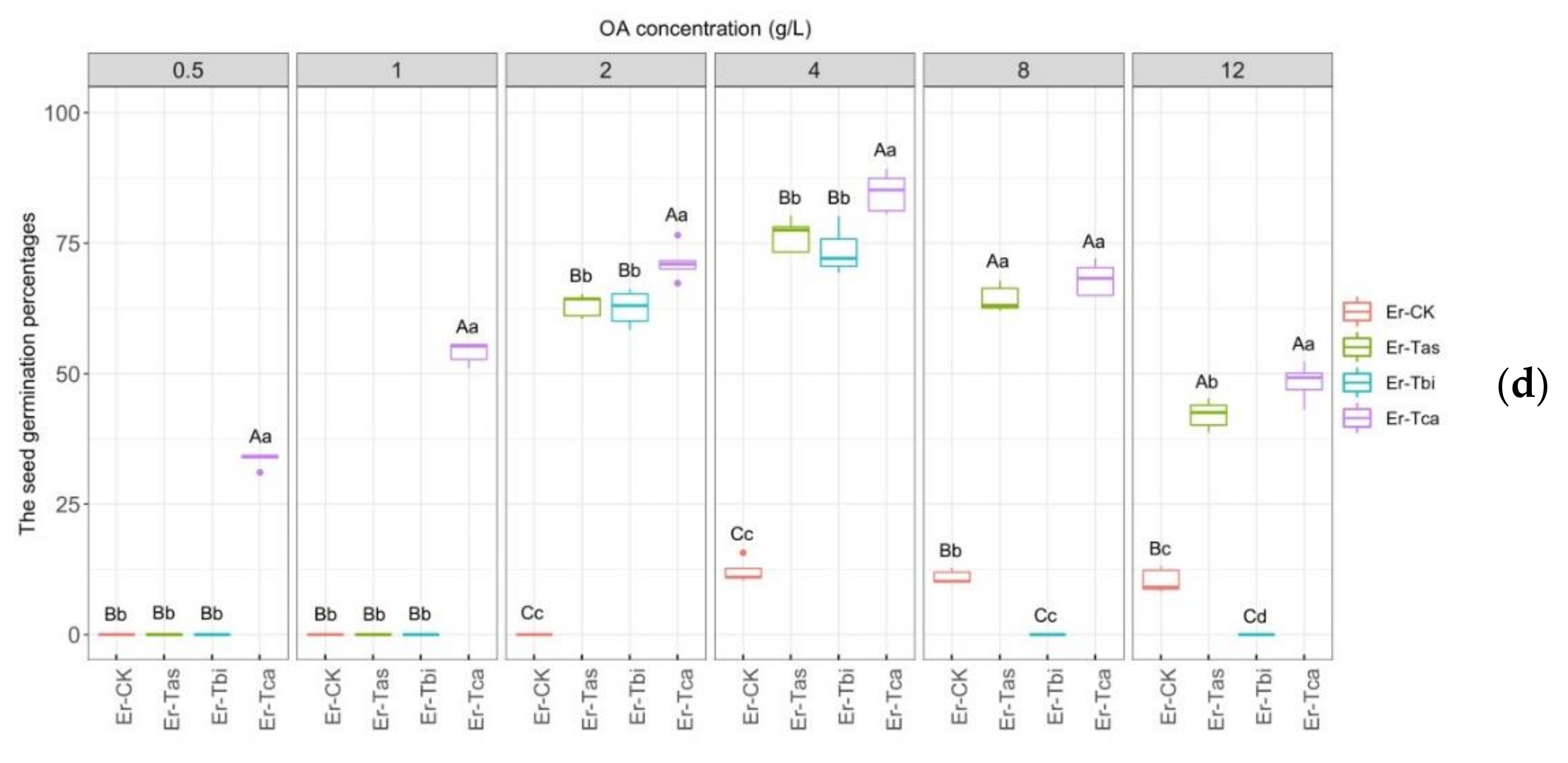
3.3. Effects of Different Fungal Strains on Seed Germination
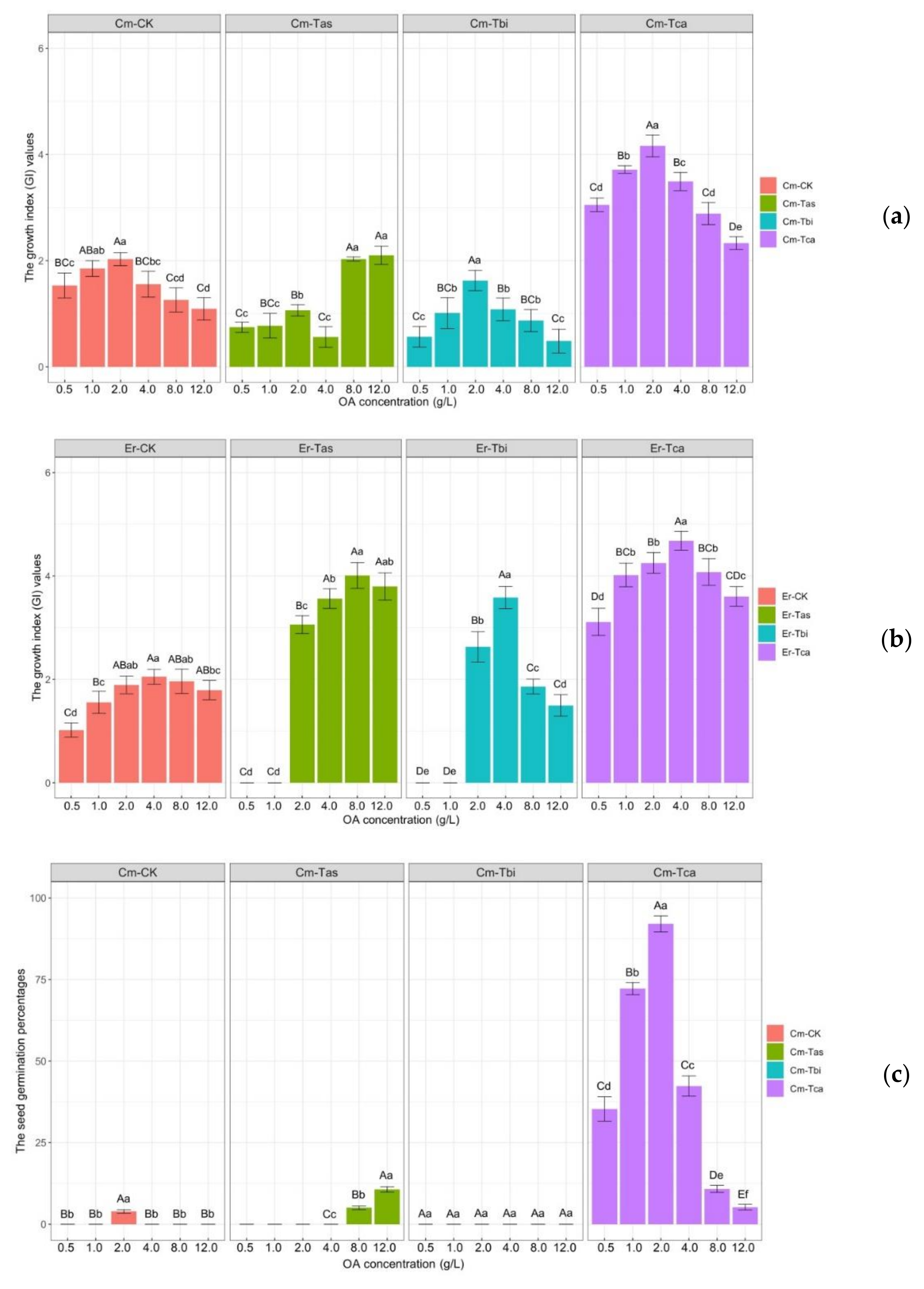
3.4. Effects of Different Concentrations of OA on Seed Germination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Stokstad, E. Orchids’ dazzling diversity explained. Science 2015, 349, 914. [Google Scholar] [CrossRef]
- Rafter, M.; Yokoya, K.; Schofield, E.J.; Zettler, L.W.; Sarasan, V. Non-specific symbiotic germination of Cynorkis purpurea (Thouars) Kraezl., a habitat-specific terrestrial orchid from the Central Highlands of Madagascar. Mycorrhiza 2016, 26, 541–552. [Google Scholar] [CrossRef]
- Suetsugu, K.; Kawakita, A.; Kato, M. Avian seed dispersal in a mycoheterotrophic orchid Cyrtosia septentrionalis. Nat. Plants 2015, 1. [Google Scholar] [CrossRef]
- Sheng, C.-L.; Lee, Y.-I.; Gao, J.-Y. Ex situ symbiotic seed germination, isolation and identification of effective symbiotic fungus in Cymbidium mannii (Orchidaceae). Chin. J. Plant Ecol. 2012, 36, 859–869. [Google Scholar] [CrossRef]
- Clements, M.A. Orchid mycorrhizal associations. Lindleyana 1988, 3, 73–86. [Google Scholar]
- Rasmussen, H.; Rasmussen, F.N. Orchid mycorrhiza: Implications of a mycophagous life style. Oikos 2009, 118, 334–345. [Google Scholar] [CrossRef]
- Khamchatra, N.-M.; Dixon, K.; Chayamarit, K.; Apisitwanich, S.; Tantiwiwat, S. Using in situ seed baiting technique to isolate and identify endophytic and mycorrhizal fungi from seeds of a threatened epiphytic orchid, Dendrobium friedericksianum Rchb.f. (Orchidaceae). Agric. Nat. Resour. 2016, 50, 8–13. [Google Scholar] [CrossRef]
- Zettler, L.W.; Poulter, S.B.; McDonald, K.I.; Stewart, S.L. Conservation-driven Propagation of an Epiphytic Orchid (Epidendrum nocturnum) with a Mycorrhizal Fungus. HortScience 2007, 42, 135–139. [Google Scholar] [CrossRef]
- Swarts, N.D.; Dixon, K. Terrestrial orchid conservation in the age of extinction. Ann. Bot. 2009, 104, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.-Y.; Shao, S.-C.; Liu, S.-J.; Gao, J.-Y. Do the fungi associated with roots of adult plants support seed germination? A case study on Dendrobium exile (Orchidaceae). Glob. Ecol. Conserv. 2019, 17, e00582. [Google Scholar] [CrossRef]
- Durán-López, M.; Caroca-Cáceres, R.; Jahreis, K.; Narváez-Vera, M.; Ansaloni, R.; Cazar, M. The micorryzal fungi Ceratobasidium sp. and Sebacina vermifera promote seed germination and seedling development of the terrestrial orchid Epidendrum secundum Jacq. South Afr. J. Bot. 2019, 125, 54–61. [Google Scholar] [CrossRef]
- Selosse, M.-A.; Faccio, A.; Scappaticci, G.; Bonfante, P. Chlorophyllous and Achlorophyllous Specimens of Epipactis microphylla (Neottieae, Orchidaceae) Are Associated with Ectomycorrhizal Septomycetes, including Truffles. Microb. Ecol. 2004, 47, 416–426. [Google Scholar] [CrossRef]
- Kottke, I.; Suárez, J.P.; Herrera, P.; Cruz, D.; Bauer, R.; Haug, I.; Garnica, S. Atractiellomycetes belonging to the ‘rust’ lineage (Pucciniomycotina) form mycorrhizae with terrestrial and epiphytic neotropical orchids. Proc. R. Soc. B Boil. Sci. 2009, 277, 1289–1298. [Google Scholar] [CrossRef]
- Riofrío, M.L.; Cruz, D.; Torres, E.; De La Cruz, M.; Iriondo, J.M.; Suárez, J.P. Mycorrhizal preferences and fine spatial structure of the epiphytic orchid Epidendrum rhopalostele. Am. J. Bot. 2013, 100, 2339–2348. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Guo, S.-X.; Xing, X.-K. Fungal diversity and mechanisms of symbiotic germination of orchid seeds: A review. Myco-Systema 2019, 38, 1808–1825. (In Chinese) [Google Scholar] [CrossRef]
- Bidartondo, M.I.; Read, D.J. Fungal specificity bottlenecks during orchid germination and development. Mol. Ecol. 2008, 17, 3707–3716. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.J.; Phillips, R.D.; Wright, M.; Linde, C.; Dixon, K. Continent-wide distribution in mycorrhizal fungi: Implications for the biogeography of specialized orchids. Ann. Bot. 2015, 116, 413–421. [Google Scholar] [CrossRef]
- Bayman, P.; Mosquera-Espinosa, A.T.; Saladini-Aponte, C.M.; Hurtado-Guevara, N.C.; Viera-Ruiz, N.L. Age-dependent mycorrhizal specificity in an invasive orchid, Oeceoclades maculata. Am. J. Bot. 2016, 103, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Ma, X.; Men, J.; Chen, Y.; Guo, S. Phylogenetic constrains on mycorrhizal specificity in eight Dendrobium (Orchidaceae) species. Sci. China Life Sci. 2017, 60, 536–544. [Google Scholar] [CrossRef]
- Reiter, N.; Reiter, N.; Phillips, R.D.; Phillips, R.D.; Swarts, N.D.; Swarts, N.D.; Wright, M.; Wright, M.; Holmes, G.; Holmes, G.; et al. Specific mycorrhizal associations involving the same fungal taxa in common and threatened Caladenia (Orchidaceae): Implications for conservation. Ann. Bot. 2020, 126, 943–955. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Honnay, O.; Cammue, B.P.A.; Brys, R.; Lievens, B. Low specificity and nested subset structure characterize mycorrhizal associations in five closely related species of the genus Orchis. Mol. Ecol. 2010, 19, 4086–4095. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.L.; Zettler, L.W.; Minso, J.; Brown, P.M. Symbiotic germination and reintroduction of Spiranthes brevilabris Lindley, an endangered orchid native to Florida. Selbyana 2003, 24, 64–70. [Google Scholar]
- Perotto, S.; Rodda, M.; Benetti, A.; Sillo, F.; Ercole, E.; Rodda, M.; Girlanda, M.; Murat, C.; Balestrini, R. Gene expression in mycorrhizal orchid protocorms suggests a friendly plant–fungus relationship. Planta 2014, 239, 1337–1349. [Google Scholar] [CrossRef]
- Øien, D.-I.; O’Neill, J.P.; Whigham, D.F.; McCormick, M.K. Germination Ecology of the Boreal-Alpine Terrestrial Orchid Dactylorhiza lapponica (Orchidaceae). Ann. Bot. Fenn. 2008, 45, 161–172. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, J. Highly compatible Epa-01 strain promotes seed germination and protocorm development of Papilionanthe teres (Orchidaceae). Plant Cell Tissue Organ Cult. 2016, 125, 479–493. [Google Scholar] [CrossRef]
- Tan, X.M.; Wang, C.L.; Chen, X.M.; Zhou, Y.Q.; Wang, Y.Q.; Luo, A.X.; Liu, Z.H.; Guo, S.X. In vitro seed germination and seedling growth of an endangered epiphytic orchid, Dendrobium officinale, endemic to China using mycorrhizal fungi (Tulasnella sp.). Sci. Hortic. 2014, 165, 62–68. [Google Scholar] [CrossRef]
- Nontachaiyapoom, S.; Sasirat, S.; Manoch, L. Symbiotic seed germination of Grammatophyllum speciosum Blume and Dendrobium draconis Rchb. f., native orchids of Thailand. Sci. Hortic. 2011, 130, 303–308. [Google Scholar] [CrossRef]
- Mala, B.; Kuegkong, K.; Sa-Ngiaemsri, N.; Nontachaiyapoom, S. Effect of germination media on in vitro symbiotic seed germination of three Dendrobium orchids. South Afr. J. Bot. 2017, 112, 521–526. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.N.; Wang, T.; Li, L.B. Symbiosis established between orchid and Tulasnella spp. fungi. J. Nucl. Agric. Sci. 2017, 31, 876–883. (In Chinese) [Google Scholar] [CrossRef]
- Motomura, H.; Yukawa, T.; Ueno, O.; Kagawa, A. The occurrence of crassulacean acid metabolism in Cymbidium (Orchidaceae) and its ecological and evolutionary implications. J. Plant Res. 2008, 121, 163–177. [Google Scholar] [CrossRef]
- Zhe, M.; Zhang, L.; Liu, F.; Huang, Y.; Fan, W.; Yang, J.; Zhu, A. Plastid RNA editing reduction accompanied with genetic variations in Cymbidium, a genus with diverse lifestyle modes. Plant Divers. in press. 2021. [Google Scholar] [CrossRef]
- Wei, J.F.; Cen, Z.Y.; Su, J. Effects of different mediums on the growth of tissue culture seedling of Cymbidium bicolor Lindl during stemporary planting period. Northern Hortic. 2012, 23, 59–61. (In Chinese) [Google Scholar]
- Thwala, M.; Wahome, P.K.; Oseni, T.O.; Masarirambi, M.T. Effects of floral preservatives on the vase life of orchid (Epidendrum radicans L.) cut flowers. J. Hort. Sci. Ornament. Plants 2013, 5, 22–29. [Google Scholar] [CrossRef]
- Huang, H.; Zi, X.-M.; Lin, H.; Gao, J.-Y. Host-specificity of symbiotic mycorrhizal fungi for enhancing seed germination, protocorm formation and seedling development of over-collected medicinal orchid, Dendrobium devonianum. J. Microbiol. 2018, 56, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.-Y.; Chen, X.-M.; Guo, S.-X.; Lee, Y.-I. Effect of different mycobionts on symbiotic germination and seedling growth of Dendrobium officinale, an important medicinal orchid. Bot. Stud. 2020, 61, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fracchia, S.; Rickert, A.M.A.; Flachsland, E.; Terada, G.; Sede, S.M. Mycorrhizal compatibility and symbiotic reproduction of Gavilea australis, an endangered terrestrial orchid from south Patagonia. Mycorrhiza 2014, 24, 627–634. [Google Scholar] [CrossRef]
- Wang, D.; Jia, S.H.; Zhang, Z.X.; Cai, Y.P.; Lin, Y. Isolation and culture of an endophytic fungus associated with Dendrobium huoshanense and its effects on the growth of plantlets. J. Fungal. Res. 2007, 5, 84–88. (In Chinese) [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K.; Battistuzzi, F.U. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Seaton, P.T.; Hu, H.; Perner, H.; Pritchard, H.W. Ex Situ Conservation of Orchids in a Warming World. Bot. Rev. 2010, 76, 193–203. [Google Scholar] [CrossRef]
- Zhao, X.-L.; Yang, J.-Z.; Liu, S.; Chen, C.-L.; Zhu, H.-Y.; Cao, J.-X. The colonization patterns of different fungi on roots of Cymbidium hybridum plantlets and their respective inoculation effects on growth and nutrient uptake of orchid plantlets. World J. Microbiol. Biotechnol. 2014, 30, 1993–2003. [Google Scholar] [CrossRef]
- Otero, J.T.; Ackerman, J.D.; Bayman, P. Differences in mycorrhizal preferences between two tropical orchids. Mol. Ecol. 2004, 13, 2393–2404. [Google Scholar] [CrossRef]
- Jiang, J.-H.; Lee, Y.-I.; Cubeta, M.A.; Chen, L.-C. Characterization and colonization of endomycorrhizal Rhizoctonia fungi in the medicinal herb Anoectochilus formosanus (Orchidaceae). Mycorrhiza 2015, 25, 431–445. [Google Scholar] [CrossRef][Green Version]
- Huber, F.K.; Kaiser, R.; Sauter, W.; Schiestl, F.P. Floral scent emission and pollinator attraction in two species of Gymnadenia (Orchidaceae). Oecologia 2004, 142, 564–575. [Google Scholar] [CrossRef]
- Herrera, H.; Valadares, R.; Contreras, D.; Bashan, Y.; Arriagada, C. Mycorrhizal compatibility and symbiotic seed germination of orchids from the Coastal Range and Andes in south central Chile. Mycorrhiza 2016, 27, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Gregg, K.B.; Kéry, M. Comparison of size vs. life-state classification in demographic models for the terrestrial orchid Cleistes bifaria. Biol. Conserv. 2006, 129, 50–58. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Guo, S.-X. Isolation and identification of endophytic and mycorrhizal fungi from seeds and roots of Dendrobium (Orchidaceae). Mycorrhiza 2011, 22, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.; Romero, C.; Suz, L.M.; Atala, C. Essential mycorrhizal partners of the endemic Chilean orchids Chloraea collicensis and C. gavilu. Flora 2014, 209, 95–99. [Google Scholar] [CrossRef]
- Dearnaley, J.D.W. Further advances in orchid mycorrhizal research. Mycorrhiza 2007, 17, 475–486. [Google Scholar] [CrossRef]
- Warcup, J.H.; Talbot, P.H.B. Perfect states of Rhizoctonias associated with orchids. New Phytol. 1967, 66, 631–641. [Google Scholar] [CrossRef]
- Moore, R.T. The genera of Rhizoctonia-like fungi: Ascorhizoctonia, Ceratorhiza gen. nov., Epulorhiza gen. nov., Moniliopsis, and Rhizoctonia. Mycotaxon 1987, 29, 91–99. [Google Scholar]
- Zi, X.-M.; Sheng, C.-L.; Goodale, U.; Shao, S.-C.; Gao, J.-Y. In situ seed baiting to isolate germination-enhancing fungi for an epiphytic orchid, Dendrobium aphyllum (Orchidaceae). Mycorrhiza 2014, 24, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Adamo, M.; Chialva, M.; Calevo, J.; De Rose, S.; Girlanda, M.; Perotto, S.; Balestrini, R. The Dark Side of Orchid Symbiosis: Can Tulasnella calospora Decompose Host Tissues? Int. J. Mol. Sci. 2020, 21, 3139. [Google Scholar] [CrossRef]
- McCormick, M.K.; Taylor, D.L.; Juhaszova, K.; Burnett, R.K.; Whigham, D.F.; O’Neill, J.P. Limitations on orchid recruitment: Not a simple picture. Mol. Ecol. 2012, 21, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Calevo, J.; Voyron, S.; Ercole, E.; Girlanda, M. Is the Distribution of Two Rare Orchis Sister Species Limited by Their Main Mycobiont? Diversity 2020, 12, 262. [Google Scholar] [CrossRef]
- Shefferson, R.P.; Bunch, W.; Cowden, C.C.; Lee, Y.; Kartzinel, T.R.; Yukawa, T.; Downing, J.; Jiang, H. Does evolutionary history determine specificity in broad ecological interactions? J. Ecol. 2019, 107, 1582–1593. [Google Scholar] [CrossRef]
- McCormick, M.K.; Whigham, D.F.; Canchani-Viruet, A. Mycorrhizal fungi affect orchid distribution and population dynamics. New Phytol. 2018, 219, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-M.; Chung, K.; Liang, C.-K.; Tsai, W.-C. New Insights into the Symbiotic Relationship between Orchids and Fungi. Appl. Sci. 2019, 9, 585. [Google Scholar] [CrossRef]
- Miyauchi, S.; Kiss, E.; Kuo, A.; Drula, E.; Kohler, A.; Sánchez-García, M.; Morin, E.; Andreopoulos, B.; Barry, K.W.; Bonito, G.; et al. Large-scale genome sequencing of mycorrhizal fungi provides insights into the early evolution of symbiotic traits. Nat. Commun. 2020, 11, 5125–5142. [Google Scholar] [CrossRef] [PubMed]



| Isolates | Species | NCBI Accession Number of ITS Sequence | Deposit Number | Original Plant |
|---|---|---|---|---|
| Tca | Tulasnella calospora | OK413294 | CPCC 401,220 | Cymbidium mannii (epiphytic) |
| Tas | Tulasnella asymmetrica | OK413349 | CPCC 401,221 | Epidendrum radicans (epiphytic) |
| Tbi | Tulasnella bifrons | OK413293 | CGMCC 14,794 | Cymbidium goeringii (terrestrial) |
| OA Concentrations (g/L) | C. mannii | E. radicans | ||||
|---|---|---|---|---|---|---|
| Tbi | Tas | Tca | Tbi | Tas | Tca | |
| 0.5 | n | n | 34.0 ± 1.0 | n | n | 25.0 ± 0.0 |
| 1.0 | n | n | 23.0 ± 0.0 | n | n | 24.0 ± 1.0 |
| 2.0 | n | n | 20.3 ± 0.6 | 18.3 ± 0.6 | 23.0 ± 0.3 | 20.0 ± 1.0 |
| 4.0 | n | n | 37.0 ± 0.0 | 19.0 ± 0.0 | 22.3 ± 0.6 | 19.3 ± 0.6 |
| 8.0 | n | 56.0 ± 0.0 | 55.3 ± 0.6 | n | 24.0 ± 0.0 | 22.0 ± 0.3 |
| 12.0 | n | 56.0 ± 0.0 | 56.3 ± 0.6 | n | 25.0 ± 0.0 | 22.0 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Xu, L.; Xia, W.; Liang, L.; Bai, X.; Li, L.; Xu, L.; Liu, L. Mycorrhizal Compatibility and Germination-Promoting Activity of Tulasnella Species in Two Species of Orchid (Cymbidium mannii and Epidendrum radicans). Horticulturae 2021, 7, 472. https://doi.org/10.3390/horticulturae7110472
Yang Q, Xu L, Xia W, Liang L, Bai X, Li L, Xu L, Liu L. Mycorrhizal Compatibility and Germination-Promoting Activity of Tulasnella Species in Two Species of Orchid (Cymbidium mannii and Epidendrum radicans). Horticulturae. 2021; 7(11):472. https://doi.org/10.3390/horticulturae7110472
Chicago/Turabian StyleYang, Qianyu, Lijun Xu, Wei Xia, Lixiong Liang, Xiao Bai, Lubin Li, Lu Xu, and Lei Liu. 2021. "Mycorrhizal Compatibility and Germination-Promoting Activity of Tulasnella Species in Two Species of Orchid (Cymbidium mannii and Epidendrum radicans)" Horticulturae 7, no. 11: 472. https://doi.org/10.3390/horticulturae7110472
APA StyleYang, Q., Xu, L., Xia, W., Liang, L., Bai, X., Li, L., Xu, L., & Liu, L. (2021). Mycorrhizal Compatibility and Germination-Promoting Activity of Tulasnella Species in Two Species of Orchid (Cymbidium mannii and Epidendrum radicans). Horticulturae, 7(11), 472. https://doi.org/10.3390/horticulturae7110472





