Abstract
Postharvest biocontrol agents are considered a viable alternative to the use of synthetic chemicals as demonstrated by extensive research conducted by scientists and companies worldwide. In the present investigation, the biocontrol potential of a carotenoid-producing basidiomycetous yeast isolated from table grape flowers was analyzed. The strain RY1 proved to be Sporobolomyces roseus. In vitro and in vivo tests were conducted to assess its efficacy against Penicillium expansum, one of the most important postharvest pathogens and producer of the mycotoxin patulin. The yeast proved to control both fungal growth and patulin production, and, in addition, to greatly affect disease incidence and severity on apples. Its mode of action is presumably related both to the competition for nutrients and the production of antifungal volatiles. As such, although further large-scale trials are needed, our S. roseus strain represents a potential interesting biocontrol agent to be applied after harvest.
1. Introduction
It has been estimated that, in favoring circumstances, postharvest losses of fruits and vegetables can reach 50% of total production, and are mainly due to the contamination by fungi [1]. Traditionally, control of fungal phytopathogens is performed by synthetic fungicides. However, their extensive use is suspected of having detrimental effects for the environment, human, and animal health [2]. As such, the consumers’ appreciation towards pesticide-free products is continually growing, and the search for alternatives to control postharvest losses has become a worldwide priority. In recent years, several strategies have been proposed to biologically control phytopathogens, mainly based on the use of natural compounds and microbial antagonists, such as bacteria, yeasts, yeast-like fungi, and non-pathogenic fungi.
In particular, the use of epiphytic microbial antagonists against postharvest pathogens has been the focus of several research articles [3,4,5] and reviews [6,7,8]. The key justification supporting this line is that, because of controlled environmental conditions and typical disease epidemiology, the postharvest environment is more suitable for the antagonist application than the open field [7]. Interestingly, the majority of reported postharvest biocontrol agents are yeasts, since they have a high tolerance to stressful environmental conditions (low/high temperatures, wide range of relative humidity, low oxygen levels, pH fluctuations, UV radiation), as well as to those of wounded tissues (high sugar concentration osmotic pressure and low pH). Furthermore, thanks to the fermentation process, yeast can be easily produced in large quantities and even colonize dry surfaces [9]. Finally, their use is safe since they do not produce allergenic or toxic metabolites.
During an investigation of the microbiota of table grape cultivated in Apulia (Southern Italy), yeast isolates with putative biocontrol ability were collected. In the present study, the most promising one was tested against one of the main postharvest pathogens, Penicillium expansum, a causal agent of blue mold on several commodities, particularly on pome fruit. Attention was paid to the ability to control patulin production, since it is a mycotoxin with detrimental effects on human health, so that the European Commission [10] set up regulatory limits for its content in apple-derived products. Finally, we tried to establish yeast’s mode of action as a fundamental prerequisite for its future practical application.
2. Materials and Methods
2.1. Collection of Putative Biocontrol Strains
During a monitoring activity of the presence of Botrytis cinerea on berries and flower parts of various cultivars of table grapes cultivated in Apulia (Southern Italy), senescent stamens were plated on a semi-selective medium for filamentous fungi and yeasts, i.e., Potato Dextrose Agar (PDA, Conda, Madrid, Spain) amended with ampicillin and streptomycin (250 mg/L each). The plates were then inoculated by stamens (12 stamens/plate) and incubated at 24 ± 1 °C for 3–5 days in the dark. Some yeast-like colonies with putative biocontrol ability were detected. They were isolated on new PDA plates and purified through repeated streaking. Mono-cellular isolates were maintained on PDA slants at 4 ± 1 °C in the collection of the Department of Soil, Plant and Food Sciences, University of Bari Aldo Moro. A representative isolate underwent identification [11].
2.2. Biocontrol Agent and Pathogen Inoculum
For the biocontrol strain inoculum, a loop of cells of the representative yeast isolate was used to inoculate flasks containing 50 mL of Potato Dextrose Broth (PDB, Conda), which were then incubated at 24 ± 1 °C in the dark in agitated culture (150 rpm) for 48 h. Then, 2 mL of the liquid culture was centrifuged at 3000× g for 3 min; the pellet was washed twice with sterile distilled water (SDW), resuspended in SDW, and quantified by a Thoma counting chamber (HGB Henneberg-Sander GmbH, Munich, Germany). A suspension at 107 cells/mL was used for all assays following Janisiewicz et al. [12].
For the phytopathogen inoculum, a strain of P. expansum (Pex6), a strong patulin producer, from the Fungi Culture Collection of the University of Bari Aldo Moro was used. It was grown for 14 days at 24 ± 1 °C on PDA plates. Each plate was then washed with 6 mL of SDW containing 0.05% (v/v) Tween 80 (Sigma Aldrich, Milan, Italy). The resulting conidial suspension was filtered through two layers of sterile gauze and the number of conidia was quantified by a Thoma counting chamber (HGB Henneberg-Sander GmbH). A suspension at 104 conidia/mL was used for the following assays.
2.3. Biocontrol Agent Identification
For microscopic observations, a Leica DMLS microscope was used (Leica, Feasterville, PA, USA). Colonies were grown on Corn Meal Agar (CMA) and PDA (Conda) according to Lorenzini et al. [11].
For molecular identification, 2 mL of a PDB culture of the selected isolate, prepared as described above, was centrifuged at 3000× g for 3 min and the pellet was washed twice with SDW. Once dry, the pellet underwent DNA extraction following Sanzani et al. [13]. DNA was diluted at concentration 50 ng/µL and amplified using primers ITS1-ITS4 [14]. Briefly, reaction mixtures (50 µL) contained 5 µL Buffer 10X, 4 µL MgCl2 50 mM, 4 μL dNTPs mix 10 µM, 2 µL of each primer 10 µM, 0.8 µL Eurotaq 5 U/µL (Euroclone, Milan, Italy), 1 µL DNA and 33.2 µL ultra-pure sterile water. Reactions were run in the thermal cycler PCR express (Thermo Hybaid, Ashford, UK), foreseeing an initial denaturation at 95 °C for 2 min, followed by 35 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min and a final extension at 72 °C for 5 min. The amplicons were checked by electrophoresis on a 1.7% agarose gel in TBE buffer (1×) stained by GelRed® (Biotium, Inc., Landing Parkway, Fremont, CA, USA), and visualized by UV illumination (Gel Doc™ EZ Gel Imager, BioRad). Amplicons (500 bp) were purified by GenElute Gel Extraction Kit (Sigma-Aldrich) and sequenced in both directions by StarSEQ (Mainz, Germany). Sequence accuracy was evaluated by FinchTV 1.4.0 software (Geospiza, Inc., Seattle, WA, USA; http://www.geospiza.com, accessed on 12 October 2021). Strand sequences were aligned using the free software MULTALIN (http://multalin.toulouse.inra.fr/multalin/, accessed on 12 October 2021). Obtained consensus sequences were submitted to the online NCBI BLAST search and deposited in the GenBank database.
Sequences of reference strains of Sporobolomyces species (Table 1) were aligned using CLUSTALW and introduced to MEGAX for phylogenetic analysis with the Maximum Likelihood method using the Tamura-Nei model [15]. Analyses were performed with 1000 bootstrap replications.

Table 1.
GenBank accession numbers of sequences of the Sporobolomyces spp. strains used as references in phylogenetic analysis.
2.4. Biocontrol Assays
In in vitro assays, PDA plates were ideally divided into two portions: on the left one an abundant loop of cells of the antagonist was stricken; whereas, on the right one a 10 µL drop of conidial suspension of the pathogen was placed. Control plates were only inoculated with the pathogen. The assay was conducted in triplicate. The plates were then incubated at 24 ± 1 °C for 7 days. By the end of incubation, the biocontrol activity was evaluated in terms of colony diameter (mm). Furthermore, patulin production was measured by HPLC according to Sanzani et al. [16].
In in vivo assays, apples of cv. Golden Delicious, freshly harvested, free of defects or injuries, and uniform in size, colour, and ripeness were used. They were superficially sterilized by immersion for 2 min in 2% sodium hypochlorite and rinsed with running tap water for 1 min. Once dry, they were divided into 4 groups of 10 apples each, wounded (3 × 3 mm) in 4 equidistant points on the area close to the peduncle, and after 30 min, treated with 10 μL of the antagonist suspension. Untreated apples were the control. After 2 h the same wounds were inoculated with 10 μL of the pathogen conidial suspension. Finally, the fruit were stored in the dark at 20 ± 1 °C and 95% relative humidity (RH). From the 5th day of incubation, apples were inspected every two days up to 9 days, for disease incidence (infected wounds, %) and severity (lesion diameter, mm).
The same experiment was conducted also on apricot fruit cv. Kioto, with the exception that each fruit had 2 wounds and the replicates were made of 20 fruit each.
2.5. Biocontrol Mode of Action
For evaluating the antibiosis ability, yeast cells were grown in a PDB culture as reported above. The supernatant was deprived of the cells of the antagonist by centrifugation at 3000× g for 3 min and filtration through a 0.22 μm filter. Previously, 9 PDA plates were ideally divided into two portions, and in the right one a 15 mm well was created. Such well was filled with 200 μL of the cell-free supernatant or regular PDB as a control. On each plate at about 2 cm from the well, a drop of 10 μL of conidial suspension of the pathogen was placed. Finally, plates were incubated at 24 ± 1 °C for 7 days. The assay was conducted in triplicate.
For evaluating the production of volatiles, three plates were prepared with three equally distant smears of yeast cells each, and incubated at 24 ± 1 °C for 48 h. The same number of plates was inoculated in the centre with a drop of the suspension of P. expansum. The plates containing the antagonist were turned upside down, on the pathogen-inoculated plates, the contact margins were sealed with parafilm, and the stacked plates were incubated at 24 ± 1 °C for 7 days. Pathogen-inoculated plates topped by a non-inoculated PDA plate served as a control. The antifungal activity carried out by volatile substances released by antagonists was evaluated by measuring the diameter of the pathogen colony (mm) grown in each plate.
For evaluating the induction of resistance, 40 apples were superficially sterilized, wounded in four places as previously reported, and divided into two groups of twenty fruits each. All wounds were treated with 10 μL of the antagonist suspension or sterile distilled water (control). Finally, 24 h later, on each apple, 4 more wounds, about 5 mm distant from the first ones, were made and, then, inoculated with 10 μL of the conidial suspension of the pathogen. Once dry, the fruit were stored at 20 ± 1 °C and 95% RH. From the 5th day of incubation, apples were inspected every two days up to 9 days, for disease incidence (infected wounds, %) and severity (lesion diameter, mm).
For evaluating the competition for nutrients, apple fruit were surface sterilized as reported above and divided into two groups. For one of them, wounds treated with the antagonist, once dry, were added with 10 μL of a glucose solution (4 g/L), as an additional source of C. Then, after 2 h from treatment with the antagonist, the same wounds were inoculated with the suspension of the pathogen, and once dry, treated with a new rate of glucose solution. Apples treated with the antagonist and after 2 h inoculated with the pathogen served as a control. Then, the two groups were incubated at 20 ± 1 °C and 95% RH. From the 5th day of incubation, apples were inspected every two days up to 9 days, for disease incidence (infected wounds, %) and severity (lesion diameter, mm).
2.6. Data Analysis
Data were subjected to ANOVA (one-way analysis of variance). Significant differences (p ≤ 0.05) between mean levels of patulin accumulation, colony diameter, disease severity/incidence were identified by the General Linear Model (GLM) procedure with the Duncan’s Multiple Range Test (DMRT). Percentage data of disease incidence were subjected to arcsine-square-root transformation before ANOVA analysis. Data were processed using the software package Statistics for Windows (StatSoft, Tulsa, OK, USA).
The extent of the control exerted by the antagonist was expressed by a control index (CI) calculated with the following formula:
where A and B correspond to the mean infected wounds (%) or lesion diameter (mm) or mean patulin level (μg/cm2) or colony diameter (mm), measured in control (A, inoculated with P. expansum) and treated fruits/plates (B, treated with biocontrol agent and inoculated with P. expansum).
CI (%) = [(A − B)/A] × 100
3. Results
3.1. Biocontrol Agent Isolation and Identification
The microorganisms isolated from stamens of grapevine flowers grown on PDA plates had a yeast-like appearance: colonies with sharp margins, characterized by a reddish coloration, reasonably due to the production of carotenoid pigments. Micro-morphologically, true or pseudohyphae were present, as well as ballistoconidia borne on large sterigmata. Those characteristics matched those of Sporobolomyces, a genus of basidiomycetous yeast. As confirmation, the rDNA portion including ITS regions and 5.8S rDNA of the representative strain RY1 was sequenced (Genbank accession no. OK20681). BLAST analysis confirmed 100% identity with Sporobolomyces roseus (teleomorph Sporidiobolus metaroseus) strains (MN922413, KY105475). The phylogenetic analysis confirmed the identification, as reference strain RY1 clustered readily and consistently with the reference strain of S. roseus (Figure 1).
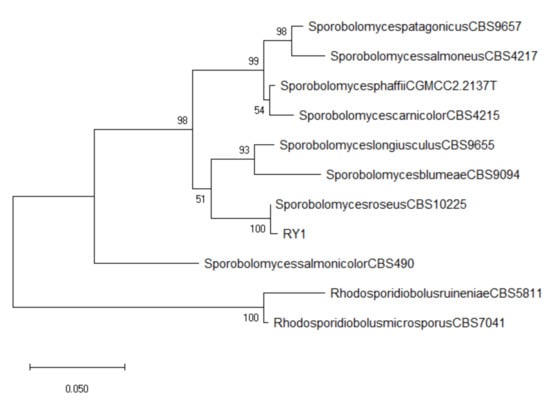
Figure 1.
Phylogenetic tree based on the ITS sequences of reference strains of Sporobolomyces spp. plus the isolate RY1 from this study. Numbers on nodes represent the maximum likelihood bootstrap percentages. Branch lengths are proportional to the number of nucleotide substitutions and measured using the bar scale (0.050). Type strains are indicated with a T.
3.2. Biocontrol Assays
Concerning the in vitro assay, the diameter of the P. expansum colonies after 5 days of incubation in presence of the biocontrol agent showed an average reduction of 52%, as compared to the control (Figure 2). In particular, while in the control plates the colony of P. expansum had an average diameter of 54.7 mm, in the presence of S. roseus the average diameter of the colony was 26.2 mm. Furthermore, patulin production in presence of the antagonist was reduced by 75%. In fact, in the control plates an average production of 181.2 µg/cm2 of plate surface was recorded, while, in presence of S. roseus, the production was 45.5 µg/cm2 (Figure 2).
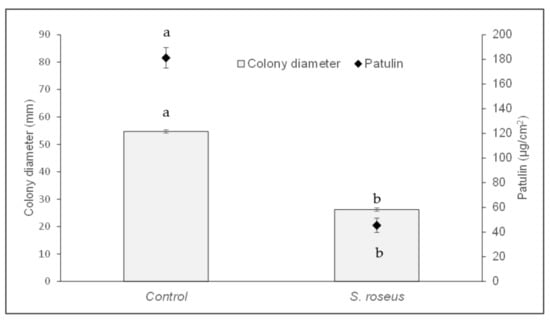
Figure 2.
Effect of Sporobolomyces roseus on Penicillium expansum colony diameter (mm) and patulin production (µg/cm2). Data are the mean of three replicates ± standard error of the mean (SEM). For each parameter, bars with different letters are statistically significant (p ≤ 0.05) according to Duncan’s Multiple Range Test (DMRT).
Concerning the in vivo assay on Golden Delicious apple, once applied into wounds, S. roseus reduced disease incidence up to 96% (Figure 3A) and the reduction remained significant for all the incubation period (9 days); similarly, a reduction of disease severity up to 98% (Figure 3B) was observed in presence of S. roseus, which remained significant for the 9 days of the monitoring.
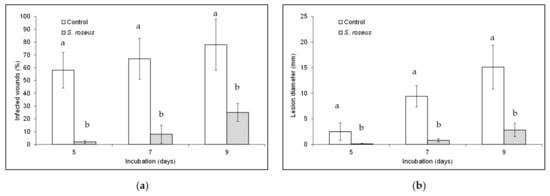
Figure 3.
Sporobolomyces roseus effect on blue mold incidence (a) and severity (b) on Golden Delicious apples. Fruit were wounded, yeast-treated, pathogen-inoculated, and stored at 20 ± 1 °C for 9 days in the dark and high relative humidity. Data are the mean of three replicates ± standard error of the mean (SEM). Bars with different letters are statistically significant (p ≤ 0.05) according to Duncan’s Multiple Range Test (DMRT).
In the same essay performed on Kioto apricots, strain RY1 proved to reduce disease incidence up to 33% (Figure 4A), while disease severity was reduced up to 58% as compared to the untreated control (Figure 4B).
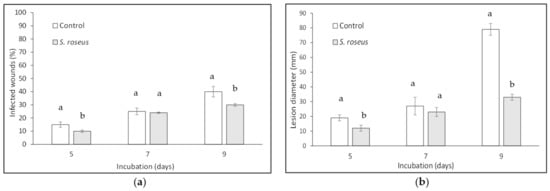
Figure 4.
Sporobolomyces roseus effect on blue mold incidence (a) and severity (b) on apricot cv. Kioto. Fruit were wounded, yeast-treated, pathogen-inoculated, and stored at 20 ± 1 °C for 9 days in the dark and high relative humidity. Data are the mean of three replicates ± standard error of the mean (SEM). Bars with different letters are statistically significant (p ≤ 0.05) according to Duncan’s Multiple Range Test (DMRT).
3.3. Evaluation of the Mode of Action
The antibiosis tests revealed no significant reduction of fungal growth by the antagonist as far as the release of water-soluble compounds concerns (Table 2). On the contrary, a 35% reduction of fungal growth was observed when the release of volatile compounds was assessed (Table 2).

Table 2.
Results of antibiosis and production of volatiles in vitro tests. Data (colony diameter, mm) are the mean of three replicates ± standard error of the mean (SEM). For each parameter, values with different letters are statistically significant (p ≤ 0.05) according to Duncan’s Multiple Range Test (DMRT).
Concerning the essay on the yeast’s ability to induce resistance in wound-treated apples, no reduction of disease incidence and severity was observed (data not shown). Whereas, when exogenous nutrients were applied together with S. roseus treatment and inoculation with P. expansum, the control exerted by the antagonist, as far as both disease incidence and severity concerned, was not significant, thus suggesting a competition for nutrients between the antagonist and the pathogen (Figure 5).
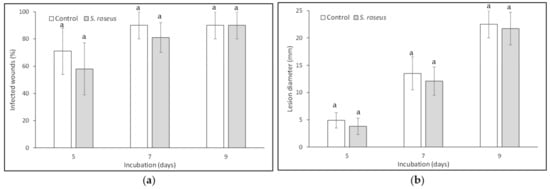
Figure 5.
Sporobolomyces roseus effect on blue mold incidence (a) and severity (b) on Golden Delicious apples. Fruit were wounded, yeast-treated, pathogen-inoculated, and stored at 20 ± 1 °C for 9 days in the dark and high relative humidity. An exogenous source of C was also added. Data are the mean of three replicates ± standard error of the mean (SEM). Bars with different letters are statistically significant (p ≤ 0.05) according to Duncan’s Multiple Range Test (DMRT).
4. Discussion
The emerging technologies for the control of postharvest fungal diseases are essentially based on the application of microbial antagonists, natural substances, and/or sanitizing products [17]. Concerning the use of biocontrol agents, much of the research has involved the use of yeasts, because of their adaptability, safety, and reproductive ability. Indeed, there are several yeast-based formulations approved for the use in the EU against postharvest diseases, as, for example, Botector (Manica S.p.A, Rovereto, Italy) based on Aureobasidium pullulans strains DSM 14940 and DSM 14941, and Next-Agrauxine based on Candida oleophila strain O (Lesaffre, France).
Epiphytic populations present in nature represent an important source of putative antagonists. Within Basidiomycetous yeasts, species of the genus Sporobolomyces are particularly represented since ubiquitous in temperate zones, being isolated frequently from various plant parts [12]. Recently, the genus has been revised to eliminate polyphyly and the dual nomenclature for asexual and sexual taxa, so that a total of 15 Sporobolomyces species are currently accepted [18]. Species belonging to the genus proved to have a high colonizing ability of plant tissues [19] and to be effective against various fungal pathogens [20,21]. In particular, Janisiewicz et al. [12] tested S. roseus strain FS-43-238 against P. expansum and B. cinerea on harvested apples; however, no data were provided on its direct effect on the fungi. Being a red yeast, S. roseus produces carotenoids. Although the physiological role of carotenoids is difficult to prove, several lines of evidence point to an antioxidant function [22]. The typical phylloplane habitat of red yeasts foresees exposure to high UV irradiation, and thus its ecological fitness would greatly benefit from a system that protects from oxidative stress.
In our investigation, we selected S. roseus strain RY1 identified by morphological observation and molecular approaches as reported by Lorenzini et al. [11]. It proved to be effective in vitro against the growth of a P. expansum strain known to be a strong patulin producer, thus suggesting having a direct antifungal effect on the pathogen. Furthermore, it proved to control patulin production, a trait which is particularly interesting, as fruits, especially of second choice, are not only consumed fresh but also destined to processing industry for producing juices, jams, purees, etc. [23]. Furthermore, apart from having a food safety significance, patulin seems to be involved in P. expansum virulence and thus its control might have significance from a disease control perspective [24]. Similarly, Ianiri et al. [25] reported that Sporobolomyces sp. strain IAM 13481 was found to be able to degrade patulin to form two different breakdown products, desoxypatulinic acid and (Z)-ascladiol. Most importantly when tested in vivo on Golden Delicious apples, our antagonist strain RY1 confirmed its ability in reducing disease incidence and severity, in agreement with Janisiewicz et al. [12]. An effective, although reduced, control ability was observed on apricot, which supported the idea that the host composition might be involved in the biocontrol strain mode of action.
As such, we also investigated the mode of action of our strain RY1, as far as the antibiosis, competition for nutrients, emission of volatiles, and induction of resistance concern. Indeed, understanding the mode of action of postharvest biocontrol agents is a fundamental prerequisite for product development and registration. We found out that RY1 seems to exert its biocontrol ability by competing for nutrients, particularly for the glucose as carbon source, with the pathogen P. expansum, as confirmed by the reversing of its control effect in presence of an exogenous additional C source. This finding agrees with the results of Fokkema [26] on the interaction between S. roseus and Cochliobolus sativus on wheat leaves. The main role of sugars in yeast is to provide energy in the form of ATP, which is necessary for cell maintenance and synthesis of essential biomass components [27]; as such, the high competitivity of RY1 for the glucose assimilation might support its efficiency as biocontrol agent. Furthermore, our results suggest a lack in the release of water-soluble compounds by S. roseus, but a significant emission of volatiles with putative antifungal properties. The production of volatile substances underlines the antagonistic activity of numerous microorganisms as Muscodor albus [28]. However, attention should be paid to assess the putative toxicity of those volatiles to humans and animals that might eventually cause a withdrawal of the biocontrol agent, as in the case mentioned above. In a recent study by Sohby et al. [29], S. roseus proved to produce substances with deterrent effects for the aphid parasitoid Aphidius ervi. This finding supports the hypothesis that the S. roseus strain RY1 releases volatile compounds, which might be detrimental or discourage pest attacks. In the above-mentioned study, acetaldehyde, ethyl acetate, and dimethyl disulphide were reported as main volatile organic compounds, whose role in yeasts’ antifungal and biofilm forming ability has been described [30,31]. In particular, acetaldehyde is known to be produced by yeasts from ethanol as well as from glucose by fermentation [32]. As such, the competitivity for glucose could be related to the production of antifungal volatiles as acetaldehyde. In fact, it has been reported that in some antagonistic microorganisms the competition for nutrients is accompanied by the production of toxic substances for the pathogens [33]. Acetaldehyde was found to affect spore germination, germ tube elongation and sporulation of Botrytis cinerea and Rhizopus stolonifer, acting on membrane integrity and respiration [34]. The multiple modes of action of RY1 might represent a useful distinctive feature for the biocontrol agent, considering that pathogens can easily develop resistance mechanisms, whereas multiple hurdles might be more difficult to overcome. Furthermore, the complex tritrophic interactions between host, pathogen, and biocontrol agent can have different outcomes from what is initially expected, so that new combinations of mechanisms may be revealed with the aid of the increasing genomic and transcriptomic information [35].
Finally, as reported by Janisiewicz et al. [12], Sporobolomyces could represent an interesting biocontrol agent, as it grows well even in absence of free water but not at 36 °C (average temperature of the human body) and can withstand the pressure of nozzles and pumps. Furthermore, S. roseus, being a psychrotrophic yeast [36], could be efficiently used for those commodities stored at low temperature, such as apples. However, specific studies on its safety for humans and animals, as well as of its released volatiles, should be conducted before a practical application.
5. Conclusions
Our strain of S. roseus RY1 proved to be effective on P. expansum primary and secondary metabolism. Its mode of action seems related to the competition for nutrients as well as the production of antifungal volatiles, although further studies are needed for their characterization and safety prior to a large-scale application.
Author Contributions
Conceptualization, S.M.S. and A.I.; methodology, S.M.S., M.S. (Michele Solfrizzo), and A.I.; validation, S.M.S., M.S. (Michele Solfrizzo), and A.I.; formal analysis, S.M.S.; investigation, S.M.S., M.S. (Michele Sgaramella), and S.M.; resources, M.S. (Michele Solfrizzo) and A.I.; data curation, S.M.S.; writing—original draft preparation, S.M.S.; writing—review and editing, S.M.S., M.S. (Michele Sgaramella), S.M., M.S. (Michele Solfrizzo), and A.I.; visualization, S.M.S.; supervision, S.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carmona-Hernandez, S.; Reyes-Pérez, J.J.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: A review. Agronomy 2019, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Nunes, C.A. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Spadaro, D.; Vola, R.; Piano, S.; Gullino, M.L. Mechanisms of action and efficacy of four isolates of the yeast Metschnikowia pulcherrima active against postharvest pathogens on apples. Postharvest Biol. Technol. 2002, 24, 123–134. [Google Scholar] [CrossRef]
- Carbó, A.; Torres, R.; Teixidó, N.; Usall, J.; Magan, N.; Medina, A. Predicted ecological niches and environmental resilience of different formulations of the biocontrol yeast Candida sake CPA-1 using the Bioscreen C. BioControl 2018, 63, 855–866. [Google Scholar] [CrossRef] [Green Version]
- Ippolito, A.; Schena, L.; Pentimone, I.; Nigro, F. Control of postharvest rots of sweet cherries by pre-and postharvest applications of Aureobasidium pullulans in combination with calcium chloride or sodium bicarbonate. Postharvest Biol. Technol. 2005, 36, 245–252. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef]
- Spadaro, D.; Ciavorella, A.; Zhang, D.; Garibaldi, A.; Gullino, M.L. Effect of culture media and pH on the biomass production and biocontrol efficacy of a Metschnikowia pulcherrima strain to be used as a biofungicide for postharvest disease control. Can. J. Microbiol. 2010, 56, 128–137. [Google Scholar] [CrossRef]
- European Commission, Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 365, 5–24.
- Lorenzini, M.; Zapparoli, G.; Azzolini, M.; Carvalho, C.; Sampaio, J.P. Sporobolomyces agrorum sp. nov. and Sporobolomyces sucorum sp. nov., two novel basidiomycetous yeast species isolated from grape and apple must in Italy. Int. J. Syst. Evol. Microbiol. 2019, 69, 3385–3391. [Google Scholar] [CrossRef] [PubMed]
- Janisiewicz, W.J.; Peterson, D.L.; Bors, R. Control of storage decay of apples with Sporobolomyces roseus. Plant Dis. 1994, 78, 466–470. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Schena, L.; De Cicco, V.; Ippolito, A. Early detection of Botrytis cinerea latent infections as a tool to improve postharvest quality of table grapes. Postharvest Biol. Technol. 2012, 68, 64–71. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogeneties. In PCR Protocols. A guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, J.W., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Sanzani, S.M.; De Girolamo, A.; Schena, L.; Solfrizzo, M.; Ippolito, A.; Visconti, A. Control of Penicillium expansum and patulin accumulation on apples by quercetin and umbelliferone. Eur. Food Res. Technol. 2009, 228, 381–389. [Google Scholar] [CrossRef]
- Mari, M.; Bertolini, P.; Pratella, G.C. Non-conventional methods for the control of post-harvest pear diseases. J. Appl. Microbiol. 2003, 94, 761–766. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.M.; Groenewald, M.; Takashima, M.; Theelen, B.; Han, P.J.; Liu, X.-Z.; Boekhout, T.; Bai, F.-Y. Phylogeny of yeasts and related filamentous fungi within Pucciniomycotina determined from multigene sequence analyses. Stud. Mycol. 2015, 81, 27–53. [Google Scholar] [CrossRef] [Green Version]
- Dik, A.J.; Fokkema, N.J.; van Pelt, J.A. Influence of climatic and nutritional factors on yeast population dynamics in the phyllosphere of wheat. Micro. Ecol. 1992, 23, 41–52. [Google Scholar] [CrossRef]
- Fokkema, N.J.; Den Houter, J.G.; Kosterman, Y.J.C.; Nelis, A.L. Manipulation of yeasts on field-grown wheat leaves and their antagonistic effect on Cochliobolus sativus and Septoria nodorum. Trans. Br. Mycol. Soc. 1979, 72, 19–29. [Google Scholar] [CrossRef]
- Tyagi, S.; Dube, V.P.; Charaya, M.U. Biological control of the purple blotch of onion caused by Alternaria porri (Ellis) Ciferri. Int. J. Pest Manag. 1990, 36, 384–386. [Google Scholar]
- Davoli, P.; Mierau, V.; Weber, R.W.S. Carotenoids and fatty acids in red yeasts Sporobolomyces roseus and Rhodotorula glutinis. Appl. Biochem. Microbiol. 2004, 40, 392–397. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Reverberi, M.; Geisen, R. Mycotoxins in harvested fruits and vegetables: Insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biol. Technol. 2016, 122, 95–105. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Reverberi, M.; Punelli, M.; Ippolito, A.; Fanelli, C. Study on the role of patulin on pathogenicity and virulence of Penicillium expansum. Int. J. Food Microbiol. 2012, 153, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Ianiri, G.; Idnurm, A.; Wright, S.A.; Durán-Patrón, R.; Mannina, L.; Ferracane, R.; Ritieni, A.; Castoria, R. Searching for genes responsible for patulin degradation in a biocontrol yeast provides insight into the basis for resistance to this mycotoxin. Appl. Environ. Microbiol. 2013, 79, 3101–3115. [Google Scholar] [CrossRef] [Green Version]
- Fokkema, N.J. Competition for endogenous and exogenous nutrients between Sporobolomyces roseus and Cochliobolus sativus. Can. J. Bot. 1984, 62, 2463–2468. [Google Scholar] [CrossRef]
- Fredlund, E.; Druvefors, U.; Lingsten, K.-J.; Boysen, M.E.; Schnürer, J. 2002. Physiological characteristics of the biocontrol yeast Pichia anomala J121. FEMS Yeast Res. 2002, 2, 395–402. [Google Scholar] [PubMed]
- Strobel, G.A.; Kluck, K.; Hess, W.M.; Sears, J.; Ezra, D.; Vargas, P.N. Muscodor albus E-6, an endophyte of Guazuma ulmifolia making volatile antibiotics: Isolation, characterization and experimental establishment in the host plant. Microbiology 2007, 153, 2613–2620. [Google Scholar] [CrossRef] [Green Version]
- Sobhy, I.S.; Goelen, T.; Herrera-Malaver, B.; Verstrepen, K.J.; Wäckers, F.; Jacquemyn, H.; Lievens, B. Associative learning and memory retention of nectar yeast volatiles in a generalist parasitoid. Anim. Behav. 2019, 153, 137–146. [Google Scholar] [CrossRef]
- Chauhan, N.M.; Raut, J.S.; Karuppayil, S.M. Acetaldehyde inhibits the yeast-to-hypha conversion and biofilm formation in Candida albicans. Mycoscience 2011, 52, 356–360. [Google Scholar] [CrossRef]
- Contarino, R.; Brighina, S.; Fallico, B.; Cirvilleri, G.; Parafati, L.; Restuccia, C. Volatile organic compounds (VOCs) produced by biocontrol yeasts. Food Microbiol. 2019, 82, 70–74. [Google Scholar] [CrossRef]
- Uittamo, J.; Siikala, E.; Kaihovaara, P.; Salaspuro, M.; Rautemaa, R. Chronic candidosis and oral cancer in APECED-patients: Production of carcinogenic acetaldehyde from glucose and ethanol by Candida albicans. Int. J. Cancer 2009, 124, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.K.; McSpadden Gardener, B. Biological Control of Plant Pathogens. The Plant Health Instructor. Available online: https://www.apsnet.org/edcenter/disimpactmngmnt/topc/Documents/PHI-BiologicalControl.pdf (accessed on 20 September 2021).
- Avissar, I.; Droby, S.; Pesis, E. Characterisation of acetaldehyde effects on Rhizopus stolonifer and Botrytis cinerea. Ann. Appl. Biol. 1990, 116, 213–220. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Białkowska, A.M.; Krysiak, J.; Florczak, T.; Szulczewska, K.M.; Wanarska, M.; Turkiewicz, M. The psychrotrophic yeast Sporobolomyces roseus LOCK 1119 as a source of a highly active aspartic protease for the in vitro production of antioxidant peptides. Biotechnol. Appl. Biochem. 2018, 65, 726–738. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).