Morpho-Anatomical and Biochemical Characterization of Embryogenic and Degenerative Embryogenic Calli of Phoenix dactylifera L.
Abstract
1. Introduction
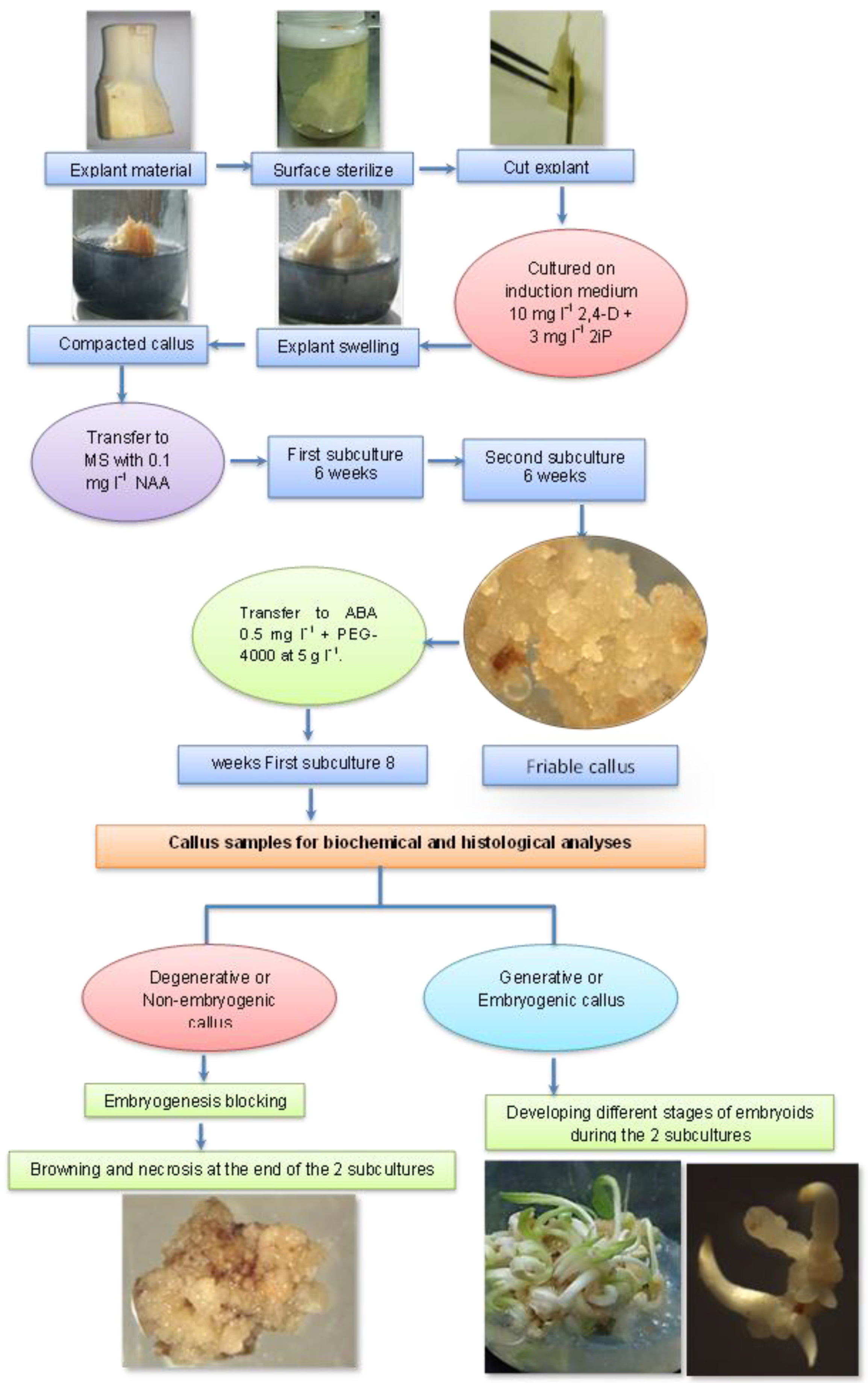
2. Material and Methods
2.1. Experimental Layout
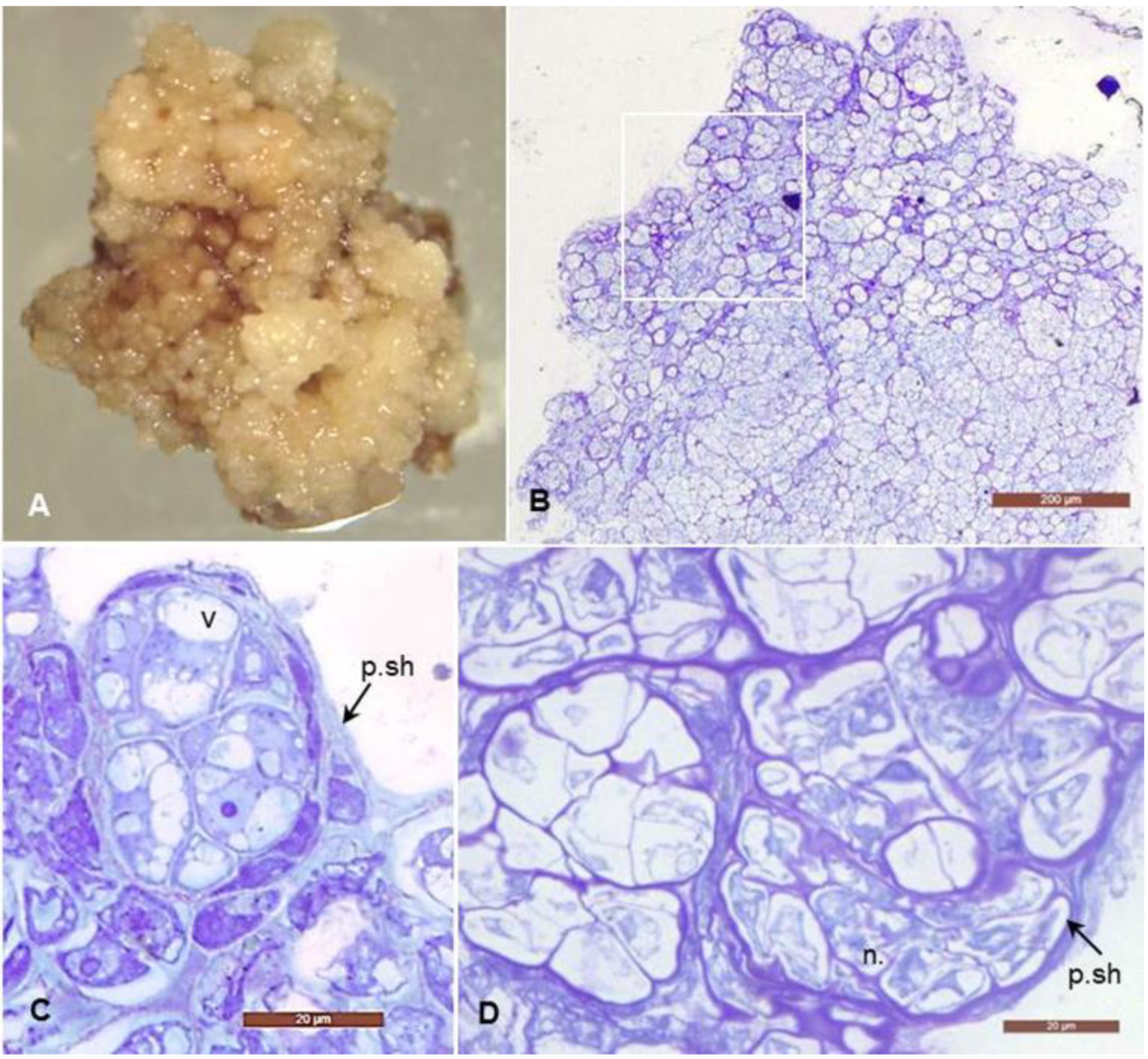
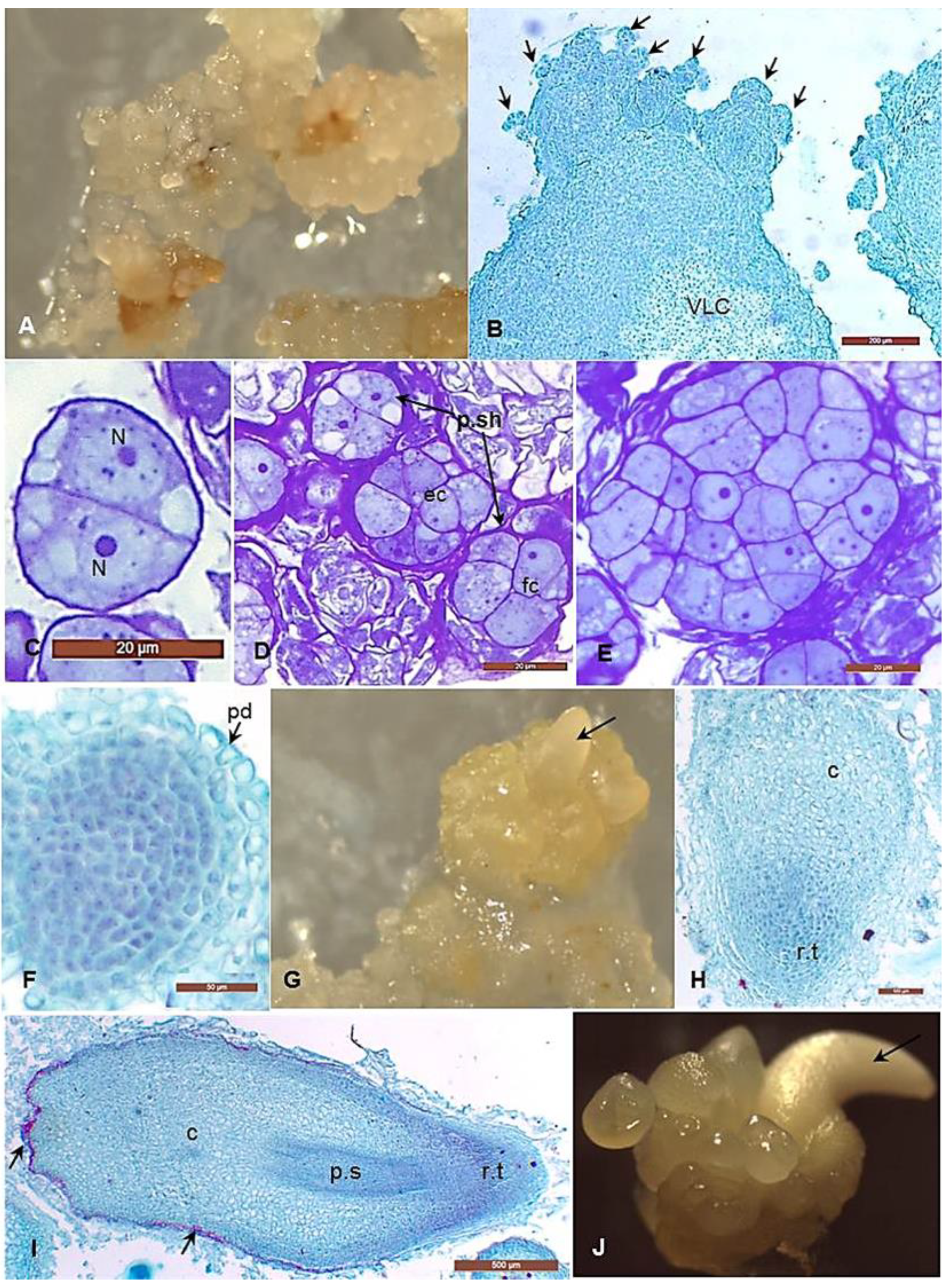
2.2. Histological Analyses
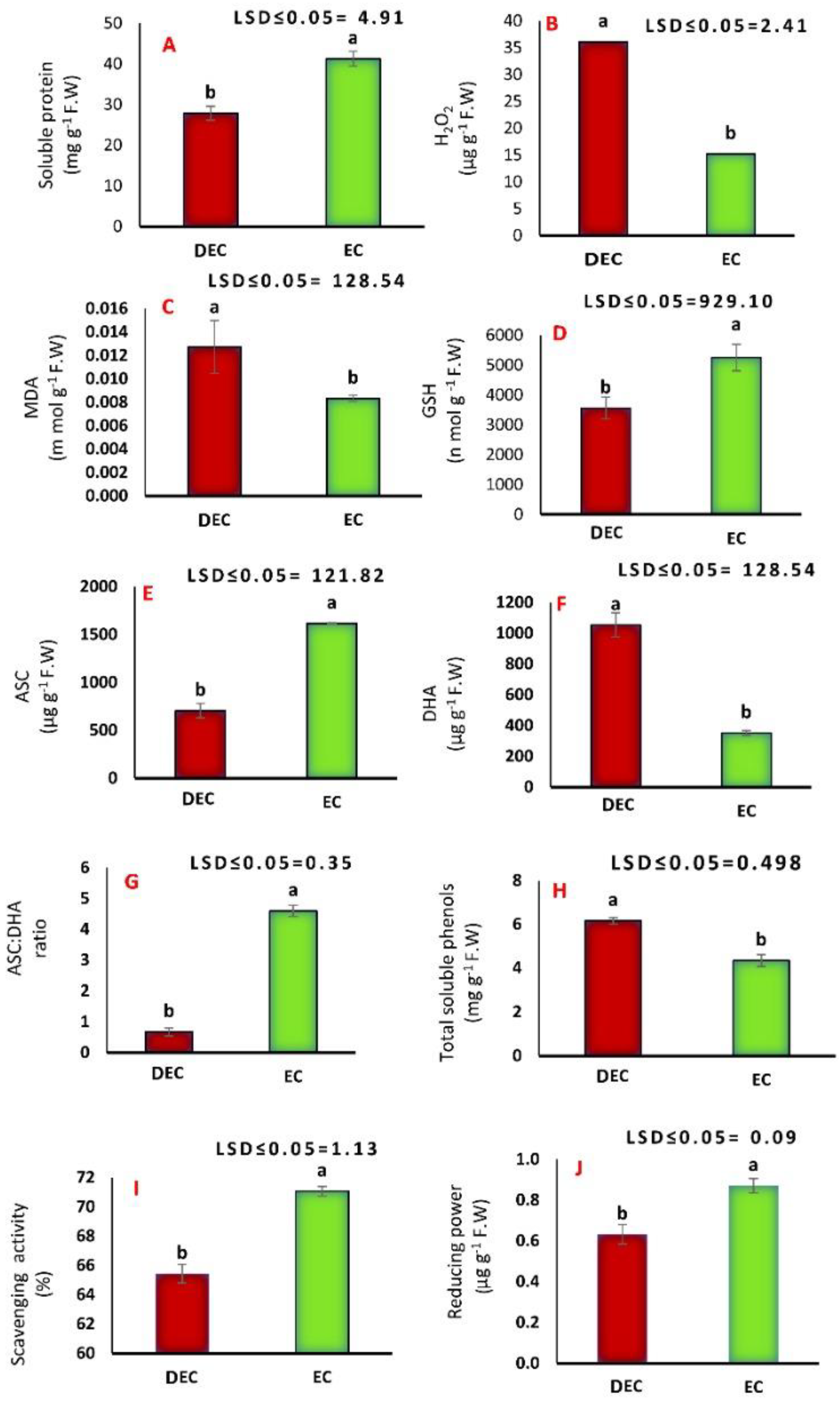
2.3. Biochemical Analyses
2.3.1. Soluble Proteins, Hydrogen Peroxide and Malondialdehyde Determination
2.3.2. Reduced Glutathione, Ascorbic Acid, Dehydroascorbate, Total Phenolic Compounds and Antioxidant Activity Determination
2.3.3. Polyphenolic Compounds Analysis by HPLC
2.4. Statistical Analysis
3. Results
3.1. Morphological and Histological Analyses of Degenerative Embryogenic Callus (DEC)
3.2. Morphological and Histological Analyses of the Embryogenic Callus and the Successive Developmental Stages of Somatic Embryos
3.3. Biochemical Analyses
3.4. Fractionation of Polyphenolic Compounds
4. Discussion
4.1. Histological Analysis
4.2. Biochemical Analyses
4.3. Fractionation of Polyphenolic Compounds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bellaj, E.M.; Hadrami, E.I. Characterization of two constitutive hyroxycinnamic acids derivatives in date palm (Phoenix dactylifera L.) callus in relation with tissue browning. Biotechnology 2004, 3, 155–159. [Google Scholar] [CrossRef][Green Version]
- Morel, A.; Trontin, J.F.; Corbineau, F.; Lomenech, A.M.; Beaufour, M.; Reymond, I.; Le Metté, C.; Ader, K.; Harvengt, L.; Cadene, M.; et al. Cotyledonary somatic embryos of Pinus pinaster Ait. most closely resemble fresh, maturing cotyledonary zygotic embryos: Biological, carbohydrate and proteomic analyses. Planta 2014, 240, 1075–1095. [Google Scholar] [CrossRef] [PubMed]
- Nic-Can, G.I.; Galaz-Ávalos, R.M.; De-la-Peña, C.; Alcazar-Magaña, A.; Wrobel, K.; Loyola-Vargas, V.M. Somatic Embryogenesis: Identified Factors that Lead to Embryogenic Repression. A Case of Species of the Same Genus. PLoS ONE 2015, 10, e0126414. [Google Scholar] [CrossRef]
- Silveira, V.; de Vita, A.M.; Macedo, A.F.; Dias, M.F.R.; Floh, E.I.S.; Santa-Catarina, C. Morphological and polyamine content changes in embryogenic and non-embryogenic callus of sugarcane. Plant Cell Tissue Organ Cult. 2013, 114, 351–364. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Naik, P.M. Date palm micropropagation: Advances and applications. Ciência Agrotecnologia 2017, 41, 347–358. [Google Scholar] [CrossRef]
- Cangahuala-Inocente, G.C.; Silveira, V.; Caprestano, C.A.; Floh, E.I.S.; Guerra, M.P. Dynamics of physiological and biochemical changes during somatic embryogenesis of Acca sellowiana. In Vitro Cell. Dev. Biol. Plant. 2014, 50, 166–175. [Google Scholar] [CrossRef]
- Cui, K.R.; Xing, G.S.; Liu, X.M.; Xing, G.M.; Wang, Y.F. Effect of the hydrogen peroxide on somatic embryogenesis of Lycium barbatum L. Plant Sci. 1999, 146, 9–16. [Google Scholar] [CrossRef]
- Fatima, S.; Mujib, A.; Samaj, J. Anti-oxidant enzyme responses during in vitro embryogenesis in Catharanthus roseus. J. Hortic. Sci. Biotechnol. 2011, 86, 569–574. [Google Scholar] [CrossRef]
- Manivannan, A.; Jana, S.; Soundararajan, P.; Ko, C.H.; Jeong, B.R. Antioxidant enzymes metabolism and cellular differentiation during the developmental stages of somatic embryogenesis in Torilis japonica (Houtt.) DC. Plant Omics J. 2015, 8, 461–471. [Google Scholar]
- Eldin, Z.A.F.M.; Ibrahim, H.A. Some biochemical changes and activities of antioxidant enzymes in developing date palm somatic and zygotic embryos in vitro. Ann. Agric. Sci. 2015, 60, 121–130. [Google Scholar] [CrossRef]
- Pullman, G.S.; Zeng, X.; Copeland-Kamp, B.; Crockett, J.; Lucrezi, J.; May, S.W.; Bucalo, K. Conifer somatic embryogenesis: Improvements by supplementation of medium with oxidation–reduction agents. Tree Physiol. 2015, 35, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Reis, E.; Batista, M.T.; Canhoto, J.M. Effect and analysis of phenolic compounds during somatic embryogenesis induction in Feijoa sellowiana Berg. Protoplasma 2008, 232, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.C.; de los Angeles Blanco, M.; Peláez, O.; González, A.; Cid, M.; Iglesias, A.; González, B.; Escalona, M.; Espinosa, P.; Borroto, C. Sugarcane micropropagation and phenolic excretion. Plant Cell Tissue Organ Cult. 2001, 65, 1–8. [Google Scholar] [CrossRef]
- Cvikrová, M.; Hrubcová, M. The role of phenolic substances in the processes of differentiation and morphogenesis. In Advances in Regulation of Plant Development; Strnad, M., Pec, P., Beck, E., Eds.; Peres Publications: Prague, Czech Republic, 1999; pp. 213–220. [Google Scholar]
- Fry, S.C. Cross-Linking of Matrix Polymers in the Growing Cell Walls of Angiosperms. Annu. Rev. Plant Physiol. 1986, 37, 165–186. [Google Scholar] [CrossRef]
- Othmani, A.; Bayoudh, C.; Drira, N.; Marrakchi, M.; Trifi, M. Somatic embryogenesis and plant regeneration in date palm Phœnix dactylifera L., cv. Boufeggous is significantly improved by fine chopping and partial desiccation of embryogenic callus. Plant Cell Tissue Organ Cult. 2009, 97, 71–79. [Google Scholar] [CrossRef]
- Tisserat, B.; Demason, D.A. A Histological Study of Development of Adventive Embryos in Organ Cultures of Phoenix dactylifera L. Ann. Bot. 1980, 46, 465–472. [Google Scholar] [CrossRef]
- Sane, D.; Aberlenc-Bertossi, F.; Gassama-Dia, Y.K.; Sagna, M.; Trouslot, M.F.; Duval, Y.; Borgel, A. Histocytological Analysis of Callogenesis and Somatic Embryogenesis from Cell Suspensions of Date Palm (Phoenix dactylifera). Ann. Bot. 2006, 98, 301–308. [Google Scholar] [CrossRef]
- Abd El Bar, O.H.; El Dawayati, M.M. Histological changes on regeneration in vitro culture of date palm 457 (Phoenix dactylifera) leaf explants. Aust. J. Crop. Sci. 2014, 8, 848–855. [Google Scholar]
- Zayed, E.M.M.; Abdelbar, O.H. Morphogenesis of immature female inflorescences of date palm in vitro. Ann. Agric. Sci. 2015, 60, 113–120. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Frankl, A.; Mari, M. Electron microscopy for ultrastructural analysis and protein localization in Saccharomyces cerevisiae. Microb. Cell 2015, 2, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Abdelbar, O.H. Histological Analysis of the Developmental Stages of Direct Somatic Embryogenesis Induced from in Vitro Leaf Explants of Date Palm. Methods Mol. Biol. 2017, 1637, 145–162. [Google Scholar] [CrossRef]
- Berlyn, G.P.; Miksche, J.P.; Sass, J.E. Botanical Microtechnique and Cytochemistry; Iowa State University Press: Ames, IA, USA, 1976; pp. 24–100. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Guo, Z.; Tan, H.; Zhu, X. A simple colorimetric method for determination of hydrogen peroxide in plant tissues. Plant Growth Regul. 2006, 49, 113–118. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Kapour, A.; Haskovic, A.; Copra Janicijevic, A.; Klepo, L.; Topcagic, A.; Tahirovic, I.; Sofic, E. Spectrophotometric analysis of total ascorbic content in various fruits and vegetables. Bull. Chem. Technol. Bosnia Herzeg. 2012, 38, 39–42. [Google Scholar]
- Shahidi, F.; Naczk, M. Methods of analysis and quantification of phenolic compounds. In Food Phenolic: Sources, Chemistry, Effects and Applications; Technomic Publishind Company, Inc.: Lancaster, PA, USA, 1995; pp. 287–293. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Jananese J. Nutr. 1986, 44, 307–325. [Google Scholar]
- Gökbulut, A. Validated RP-HPLC Method for Quantification of Phenolic Compounds in Methanol Extracts of Aerial Parts and Roots of Thymus sipyleus and Evaluation of Antioxidant Potential. Trop. J. Pharm. Res. Oct. 2015, 14, 1871–1877. [Google Scholar] [CrossRef]
- Statistix. Statistix 9: Analytical Software; Statistix: Tallahassee, FL, USA, 2009. [Google Scholar]
- López Arnaldos, T.; Muñoz, R.; Ferrer, M.A.; Calderón, A.A. Changes in phenol content during strawberry (Fragaria×ananassa, cv. Chandler) callus culture. Physiol. Plant. 2001, 113, 315–322. [Google Scholar] [CrossRef]
- Nakamura, M.; Seki, M.; Furusaki, S. Enhanced anthocyanin methylation by growth limitation in strawberry suspension culture. Enzym. Microb. Technol. 1998, 22, 404–408. [Google Scholar] [CrossRef]
- Fki, L. Date Palm Micropropagation via Somatic Embryogenesis. In Date Palm Biotechnology; Jain, S., Al-Khayri, J., Johnson, D., Eds.; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Gomes, H.T.; Bartos, P.M.C.; Silva, C.O.; do Amaral, L.I.V.; Scherwinski-Pereira, J.E. Comparative biochemical profiling during the stages of acquisition and development of somatic embryogenesis in African oil palm (Elaeis guineensis Jacq.). Plant Growth Regul. 2014, 74, 199–208. [Google Scholar] [CrossRef]
- Kairong, C.; Ji, L.; Gengmei, X.; Jianlong, L.; Lihong, W.; Yafu, W. Effect of hydrogen peroxide on synthesis of proteins during somatic embryogenesis in Lycium barbarum. Plant Cell Tissue Organ Cult. 2002, 68, 187–193. [Google Scholar] [CrossRef]
- El-Hadrami, I.; Baaziz, M. Somatic embryogenesis and analysis of peroxidases in Phoenix dactyfifera L. Biol. Plant. 1995, 37, 196–203. [Google Scholar]
- Nurnaeimah, N.; Mat, N.; Suryati Mohd, K.; Badaluddin, N.A.; Yusoff, N.; Sajili, M.H.; Mahmud, K.; Mohd Adnan, A.F.; Khandaker, M.M. The Effects of Hydrogen Peroxide on Plant Growth, Mineral Accumulation, as Well as Biological and Chemical Properties of Ficus deltoidea. Agronomy 2020, 10, 599. [Google Scholar] [CrossRef]
- Pasternak, T.; Potters, G.; Caubergs, R.; Jansen, M.A. Complementary interactions between oxidative stress and auxins control plant growth responses at plant, organ, and cellular level. J. Exp. Bot. 2005, 56, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Zhou, L.; Xu, X.; Cao, J.; Xi, T. The role of salicylic acid and carrot embryogenic callus extracts in somatic embryogenesis of naked oat (Avena nuda). Plant Cell Tissue Organ Cult. 2006, 85, 109–113. [Google Scholar] [CrossRef]
- Adams, L.K.; Benson, E.E.; Staines, H.J.; Bremnerl, D.H.; Millam, S.; Deighton, N. Effects of the Lipid Peroxidation Products 4-Hydroxy-2- Nonenal and Malondialdehyde on the Proliferation and Morphogenetic Development of in vitro Plant Cells. J. Plant Physiol. 1999, 155, 376–386. [Google Scholar] [CrossRef]
- Taqi, A.K.; Mazid, M.; Mohammad, F. A review of ascorbic acid potentialities against oxidative stress induced in plants. J. Agrobiol. 2011, 28, 97–111. [Google Scholar] [CrossRef]
- Mapson, L.W.; Moustafa, E.M. Ascorbic acid and glutathione as respiratory carriers in the respiration of pea seedlings. Biochem. J. 1956, 62, 248–259. [Google Scholar] [CrossRef]
- Takahama, U.; Oniki, T. The Association of Ascorbate and Ascorbate Oxidase in the Apoplast with IAA-Enhanced Elongation of Epicotyls from Vigna angularis. Plant Cell Physiol. 1994, 35, 257–266. [Google Scholar] [CrossRef]
- Abdellatif, Y.M.R.; Ibrahim, M.T.S. Non-enzymatic anti-oxidants potential in enhancing Hibiscus sabdariffa L. tolerance to oxidative stress. Int. J. Bot. 2018, 14, 43–58. [Google Scholar] [CrossRef]
- Stasolla, C.; Yeung, E.C. Ascorbic acid improves conversion of white spruce somatic embryos. In Vitro Cell. Dev. Biol. Plant 1999, 35, 316–319. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A.H. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamary, M.; Al-Habori, M.; Al-Zubairi, A.S. The in vitro antioxidant activity of different types of palm dates(Phoenix dactylifera) syrups Arab. J. Chem. 2014, 7, 964–971. [Google Scholar]
- Cvikrová, M.; Malá, J.; Hrubcová, M.; Eder, J.; Zoń, J.; Macháčková, I. Effect of inhibition of biosynthesis of phenylpropanoids on sessile oak somatic embryogenesis. Plant Physiol. Biochem. 2003, 41, 251–259. [Google Scholar] [CrossRef]
- Bido, G.D.; Ferrarese, M.D.L.; Marchiosi, R.; Ferrarese, O. Naringenin inhibits the growth and stimulates the lignification of soybean root. Braz. Arch. Biol. Technol. 2010, 53, 533–542. [Google Scholar] [CrossRef]
- Steenackers, W.; Klíma, P.; Quareshy, M.; Cesarino, I.; Kumpf, R.P.; Corneillie, S.; Araújo, P.; Viaene, T.; Goeminne, G.; Nowack, M.K.; et al. cis-Cinnamic Acid Is a Novel, Natural Auxin Efflux Inhibitor That Promotes Lateral Root Formation. Plant Physiol. 2017, 173, 552–565. [Google Scholar] [CrossRef]
- Kurepa, J.; Shull, T.E.; Karunadasa, S.S.; Smalle, J.A. Modulation of auxin and cytokinin responses by early steps of the phenylpropanoid pathway. BMC Plant Biol. 2018, 18, 278. [Google Scholar] [CrossRef]
- Volpert, R.; Osswald, W.; Elstner, E.F. Effects of cinnamic acid derivatives on indole acetic acid oxidation by peroxidase. Phytochemistry 1995, 38, 19–22. [Google Scholar] [CrossRef]
- Mnich, E.; Bjarnholt, N.; Eudes, A.; Harholt, J.; Holland, C.; Jørgensen, B.; Larsen, F.H.; Liu, M.; Manat, R.; Meyer, A.S.; et al. Phenolic cross-links: Building and de-constructing the plant cell wall. Nat. Prod. Rep. 2020, 37, 919–961. [Google Scholar] [CrossRef]
- Bunzel, M. Phenolic Compounds as Cross-Links of Plant Derived Polysaccharides. Czech J. Food Sci. Potravinářské Vědy 2004, 22, 64. [Google Scholar] [CrossRef]
- Oudgenoeg, G.; Hilhorst, R.; Piersma, S.R.; Boeriu, C.G.; Gruppen, H.; Hessing, M.; Voragen, A.G.; Laane, C. Peroxidase-mediated cross-linking of a tyrosine-containing peptide with ferulic acid. J. Agric. Food Chem. 2001, 49, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Alturki, S.M.; Wael, F.S.; Mohammed, I.A. Influence of Nutrient Medium on Antioxidants Production of Date Palm (Phoenix dactylifera L.) Cultivars in vitro. Asian J. Plant Sci. 2013, 12, 119–127. [Google Scholar] [CrossRef][Green Version]
- Khan, A.; Nazar, S.; Lang, I.; Nawaz, H.; Hussain, M.A. Effect of ellagic acid on growth and physiology of canola (Brassica napus L.) under saline conditions. J. Plant Interact. 2017, 12, 520–525. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Kandasamy, D.; Ullah, C.; Schmidt, A.; Wright, L.P.; Gershenzon, J. Flavanone-3-Hydroxylase Plays an Important Role in the Biosynthesis of Spruce Phenolic Defenses Against Bark Beetles and Their Fungal Associates. Front. Plant Sci. 2019, 10, 208. [Google Scholar] [CrossRef]
- Torel, J.; Cillard, J.; Cillard, P. Antioxidant activity of flavonoids and reactivity with peroxy radical. Phytochemistry 1986, 25, 383–385. [Google Scholar] [CrossRef]
- Mathesius, U. Flavonoids induced in cells undergoing nodule organogenesis in white clover are regulators of auxin breakdown by peroxidase. J. Exp. Bot. 2001, 52, 419–426. [Google Scholar] [CrossRef] [PubMed]
| Phenolic Compound µg mg−1 F.W | Degenerative Embryogenic Callus | Embryogenic Callus | LSD ≤ 0.05 |
|---|---|---|---|
| Cinnamic | 0.014 a ± 0.002 | 0.000 b ± 0.00 | 4.241 × 10−3 |
| P-Coumaric | 0.000 b ± 0.00 | 0.603 a ± 0.03 | 0.0485 |
| Ferulic | 0.271 a ± 0.03 | 0.133 b ± 0.00 | 0.0514 |
| Chlorogenic | 43.991 a ± 14.76 | 37.082 a ± 2.13 | 23.897 |
| Caffeic | 1.351 a ± 0.32 | 1.656 a ± 0.43 | 0.8483 |
| Gallic | 43.323 b ± 10.09 | 58.820 a ± 9.68 | 14.808 |
| Vanillic | 0.000 b ± 0.00 | 0.146 a ± 0.03 | 0.040 |
| Ellagic | 1.016 b ± 0.08 | 2.226 a ± 0.13 | 0.2504 |
| Naringenin | 16.247 a ± 0.77 | 2.527 b ± 0.40 | 1.3894 |
| Catechin | 0.743 a ± 0.088 | 0.000 b ± 0.00 | 0.1401 |
| Rutin | 0.000 b ± 0.00 | 0.413 a ± 0.00 | 0.0515 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zein El Din, A.F.M.; Abd Elbar, O.H.; Al Turki, S.M.; Ramadan, K.M.A.; El-Beltagi, H.S.; Ibrahim, H.A.; Gadalla, E.G.; Shams El-Din, I.M.; Ibrahim, I.S.; Farag, R.; et al. Morpho-Anatomical and Biochemical Characterization of Embryogenic and Degenerative Embryogenic Calli of Phoenix dactylifera L. Horticulturae 2021, 7, 393. https://doi.org/10.3390/horticulturae7100393
Zein El Din AFM, Abd Elbar OH, Al Turki SM, Ramadan KMA, El-Beltagi HS, Ibrahim HA, Gadalla EG, Shams El-Din IM, Ibrahim IS, Farag R, et al. Morpho-Anatomical and Biochemical Characterization of Embryogenic and Degenerative Embryogenic Calli of Phoenix dactylifera L. Horticulturae. 2021; 7(10):393. https://doi.org/10.3390/horticulturae7100393
Chicago/Turabian StyleZein El Din, Amal F. M., Ola H. Abd Elbar, Saleh M. Al Turki, Khaled M. A. Ramadan, Hossam S. El-Beltagi, Hemmat A. Ibrahim, Ezzeldin G. Gadalla, Ibrahim M. Shams El-Din, Ibrahim S. Ibrahim, Reham Farag, and et al. 2021. "Morpho-Anatomical and Biochemical Characterization of Embryogenic and Degenerative Embryogenic Calli of Phoenix dactylifera L." Horticulturae 7, no. 10: 393. https://doi.org/10.3390/horticulturae7100393
APA StyleZein El Din, A. F. M., Abd Elbar, O. H., Al Turki, S. M., Ramadan, K. M. A., El-Beltagi, H. S., Ibrahim, H. A., Gadalla, E. G., Shams El-Din, I. M., Ibrahim, I. S., Farag, R., Ibrahim, M. F. M., Abd El-Aal, M. S., El-Yazied, A. A., El-Mogy, M. M., Samaan, M. S. F., & Abdellatif, Y. M. R. (2021). Morpho-Anatomical and Biochemical Characterization of Embryogenic and Degenerative Embryogenic Calli of Phoenix dactylifera L. Horticulturae, 7(10), 393. https://doi.org/10.3390/horticulturae7100393












