Water Stress in Dwarfing Cherry Rootstocks: Increased Carbon Partitioning to Roots Facilitates Improved Tolerance of Drought
Abstract
:1. Introduction
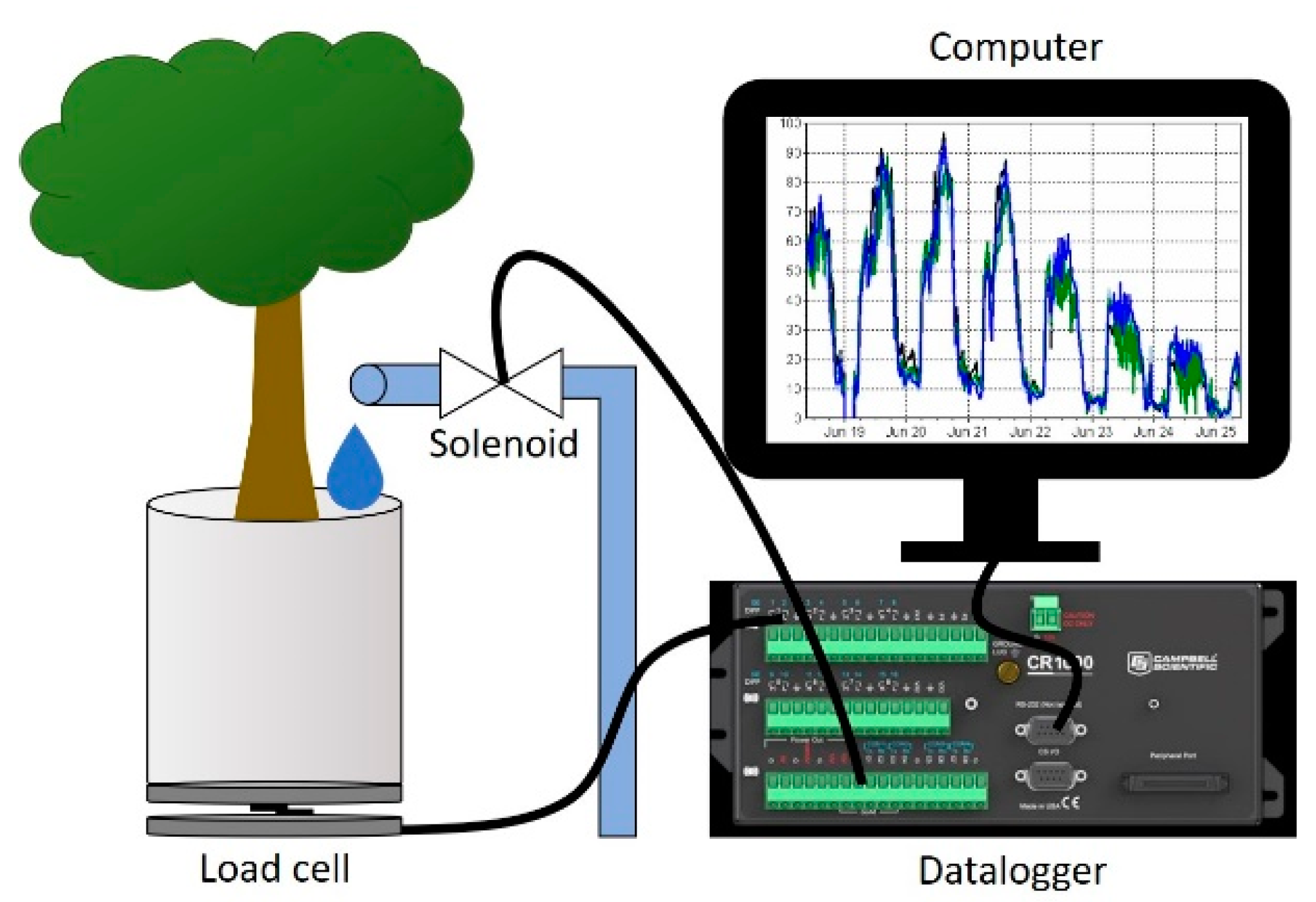
2. Materials and Methods
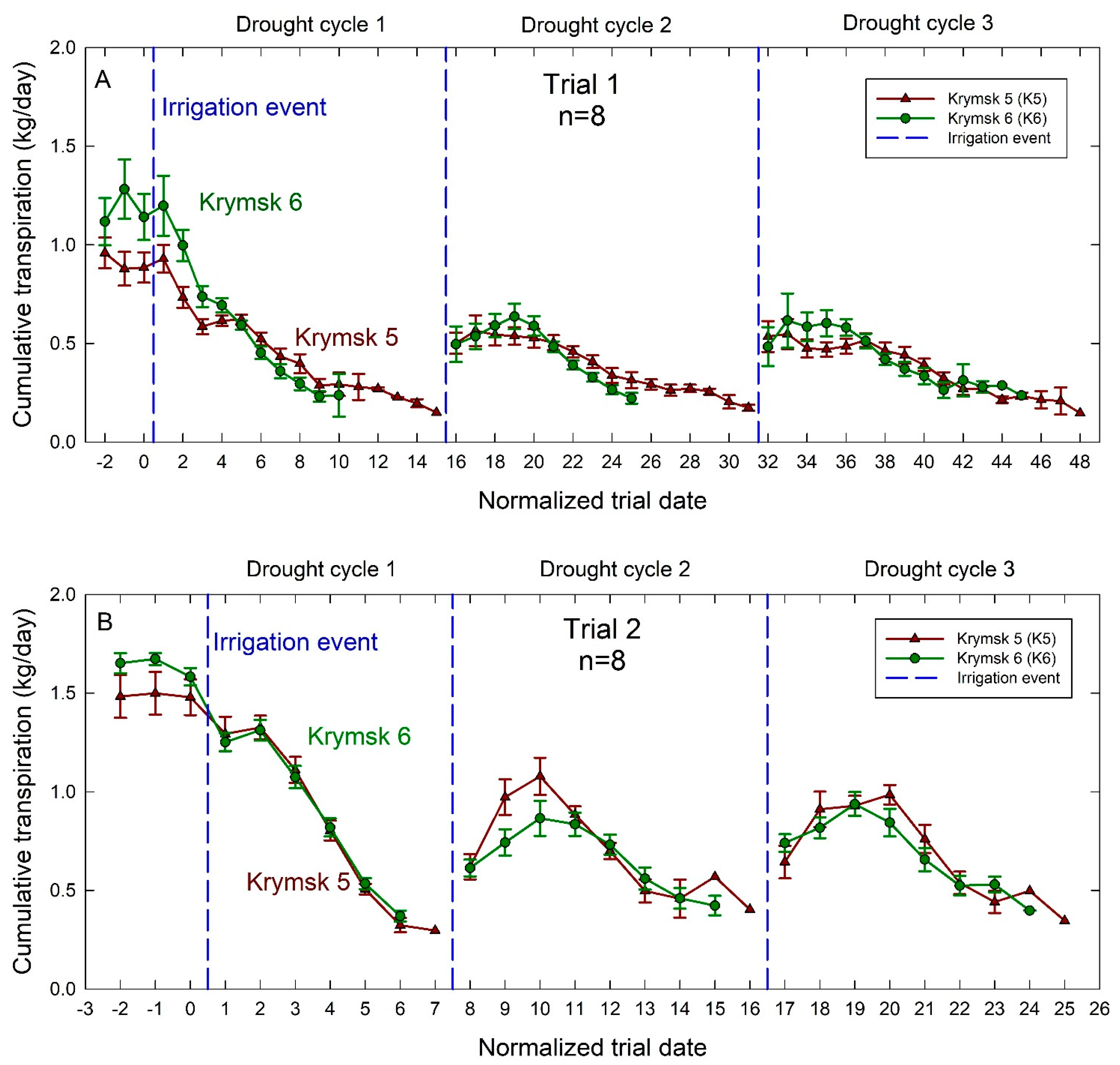
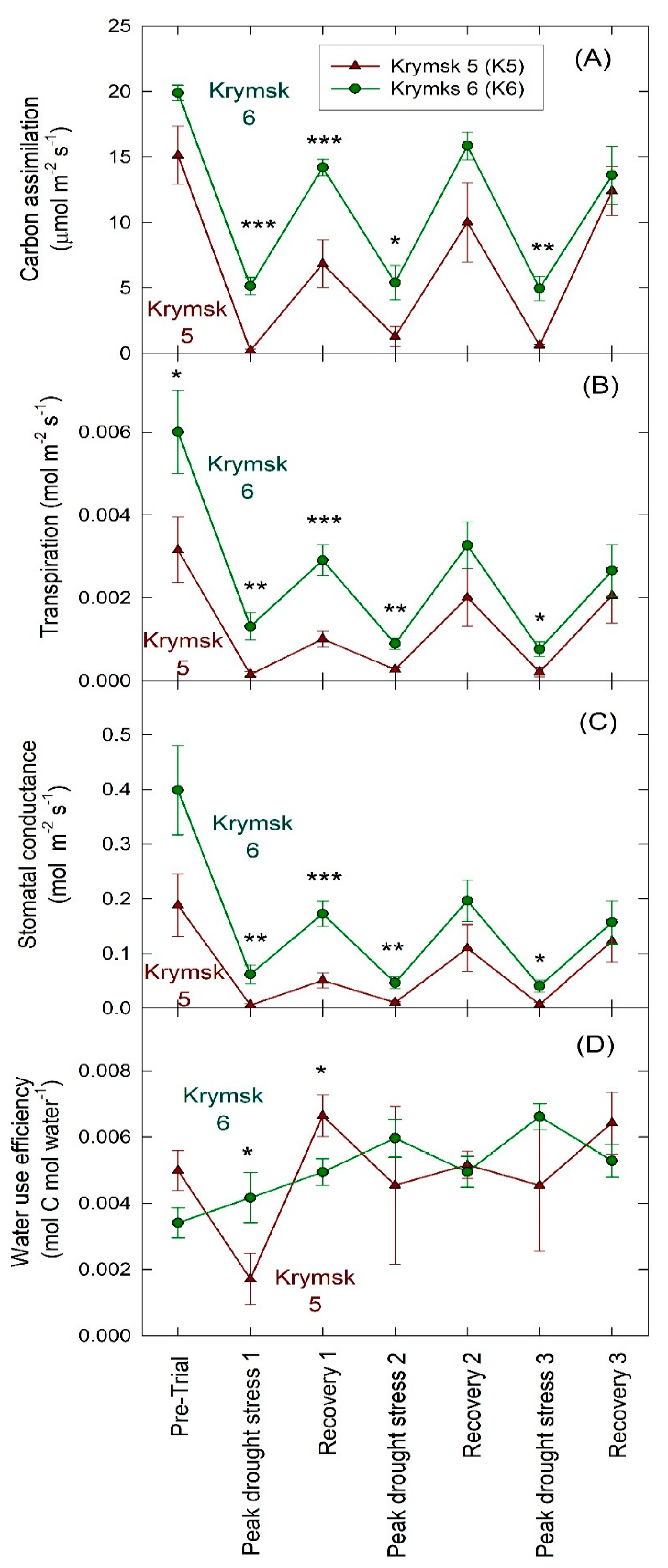
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milošević, T.; Milošević, N.; Mladenović, J. Combining fruit quality and main antioxidant attributes in the sour cherry: The role of new clonal rootstock. Sci. Hortic. 2020, 265, 109236. [Google Scholar] [CrossRef]
- Lang, G.A. The cherry industries in the USA: Current trends and future perspectives. In Proceedings of the VIII International Cherry Symposium, Yamagata, Japan, 28 February 2019; pp. 119–132. [Google Scholar]
- Morandi, B.; Manfrini, L.; Lugli, S.; Tugnoli, A.; Micheli, A.; Boini, A.; Perulli, G.; Bresilla, K.; Corelli Grappadelli, L. Physiological responses to rootstocks vigor in cherry: Why dwarfing is efficient? In Proceedings of the XXX International Horticultural Congress IHC2018: International Symposium on Cultivars, Rootstocks and Management Systems of 1281, Istanbul, Turkey, 12 August 2018; pp. 487–492. [Google Scholar]
- Robinson, T. The Evolution towards More Competitive Apple Orchard Systems in the USA. In Proceedings of the XXVII International Horticultural Congress-IHC2006: International Symposium on Enhancing Economic and Environmental 772, Seoul, Korea, 13 August 2006; pp. 491–500. [Google Scholar] [CrossRef]
- Cline, J.A. Planting density and size-controlling rootstocks influence the performance of Montmorency tart cherry (Prunus cerasus L.). Can. J. Plant Sci. 2020, 100, 16–28. [Google Scholar] [CrossRef]
- Forner-Giner, M.A.; Rodriguez-Gamir, J.; Martinez-Alcantara, B.; Quinones, A.; Iglesias, D.J.; Primo-Millo, E.; Forner, J. Performance of Navel orange trees grafted onto two new dwarfing rootstocks (Forner-Alcaide 517 and Forner-Alcaide 418). Sci. Hortic. 2014, 179, 376–387. [Google Scholar] [CrossRef]
- Elliott, J.; Deryng, D.; Müller, C.; Frieler, K.; Konzmann, M.; Gerten, D.; Glotter, M.; Flörke, M.; Wada, Y.; Best, N.; et al. Constraints and potentials of future irrigation water availability on agricultural production under climate change. Proc. Natl. Acad. Sci. USA 2014, 111, 3239–3244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An overview. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. ISBN 9783642326530. [Google Scholar]
- Tworkoski, T.; Fazio, G. Effects of Size-Controlling Apple Rootstocks on Growth, Abscisic Acid, and Hydraulic Conductivity of Scion of Different Vigor. Int. J. Fruit Sci. 2015, 15, 369–381. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Policarpo, M.; Webster, A.D.; Kingswell, G. Drought tolerance of clonal Malus determined from measurements of stomatal conductance and leaf water potential. Tree Phys. 2000, 20, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Hajagos, A.; Vegvari, G. Investigation of tissue structure and xylem anatomy of eight rootstocks of sweet cherry (Prunus avium L.). Trees 2013, 27, 53–60. [Google Scholar] [CrossRef]
- Ballesta, M.M.; Alcaraz-López, C.; Muries, B.; Mota-Cadenas, C.; Carvajal, M. Physiological aspects of rootstock–scion interactions. Sci. Hortic. 2010, 127, 112–118. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Naor, A. Irrigation Scheduling and Evaluation of Tree Water Status in Deciduous Orchards. Hortic. Rev. 2006, 32, 111–165. [Google Scholar] [CrossRef]
- Eremin, G. Prunus Plant Named “VSL-2”. U.S. Patent Application 09/880953, 20 November 2003. [Google Scholar]
- Roper, T.; Black, B.; Stasiak, R.; Marini, M.; Cline, J.; Robinson, T.; Lang, G.; Anderson, L.; Anderson, R.; Freer, J.; et al. Performance of “Montmorency” Sour Cherry (Prunus cerasus L.) on Size-Controlling Rootstocks at Six NC140 Trial Locations in North America. J. Am. Pomol. Soc. 2019, 73, 168–177. [Google Scholar]
- Long, L.E.; Brewer, L.J.; Kaiser, C. Cherry Rootstocks for the Modern Orchard. OSU Extension Service. Oregon State University: Pacific Northwest Extension Publication. 2014. Available online: http://extension.oregonstate.edu/wasco/sites/default/files/cherryrootstocksmodern-long.pdf (accessed on 15 November 2020).
- Jones, H.G. Irrigation scheduling: Advantages and pitfalls of plant-based methods. J. Exp. Bot. 2004, 55, 2427–2436. [Google Scholar] [CrossRef] [Green Version]
- Ben-Gal, A.; Kool, D.; Agam, N.; van Halsema, G.E.; Yermiyahu, U.; Yafe, A.; Presnov, E.; Erel, R.; Majdop, A.; Zipori, I.; et al. Whole-tree water balance and indicators for short-term drought stress in non-bearing “Barnea” olives. Agric. Water Manag. 2010, 98, 124–133. [Google Scholar] [CrossRef]
- Beeson, R.C., Jr. Weighing lysimeter systems for quantifying water use and studies of controlled water stress for crops grown in low bulk density substrates. Agric. Water Manag. 2011, 98, 967–976. [Google Scholar] [CrossRef]
- Chard, J.; van Iersel, M.; Bugbee, B. Mini-Lysimeters to Monitor Transpiration and Control Drought Stress: System Design and Unique Applications. 2010. Available online: https://digitalcommons.usu.edu/cpl_techniquesinstruments/18/ (accessed on 15 November 2020).
- Wheeler, W.; Wytsalucy, R.; Black, B.; Cardon, G.; Bugbee, B. Drought Tolerance of Navajo and Lovell Peach Trees: Precision Water Stress Using Automated Weighing Lysimeters. HortScience 2019, 54, 799–803. [Google Scholar] [CrossRef]
- Dos Santos, I.C.; de Almeida, A.-A.F.; Pirovani, C.P.; Costa, M.G.C.; Silva, M.F.D.G.F.D.; Bellete, B.S.; Freschi, L.; Filho, W.S.; Filho, M.A.C.; Gesteira, A.D.S. Differential accumulation of flavonoids and phytohormones resulting from the canopy/rootstock interaction of citrus plants subjected to dehydration/rehydration. Plant Physiol. Biochem. 2017, 119, 147–158. [Google Scholar] [CrossRef]
- Rieger, M.; Duemmel, M.J. Comparison of drought resistance among Prunus species from divergent habitats. Tree Physiol. 1992, 11, 369–380. [Google Scholar] [CrossRef]
- Gonçalves, B.; Moutinho-Pereira, J.; Santos, A.; Silva, A.P.; Bacelar, E.; Correia, C.; Rosa, E. Scion-rootstock interaction affects the physiology and fruit quality of sweet cherry. Tree Physiol. 2006, 26, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Sorce, C.; Mariotti, L.; Lorenzi, R.; Massai, R. Hormonal factors involved in the control of vigor of grafted peach [Prunus persica (L.) Batsch] trees and hybrid rootstocks. Adv. Hort. Sci. 2007, 21, 68–74. [Google Scholar]
- Maas, F.M.; Balkhoven-Baart, J.; van der Steeg, P.A.H. Evaluation of Krymsk® 5 (VSL-2) and Krymsk® 6 (LC-52) as rootstocks for sweet cherry “Kordia”. Acta. Hortic. 2014, 1058, 531–536. [Google Scholar] [CrossRef]
- Morandi, B.; Manfrini, L.; Lugli, S.; Tugnoli, A.; Boini, A.; Perulli, G.D.; Bresilla, K.; Venturi, M.; Grappadelli, L.C. Sweet cherry water relations and fruit production efficiency are affected by rootstock vigor. J. Plant Physiol. 2019, 237, 43–50. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Hernández, A.; López-Corrales, M.; Ruiz-Moyano, S.; Córdoba, M.G.; Martín, A. Composition of the cherry (Prunus avium L. and Prunus cerasus L.; Rosaceae). In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: London, UK, 2016; pp. 127–147. [Google Scholar]
- Barać, G.; Ognjanov, V.; Vidaković, D.O.; Dorić, D.; Ljubojević, M.; Dulić, J.; Miodragović, M.; Gašić, K. Genetic diversity and population structure of European ground cherry (Prunus fruticosa Pall.) using SSR markers. Sci. Hortic. 2017, 224, 374–383. [Google Scholar] [CrossRef]
- Kato, S.; Matsumoto, A.; Yoshimura, K.; Katsuki, T.; Iwamoto, K.; Kawahara, T.; Mukai, Y.; Tsuda, Y.; Ishio, S.; Nakamura, K.; et al. Origins of Japanese flowering cherry (Prunus subgenus Cerasus) cultivars revealed using nuclear SSR markers. Tree Genet. Genomes 2014, 10, 477–487. [Google Scholar] [CrossRef]
- Jones, M.M.; Rawson, H.M. Influence of Rate of Development of Leaf Water Deficits upon Photosynthesis, Leaf Conductance, Water Use Efficiency, and Osmotic Potential in Sorghum. Physiol. Plant. 1979, 45, 103–111. [Google Scholar] [CrossRef]
- Wang, Z.; Quebedeaux, B.; Stutte, G.W. Osmotic Adjustment: Effect of Water Stress on Carbohydrates in Leaves, Stems and Roots of Apple. Funct. Plant Biol. 1995, 22, 747–754. [Google Scholar] [CrossRef]
- Jiménez, S.; Dridi, D.J.; Gutiérrez, D.; Moret, J.J.; Irigoyen, M.A.; Moreno, Y.; Gogorcena, Y. Physiological, biochemical and molecular responses in four Prunus rootstocks submitted to drought stress. Tree Phys. 2013, 33, 1061–1075. [Google Scholar] [CrossRef] [PubMed]
- Mellisho, C.D.; Cruz, Z.N.; Conejero, W.; Ortuño, M.F.; Rodríguez, P. Mechanisms for drought resistance in early maturing cvar Flordastar peach trees. J. Agric. Sci. 2011, 149, 609–616. [Google Scholar] [CrossRef]
- Escobar-Gutiérrez, A.J.; Zipperlin, B.; Carbonne, F.; Moing, A.; Gaudillère, J.P. Photosynthesis, carbon partitioning and metabolite content during drought stress in peach seedlings. Funct. Plant Biol. 1998, 25, 197–205. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wheeler, W.; Black, B.; Bugbee, B. Water Stress in Dwarfing Cherry Rootstocks: Increased Carbon Partitioning to Roots Facilitates Improved Tolerance of Drought. Horticulturae 2021, 7, 424. https://doi.org/10.3390/horticulturae7110424
Wheeler W, Black B, Bugbee B. Water Stress in Dwarfing Cherry Rootstocks: Increased Carbon Partitioning to Roots Facilitates Improved Tolerance of Drought. Horticulturae. 2021; 7(11):424. https://doi.org/10.3390/horticulturae7110424
Chicago/Turabian StyleWheeler, Will, Brent Black, and Bruce Bugbee. 2021. "Water Stress in Dwarfing Cherry Rootstocks: Increased Carbon Partitioning to Roots Facilitates Improved Tolerance of Drought" Horticulturae 7, no. 11: 424. https://doi.org/10.3390/horticulturae7110424
APA StyleWheeler, W., Black, B., & Bugbee, B. (2021). Water Stress in Dwarfing Cherry Rootstocks: Increased Carbon Partitioning to Roots Facilitates Improved Tolerance of Drought. Horticulturae, 7(11), 424. https://doi.org/10.3390/horticulturae7110424





