Physical, Anatomical, and Biochemical Composition of Skins Cell Walls from Two Grapevine Cultivars (Vitis vinifera) of Champagne Region Related to Their Susceptibility to Botrytis cinerea during Ripening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grape Sample
2.2. Skin Characteristics
2.3. Cell Wall Characterization
2.3.1. Isolation of Cell Wall from Grape Skin
2.3.2. Extraction and Spectrophotometric Analysis of Polysaccharide Fractions of Cell Wall from Grape Skin
2.3.3. Analysis of Polysaccharides by HPSEC
2.4. Extraction of Total RNA and Gene Expression Analysis
2.5. Determination of PME Activity
2.6. Transmission Electron Microscopy Analysis of Grape Skin
2.7. Botrytis cinerea Infection Tests
2.8. Statistical Analysis
3. Results and Discussion
3.1. Grape Maturity
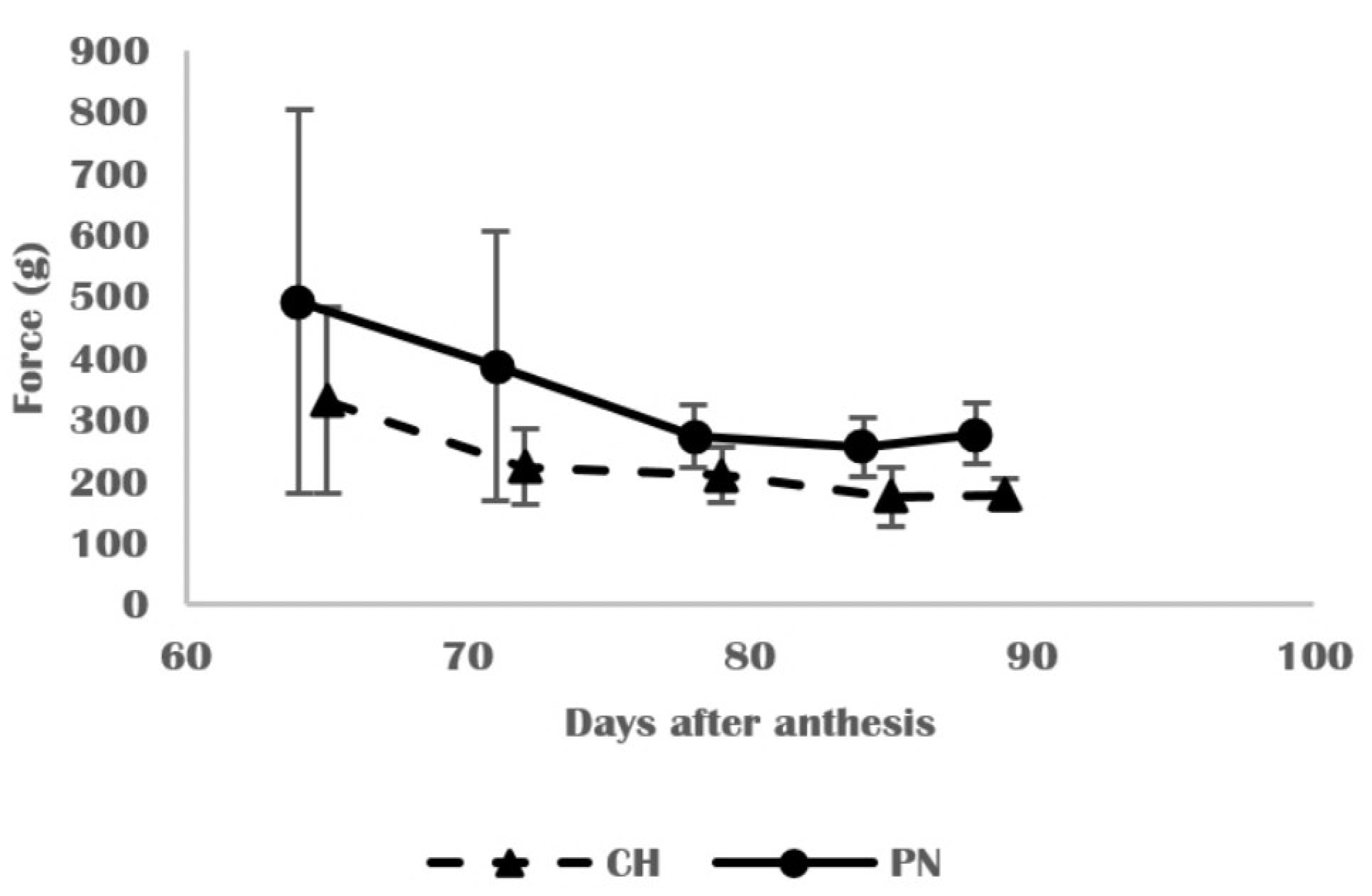
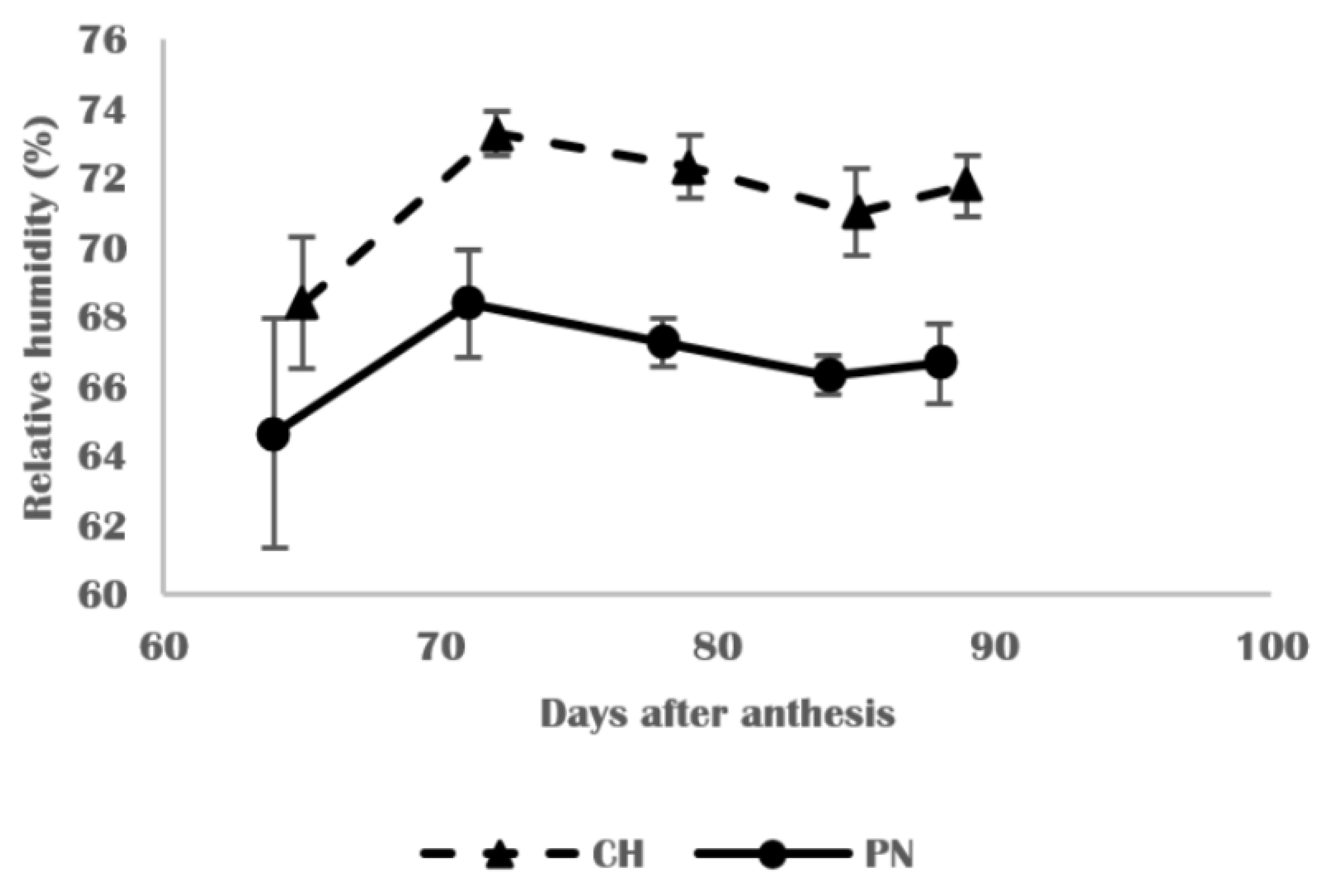
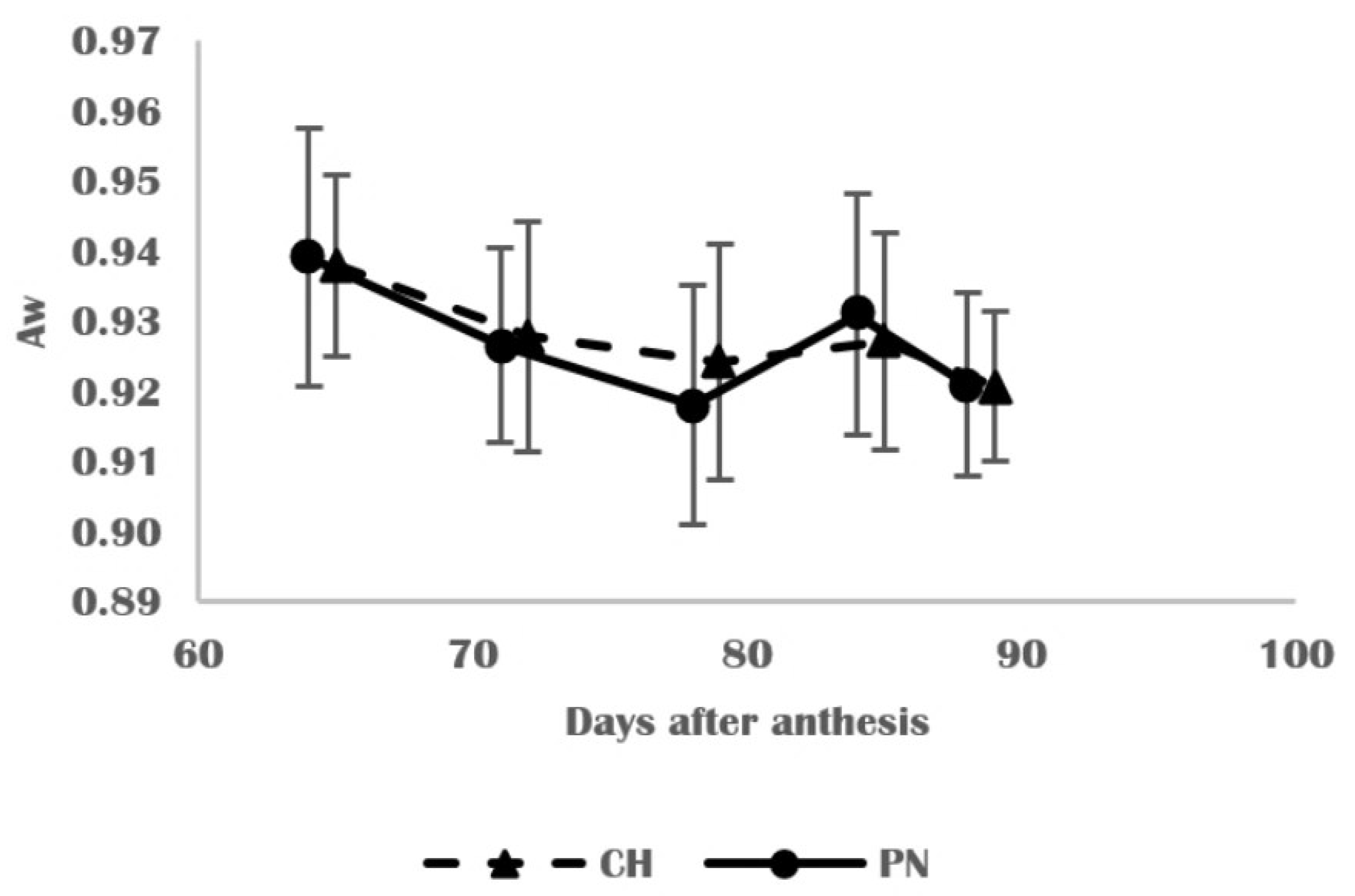
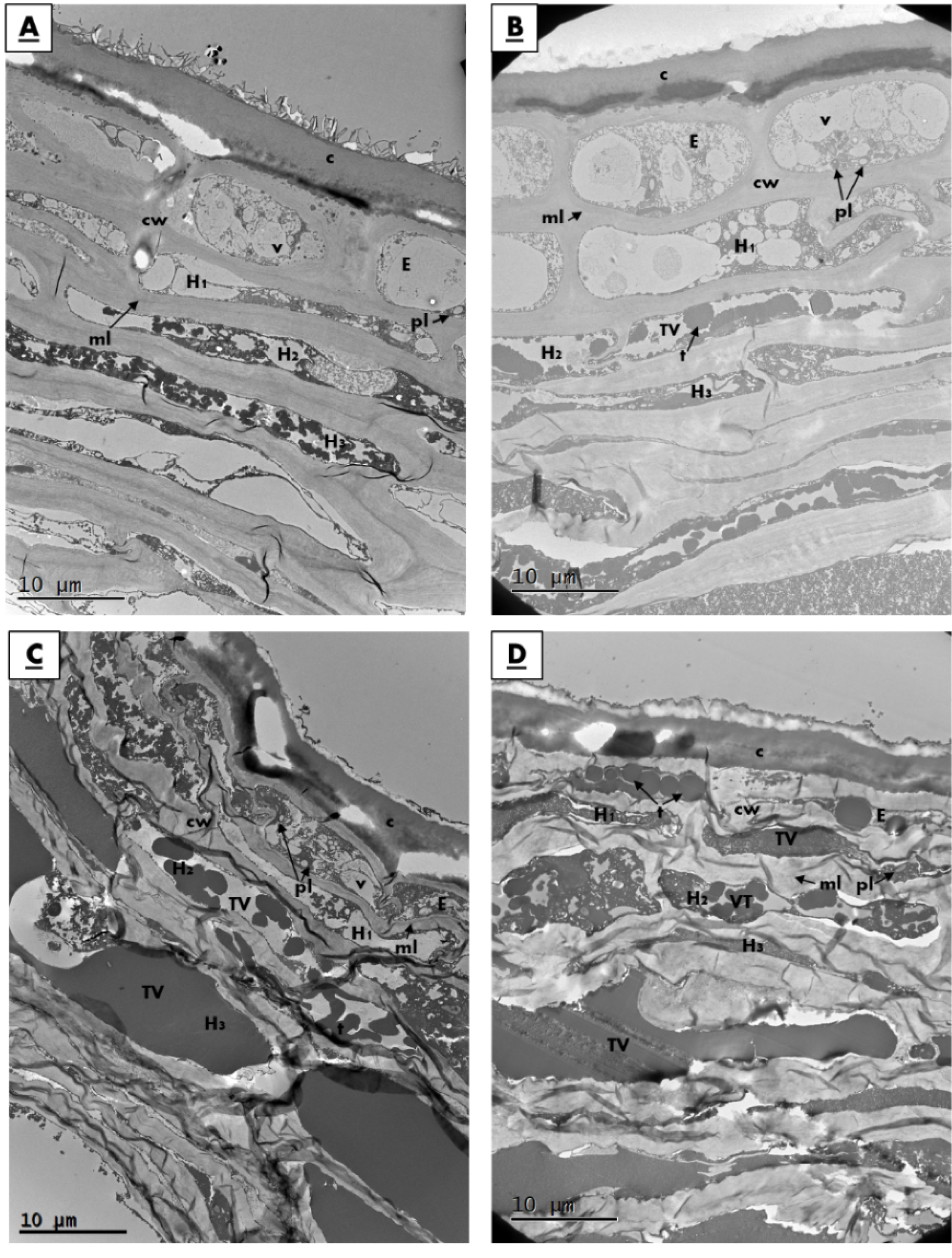
3.2. Physical and Structural Changes in Skins
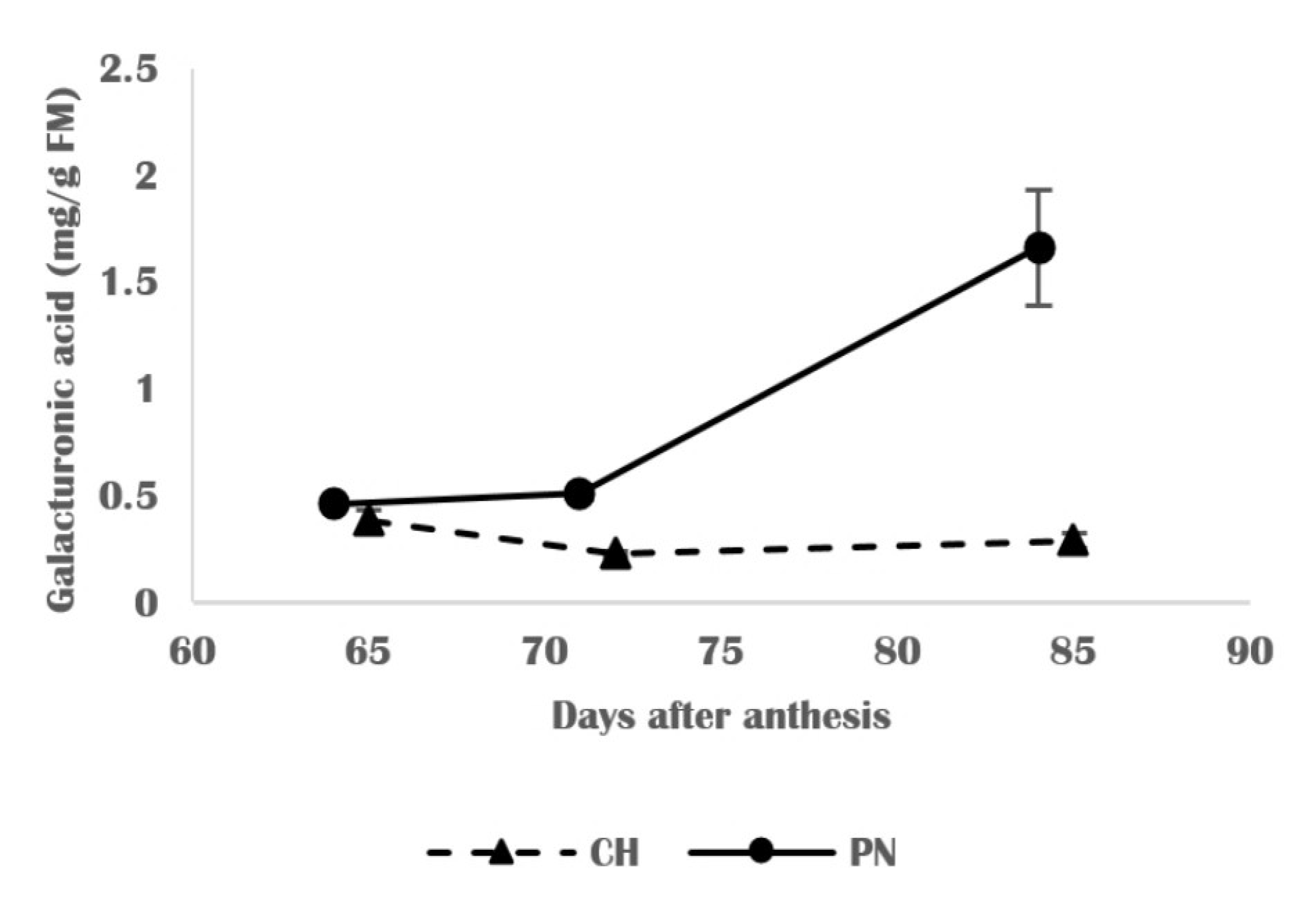
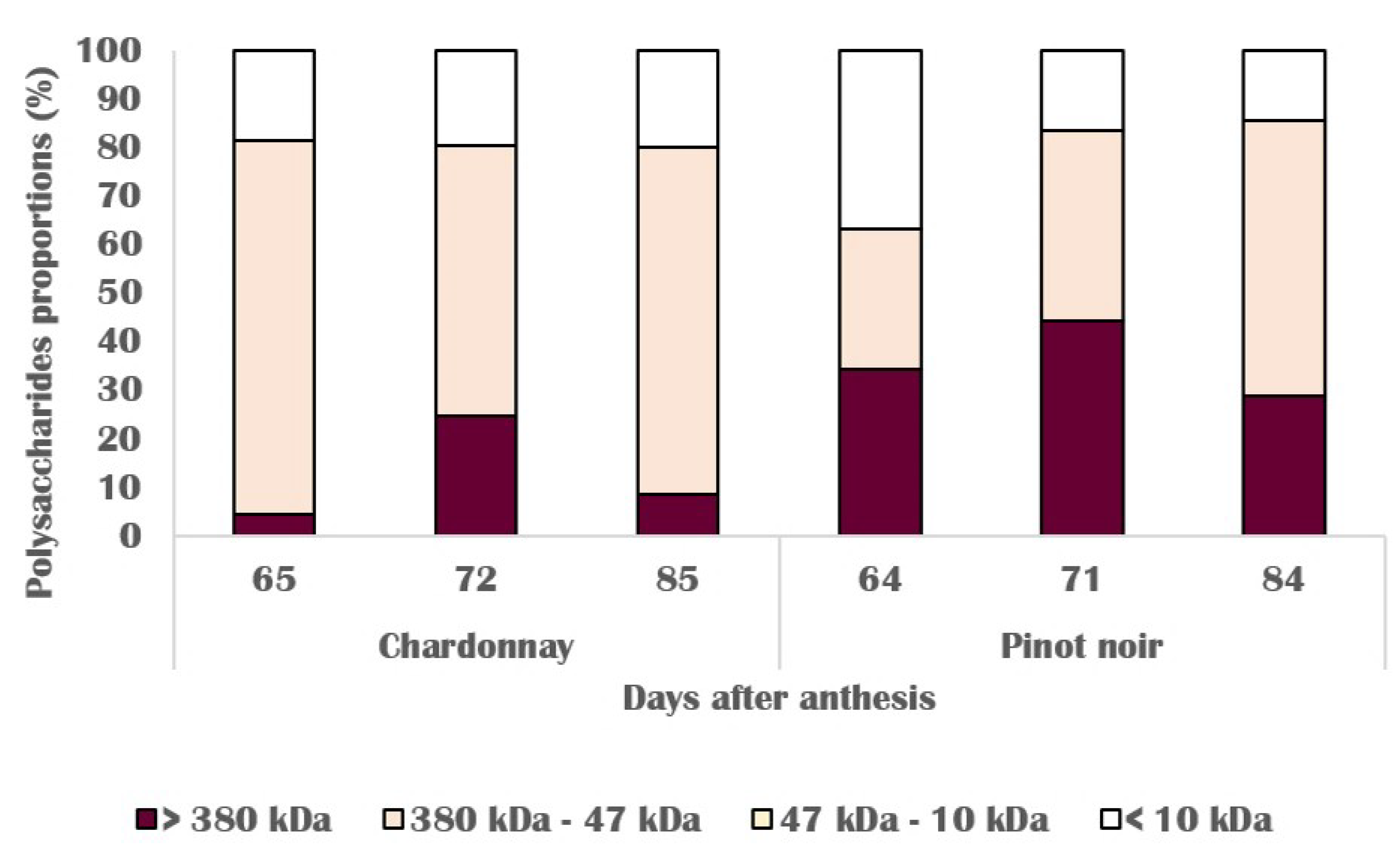
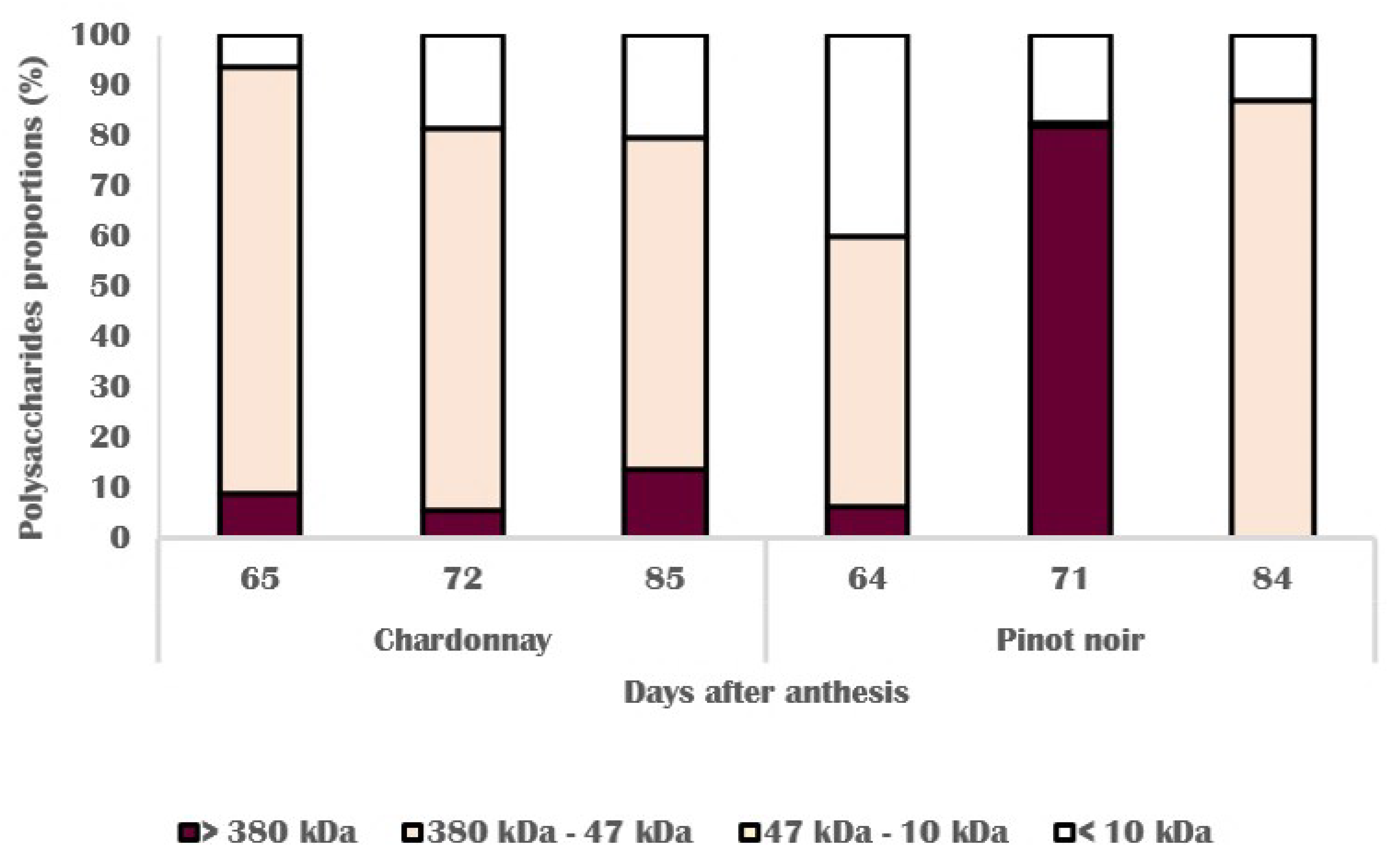
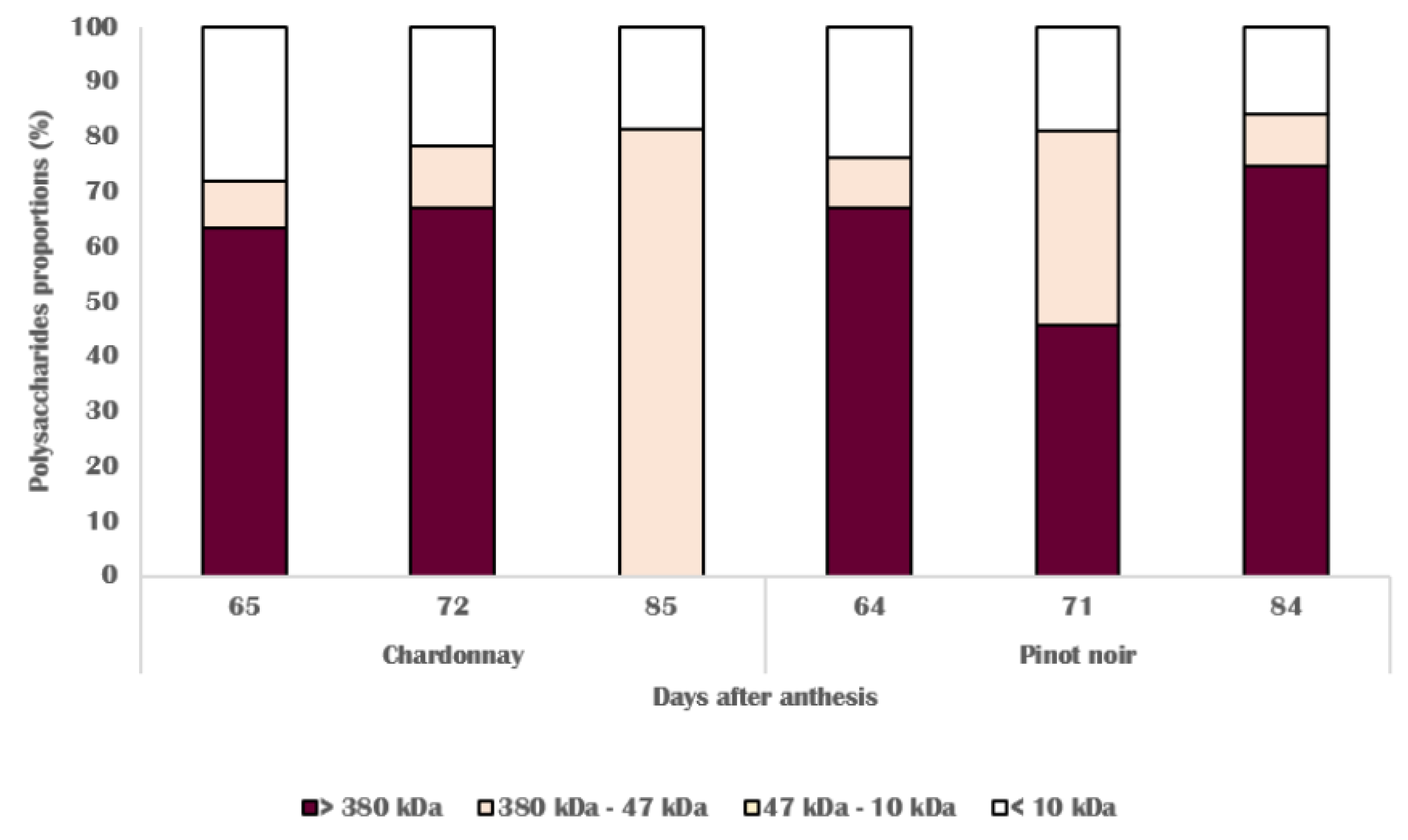
3.3. Chemical Changes in Skin Cell Wall
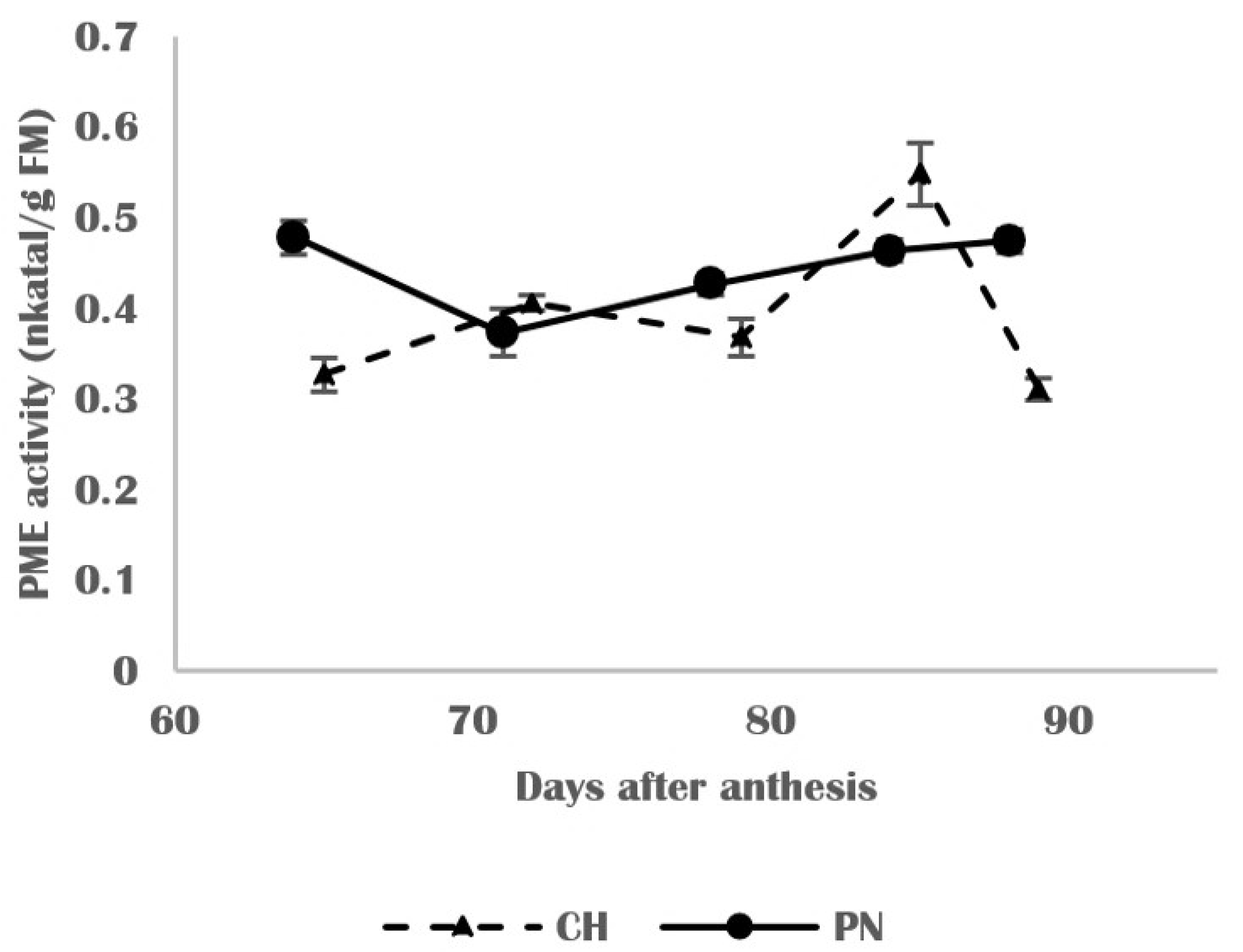
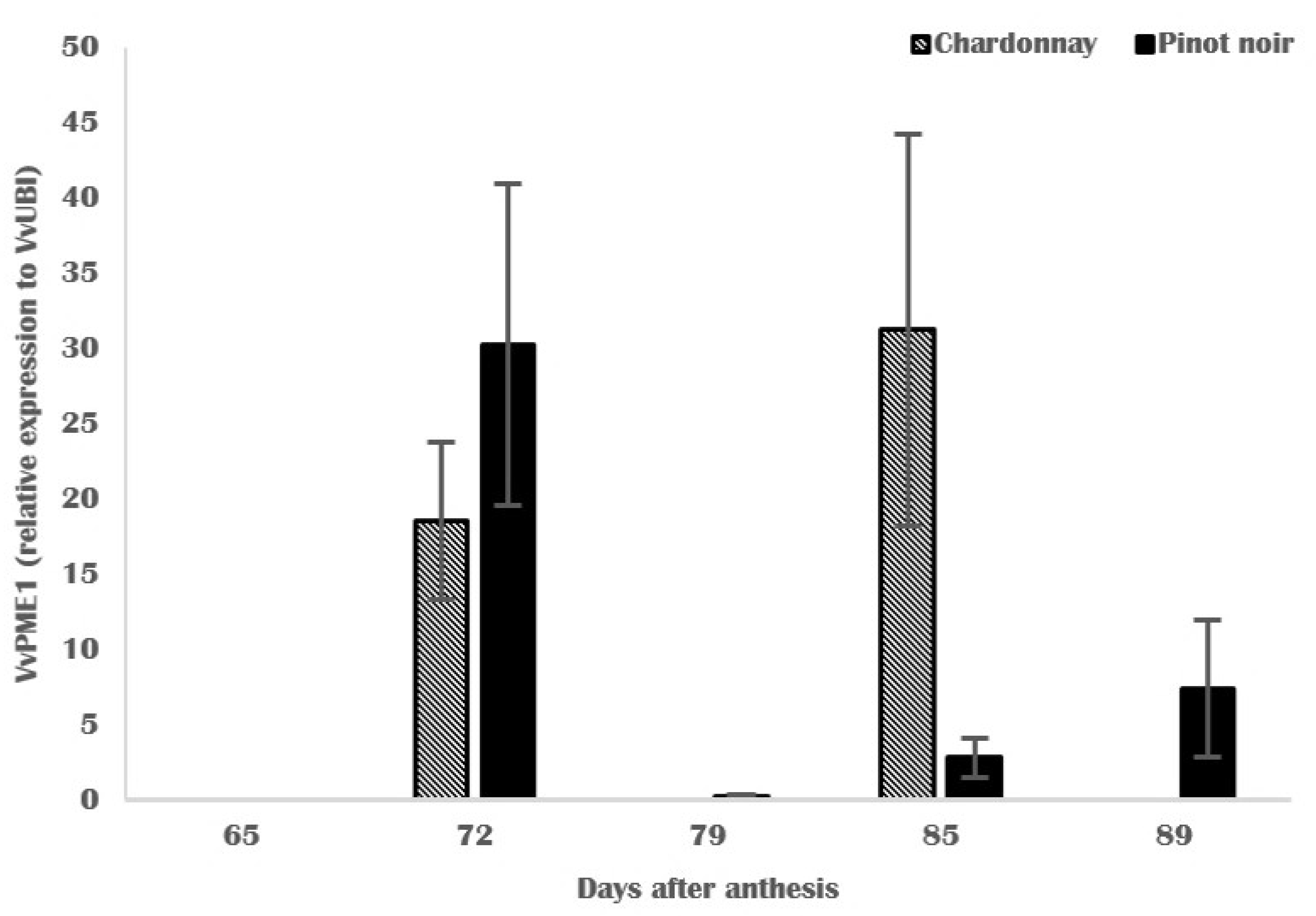
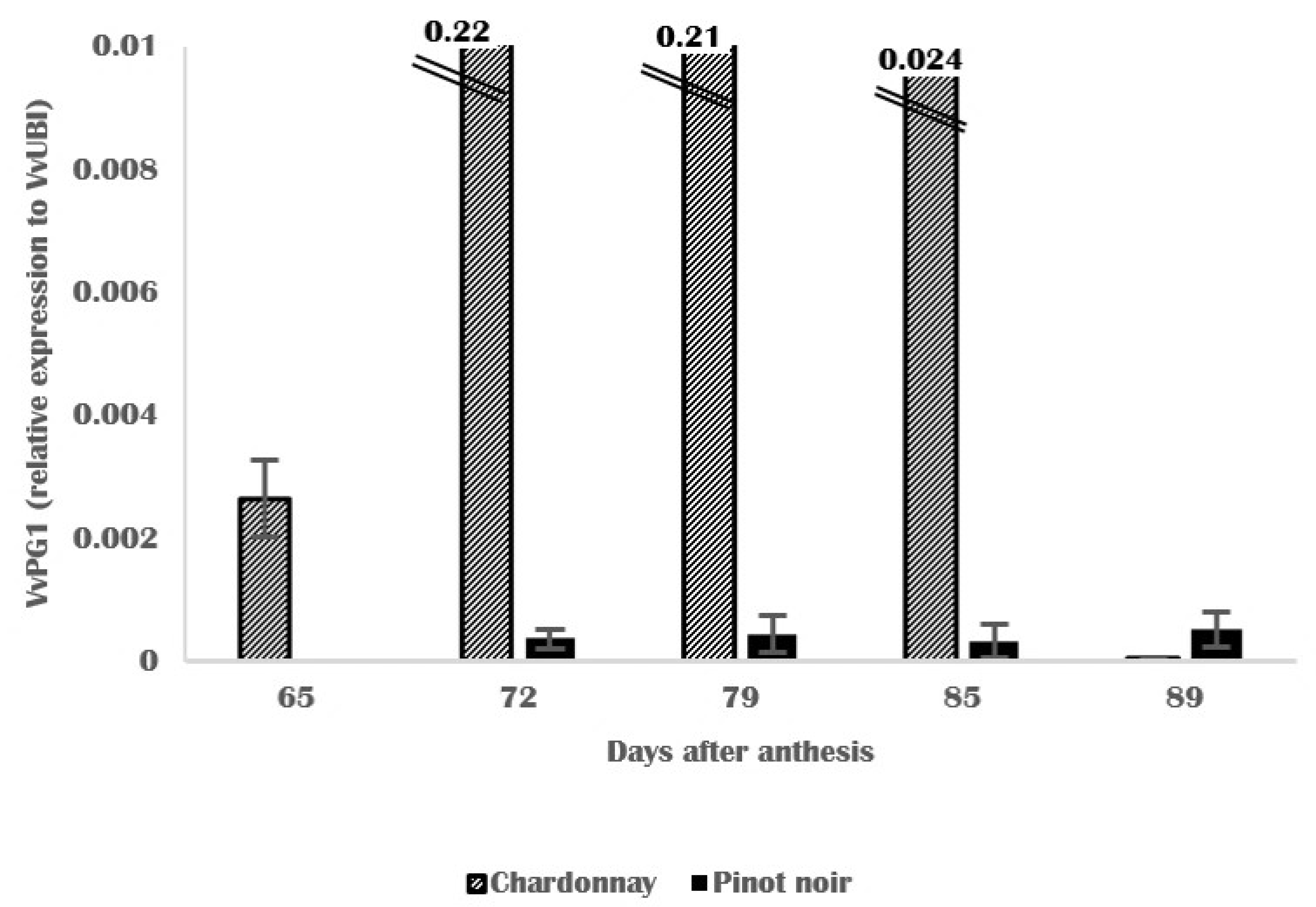
3.4. Molecular and Biochemical Changes in Skin Cell Walls
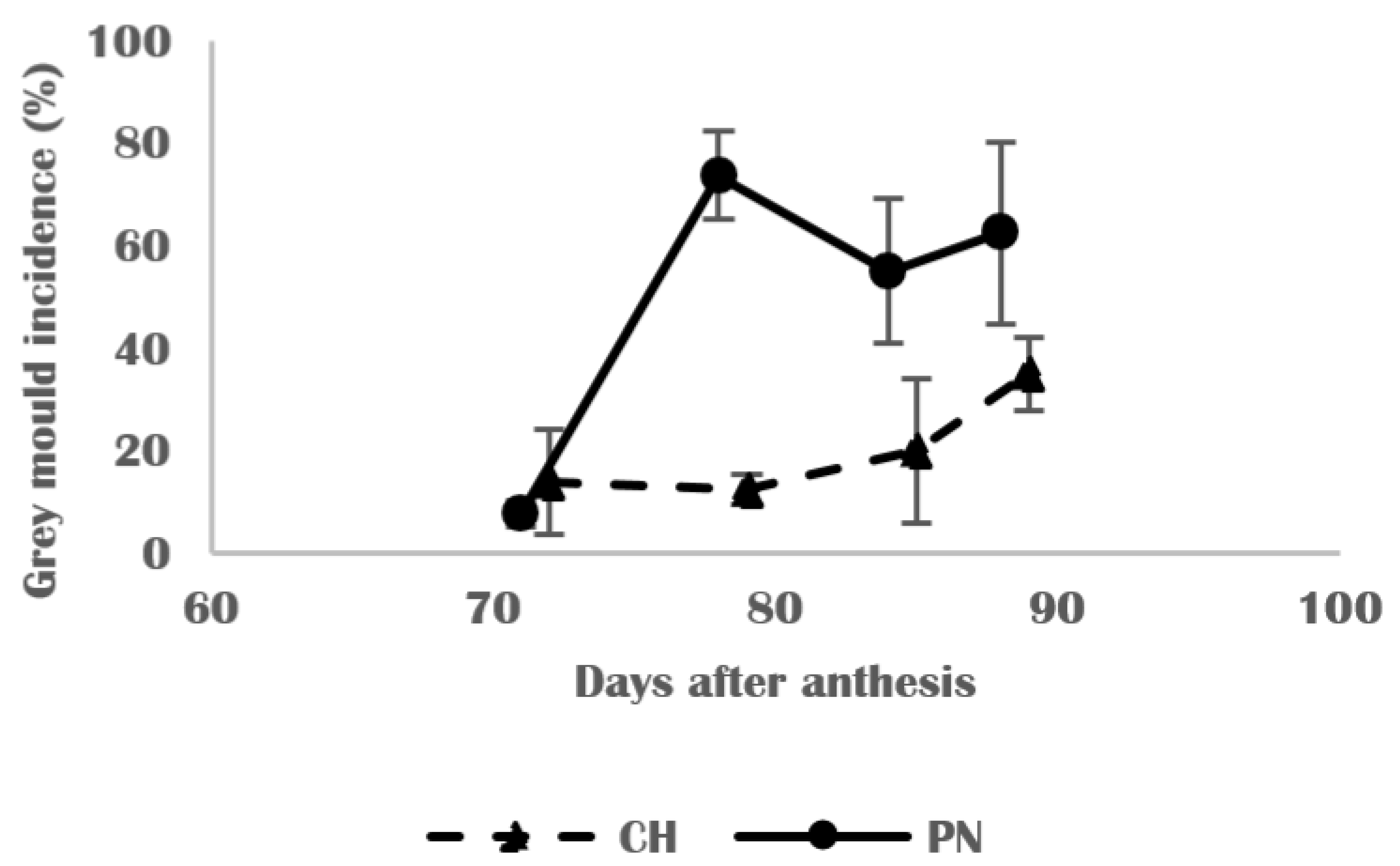
3.5. Grape Berry Susceptibility to Botrytis cinerea
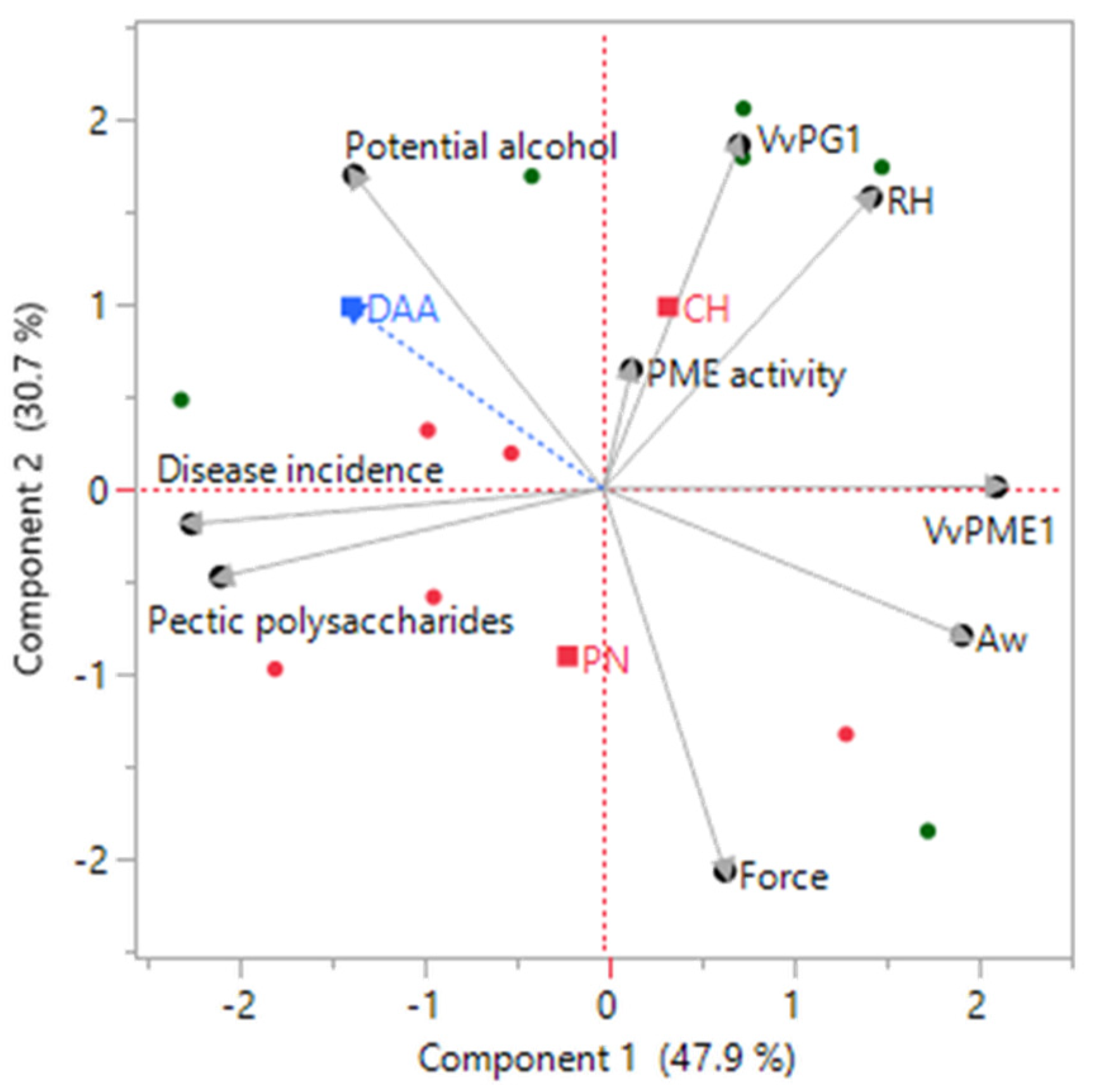
3.6. Linkage between Grape Skin Cell Wall Features and Botrytis cinerea Infection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortega-Regules, A.; Ros-García, J.M.; Bautista-Ortín, A.B.; López-Roca, J.M.; Gómez-Plaza, E. Changes in skin cell wall composition during the maturation of four premium wine grape varieties. J. Sci. Food Agric. 2008, 88, 420–428. [Google Scholar] [CrossRef]
- Fasoli, M.; Dell’Anna, R.; Dal Santo, S.; Balestrini, R.; Sanson, A.; Pezzotti, M.; Monti, F.; Zenoni, S. Pectins, Hemicelluloses and Celluloses Show Specific Dynamics in the Internal and External Surfaces of Grape Berry Skin during Ripening. Plant Cell Physiol. 2016, 57, 1332–1349. [Google Scholar] [CrossRef] [Green Version]
- Van Kan, J.A.L. Licensed to kill: The lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 2006, 11, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, F.; Moncomble, D.; Valade, M. Etat sanitaire de la vendange et qualité des vins. Le Vign. Champen. 1995, 7, 15–23. [Google Scholar]
- Marchal, R.; Tabary, I.; Valade, M.; Moncomble, D.; Viaux, L.; Robillard, B.; Jeandet, P. Effects of Botrytis cinerea infection on champagne wine foaming properties. J. Sci. Food Agric. 2001, 81, 1371–1378. [Google Scholar] [CrossRef]
- Deytieux-Belleau, C.; Geny, L.; Roudet, J.; Mayet, V.; Donèche, B.; Fermaud, M. Grape berry skin features related to ontogenic resistance to Botrytis cinerea. Eur. J. Plant Pathol. 2009, 125, 551–563. [Google Scholar] [CrossRef]
- Fermaud, M.; Deytieux-Belleau, C.; Roudet, J.; L’hyvernay, A.; Darrieutort, G.; Daguisé, F.; Donèche, B.; Geny, L. Pourriture grise du vignoble. Des indicateurs de risque en développement. Union Girondine Vins Bordx. 2010, 1062, 25–29. [Google Scholar]
- Fermaud, M.; Deytieux-Belleau, C.; Roudet, J.; Darrieutort, G.; Geny, L. Water activity at the fruit surface: A potential indicator of grape berry susceptibility to Botrytis cinerea. Integr. Prot. Prod. Vitic. IOBC 2011, 67, 155–161. [Google Scholar]
- Herzog, K.; Wind, R.; Töpfer, R. Impedance of the grape berry cuticle as a novel phenotypic trait to estimate resistance to Botrytis cinerea. Sensors 2015, 15, 12498–12512. [Google Scholar] [CrossRef] [PubMed]
- Mlikota Gabler, F.; Smilanick, J.L.; Mansour, M.; Ramming, D.W.; Mackey, B.E. Correlations of Morphological, Anatomical, and Chemical Features of Grape Berries with Resistance to Botrytis cinerea. Phytopathology 2003, 93, 1263–1273. [Google Scholar] [CrossRef] [Green Version]
- Sarig, P.; Zutkhi, Y.; Lisker, N.; Shkelerman, Y.; Ben-Arie, R. Natural and induced resistance of table grapes to bunch rots. Acta Hortic. 1998, 464, 65–70. [Google Scholar] [CrossRef]
- Comménil, P.; Brunet, L.; Audran, J.C. The development of the grape berry cuticle in relation to susceptibility to bunch rot disease. J. Exp. Bot. 1997, 48, 1599–1607. [Google Scholar] [CrossRef] [Green Version]
- Hill, G.; Stellwaag-Kittler, F.; Huth, G.; Schlosser, E. Resistance of Grapes in Different Developmental Stages to Botrytis cinerea. J. Phytopathol. 1981, 102, 328–338. [Google Scholar] [CrossRef]
- Marois, J.J.; Nelson, J.K.; Morrison, J.C.; Lile, L.S.; Bledsoe, A.M. The Influence of Berry Contact within Grape Clusters on the Development of Botrytis cinerea and Epicuticular Wax. Am. J. Enol. Vitic. 1986, 37, 293–296. [Google Scholar]
- Percival, D.C.; Sullivan, J.A.; Fisher, K.H. Effect of cluster exposure, berry contact and cultivar on cuticular membrane formation and occurrence of bunch rot (Botrytis cinerea PERS.: FR.) with 3 Vitis vinifera L. cultivars. Vitis 1993, 32, 87–97. [Google Scholar]
- Paňitrur-De La Fuente, C.; Valdés-Gómez, H.; Roudet, J.; Acevedo-Opazo, C.; Verdugo-Vásquez, N.; Araya-Alman, M.; Lolas, M.; Moreno, Y.; Fermaud, M. Classification of winegrape cultivars in Chile and France according to their susceptibility to Botrytis cinerea related to fruit maturity. Aust. J. Grape Wine Res. 2018, 24, 145–157. [Google Scholar] [CrossRef]
- Goetz, G.; Fkyerat, A.; Métais, N.; Kunz, M.; Tabacchi, R.; Pezet, R.; Pont, V. Resistance factors to grey mould in grape berries: Identification of some phenolics inhibitors of Botrytis cinerea stilbene oxidase. Phytochemistry 1999, 52, 759–767. [Google Scholar] [CrossRef]
- Orlando, R.; Magro, P.; Rugini, E. Pectic enzymes as a selective pressure tool for in vitro recovery of strawberry plants with fungal disease resistance. Plant Cell Rep. 1997, 16, 272–276. [Google Scholar] [PubMed]
- Kretschmer, M.; Kassemeyer, H.H.; Hahn, M. Age-dependent grey mould susceptibility and tissue-specific defence gene activation of grapevine berry skins after infection by Botrytis cinerea. J. Phytopathol. 2007, 155, 258–263. [Google Scholar] [CrossRef]
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Cantu, D.; Vicente, A.R.; Greve, L.C.; Dewey, F.M.; Bennett, A.B.; Labavitch, J.M.; Powell, A.L.T. The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea. Proc. Natl. Acad. Sci. USA 2008, 105, 859–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egea, J.; Romojaro, F.; Pretel, M.T.; Martinez-Madrid, M.C.; Costell, E.; Cascales, A. Application of sensory analysis to the determination of the optimum quality and harvesting moment in apricots. Acta Hortic. 2006, 701, 529–532. [Google Scholar] [CrossRef]
- Geny, L.; Saucier, C.; Bracco, S.; Daviaud, F.; Glories, Y. Composition and Cellular Localization of Tannins in Grape Seeds during Maturation. J. Agric. Food Chem. 2003, 51, 8051–8054. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, L.; Brillouet, J.-M.; Joseleau, J.-P. Structural studies of pectic substances from the pulp of grape berries. Carbohydr. Res. 1988, 182, 63–78. [Google Scholar] [CrossRef]
- Robertson, G.L. The fractional extraction and quantitative determination of pectic substances in grapes and musts. Am. J. Enol. Vitic. 1979, 30, 182–186. [Google Scholar]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Williams, P.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M.; Doco, T. Effect of winemaking techniques on polysaccharide composition of Cabernet Sauvignon, Syrah and Monastrell red wines. Aust. J. Grape Wine Res. 2014, 20, 62–71. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Martínez-Pinilla, O.; Garrido, Á.; Carrillo, J.D.; Ayestarán, B. Quantitative determination of wine polysaccharides by gas chromatography-mass spectrometry (GC-MS) and size exclusion chromatography (SEC). Food Chem. 2012, 131, 367–374. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Le Bourvellec, C.; Imberty, A.; Renard, C.M.G.C. Interactions between Pectic Compounds and Procyanidins are Influenced by Methylation Degree and Chain Length. Biomacromolecules 2013, 14, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.E.; Olsson, N.; Schlosser, J.; Peng, F.; Lund, S.T. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lacampagne, S. Localisation et Caractérisation des Tannins Dans la Pellicule du Raisin: Etude de L’impact de L’organisation Physico-Chimique des Parois Cellulaires sur la Composante Tannique, la Qualité du Fruit et la Typicité des Raisins de Bordeaux. Ph.D. Thesis, Université de Bordeaux, Bordeaux, France, 2010. Available online: http://www.theses.fr (accessed on 31 December 2011).
- Martínez, J.A.; Fernández-Trujillo, J.P. Necrotrophic fungi associated with epidermal microcracking caused by chilling injury in pickling cucumber fruit. Pesqui. Agropecu. Bras. 2007, 42, 593–598. [Google Scholar] [CrossRef]
- Maury, C.; Madieta, E.; Le Moigne, M.; Mehinagic, E.; Siret, R.; Jourjon, F. Development of a mechanical texture test to evaluate the ripening process of cabernet franc grapes. J. Texture Stud. 2009, 40, 511–535. [Google Scholar] [CrossRef]
- Deytieux, C.; Gény, L.; Donèche, B. Relation between hormonal balance and polygalacturonase activity in grape berry. Acta Hortic. 2005, 682, 163–170. [Google Scholar] [CrossRef]
- Martins, V.; Garcia, A.; Alhinho, A.T.; Costa, P.; Lanceros-Méndez, S.; Costa, M.M.R.; Gerós, H. Vineyard calcium sprays induce changes in grape berry skin, firmness, cell wall composition and expression of cell wall-related genes. Plant Physiol. Biochem. 2020, 150, 49–55. [Google Scholar] [CrossRef]
- Hardie, W.J.; O’Brien, T.P.; Jaudzems, V.G. Morphology, anatomy and development of the pericarp after anthesis in grape, Vitis vinifera L. Aust. J. Grape Wine Res. 1996, 2, 97–142. [Google Scholar] [CrossRef]
- Brizzolara, S.; Minnocci, A.; Yembaturova, E.; Tonutti, P. Ultrastructural analysis of berry skin from four grapes varieties at harvest and in relation to postharvest dehydration. OENO One 2020, 54, 1021–1031. [Google Scholar] [CrossRef]
- Battista, F.; Tomasi, D.; Porro, D.; Caicci, F.; Giacosa, S.; Rolle, L. Winegrape berry skin thickness determination: Comparison between histological observation and texture analysis determination. Ital. J. Food Sci. 2015, 27, 8–13. [Google Scholar] [CrossRef]
- Rajaei, H. Changements cytochimiques et ultrastructuraux des parois cellulaires de la pellicule du raisin, Vitis vinifera, durant la croissance et la maturation de la baie. Can. J. Bot. 1987, 65, 1343–1355. [Google Scholar] [CrossRef]
- Barnavon, L.; Doco, T.; Terrier, N.; Ageorges, A.; Romieu, C.; Pellerin, P. Analysis of cell wall neutral sugar composition, β-galactosidase activity and a related cDNA clone throughout the development of Vitis vinifera grape berries. Plant Physiol. Biochem. 2000, 38, 289–300. [Google Scholar] [CrossRef]
- Barnavon, L.; Doco, T.; Terrier, N.; Ageorges, A.; Romieu, C.; Pellerin, P. Involvement of pectin methyl-esterase during the ripening of grape berries: Partial cDNA isolation, transcript expression and changes in the degree of methyl-esterification of cell wall pectins. Phytochemistry 2001, 58, 693–701. [Google Scholar] [CrossRef]
- Rosli, H.G.; Civello, P.M.; Martínez, G.A. Changes in cell wall composition of three Fragaria x ananassa cultivars with different softening rate during ripening. Plant Physiol. Biochem. 2004, 42, 823–831. [Google Scholar] [CrossRef]
- Posé, S.; Kirby, A.R.; Mercado, J.A.; Morris, V.J.; Quesada, M.A. Structural characterization of cell wall pectin fractions in ripe strawberry fruits using AFM. Carbohydr. Polym. 2012, 88, 882–890. [Google Scholar] [CrossRef]
- Jarvis, M.C. Plant cell walls: Supramolecular assemblies. Food Hydrocoll. 2011, 25, 257–262. [Google Scholar] [CrossRef]
- Wu, H.C.; Bulgakov, V.P.; Jinn, T.L. Pectin methylesterases: Cell wall remodeling proteins are required for plant response to heat stress. Front. Plant Sci. 2018, 871, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Deytieux-Belleau, C.; Vallet, A.; Donèche, B.; Geny, L. Pectin methylesterase and polygalacturonase in the developing grape skin. Plant Physiol. Biochem. 2008, 46, 638–646. [Google Scholar] [CrossRef]
- Nunan, K.J.; Davies, C.; Robinson, S.P.; Fincher, G.B. Expression patterns of cell wall-modifying enzymes during grape berry development. Planta 2001, 214, 257–264. [Google Scholar] [CrossRef]
- Khan, N.; Fatima, F.; Haider, M.S.; Shazadee, H.; Liu, Z.; Zheng, T.; Fang, J. Genome-wide identification and expression profiling of the polygalacturonase (PG) and pectin methylesterase (PME) genes in grapevine (Vitis vinifera L.). Int. J. Mol. Sci. 2019, 20, 3180. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Mujumdar, A.S.; Deng, L.Z.; Gao, Z.J.; Xiao, H.W.; Raghavan, G.S.V. High-humidity hot air impingement blanching alters texture, cell-wall polysaccharides, water status and distribution of seedless grape. Carbohydr. Polym. 2018, 194, 9–17. [Google Scholar] [CrossRef]
- Coombe, B.G.; McCarthy, M.G. Dynamics of grape berry growth and physiology of ripening. Aust. J. Grape Wine Res. 2000, 6, 131–135. [Google Scholar] [CrossRef]
- Goulao, L.F.; Oliveira, C.M. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef] [Green Version]
- Mercado, J.A.; Matas, A.J.; Posé, S. Fruit and vegetable texture: Role of their cell walls. Encycl. Food Chem. 2018, 3, 1–7. [Google Scholar] [CrossRef]
- Gapper, N.E.; McQuinn, R.P.; Giovannoni, J.J. Molecular and genetic regulation of fruit ripening. Plant Mol. Biol. 2013, 82, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Sénéchal, F.; Wattier, C.; Rustérucci, C.; Pelloux, J. Homogalacturonan-modifying enzymes: Structure, expression, and roles in plants. J. Exp. Bot. 2014, 65, 5125–5160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Cultivar | Days after Anthesis | Potential Alcohol (% vol.) | Skin Material (mg/Berry) |
|---|---|---|---|
| Chardonnay | 65 | 4.7 | 101.3 |
| 72 | 8.2 | 107.8 | |
| 79 | 9.5 | 122.1 | |
| 85 | 10.4 | 135.1 | |
| 89 | 11.1 | 137.7 | |
| Pinot noir | 64 | 5.4 | 113.1 |
| 71 | 7.0 | 114.9 | |
| 78 | 9.5 | 124.8 | |
| 84 | 8.1 | 108.3 | |
| 88 | 9.3 | 139.6 |
| Measured Parameters | Veraison | Harvest | ||
|---|---|---|---|---|
| Chardonnay | Pinot Noir | Chardonnay | Pinot Noir | |
| Epidermis (µm) | 1.23 ± 0.11 a | 0.95 ± 0.06 a | 1.14 ± 0.12 a | 0.94 ± 0.07 a |
| Epidermis-Hypodermis 1 (µm) | 1.65 ± 0.17 b | 1.13 ± 0.11 a | 1.55 ± 0.16 b | 1.22 ± 0.10 b |
| Hypodermis 1-Hypodermis 2 (µm) | 2.03 ± 0.28 c | 1.59 ± 0.15 b | 1.70 ± 0.14 b | 1.46 ± 0.11 c |
| Hypodermis 2-Hypodermis 3 (µm) | 2.39 ± 0.22 c | 1.89 ± 0.22 c | 2.04 ± 0.28 c | 2.22 ± 0.16 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, M.; Lacampagne, S.; Barsacq, A.; Gontier, E.; Petrel, M.; Mercier, L.; Courot, D.; Gény-Denis, L. Physical, Anatomical, and Biochemical Composition of Skins Cell Walls from Two Grapevine Cultivars (Vitis vinifera) of Champagne Region Related to Their Susceptibility to Botrytis cinerea during Ripening. Horticulturae 2021, 7, 413. https://doi.org/10.3390/horticulturae7100413
André M, Lacampagne S, Barsacq A, Gontier E, Petrel M, Mercier L, Courot D, Gény-Denis L. Physical, Anatomical, and Biochemical Composition of Skins Cell Walls from Two Grapevine Cultivars (Vitis vinifera) of Champagne Region Related to Their Susceptibility to Botrytis cinerea during Ripening. Horticulturae. 2021; 7(10):413. https://doi.org/10.3390/horticulturae7100413
Chicago/Turabian StyleAndré, Marie, Soizic Lacampagne, Audrey Barsacq, Etienne Gontier, Melina Petrel, Laurence Mercier, Diane Courot, and Laurence Gény-Denis. 2021. "Physical, Anatomical, and Biochemical Composition of Skins Cell Walls from Two Grapevine Cultivars (Vitis vinifera) of Champagne Region Related to Their Susceptibility to Botrytis cinerea during Ripening" Horticulturae 7, no. 10: 413. https://doi.org/10.3390/horticulturae7100413
APA StyleAndré, M., Lacampagne, S., Barsacq, A., Gontier, E., Petrel, M., Mercier, L., Courot, D., & Gény-Denis, L. (2021). Physical, Anatomical, and Biochemical Composition of Skins Cell Walls from Two Grapevine Cultivars (Vitis vinifera) of Champagne Region Related to Their Susceptibility to Botrytis cinerea during Ripening. Horticulturae, 7(10), 413. https://doi.org/10.3390/horticulturae7100413






