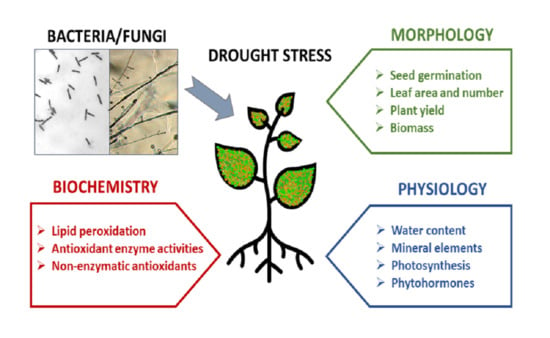
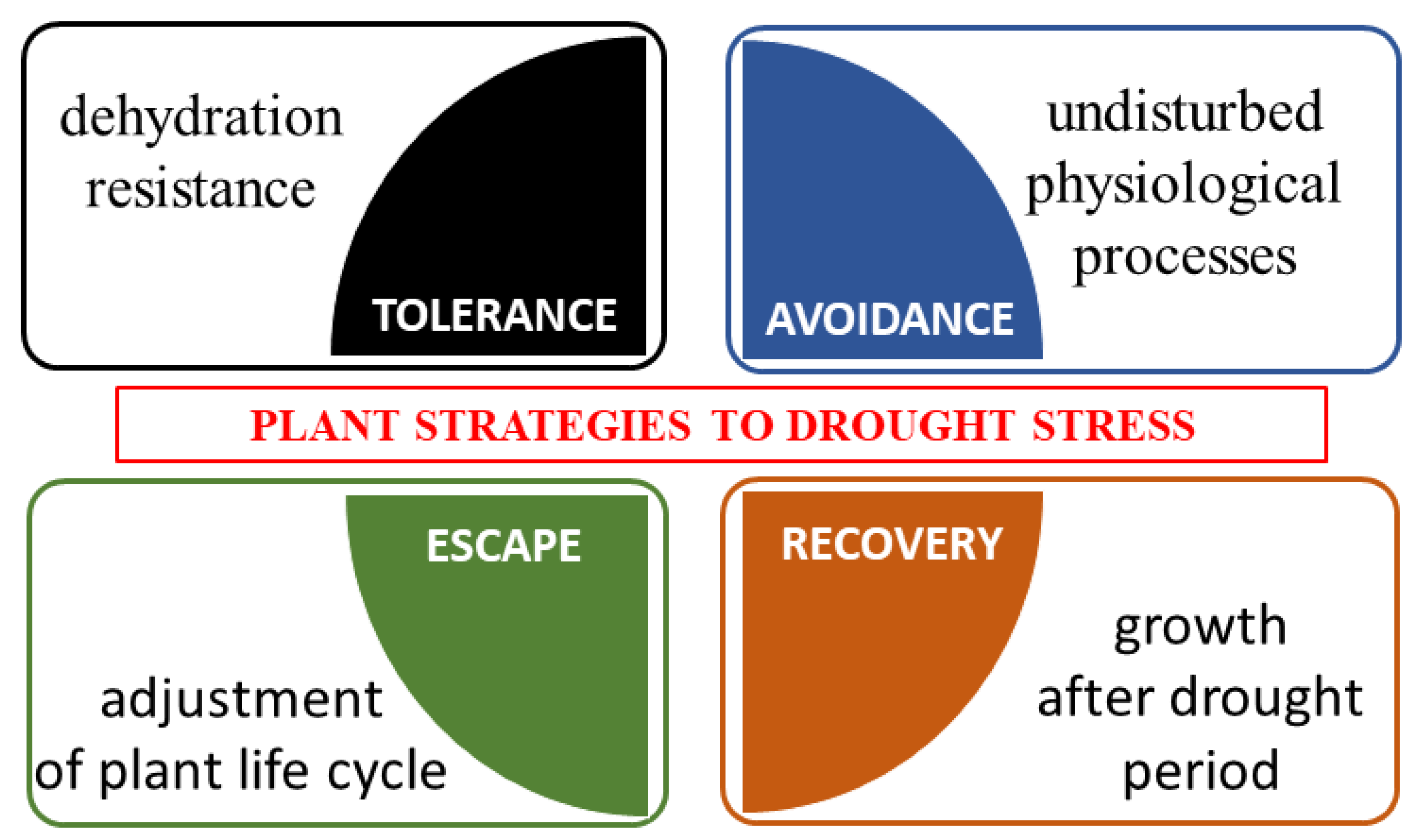
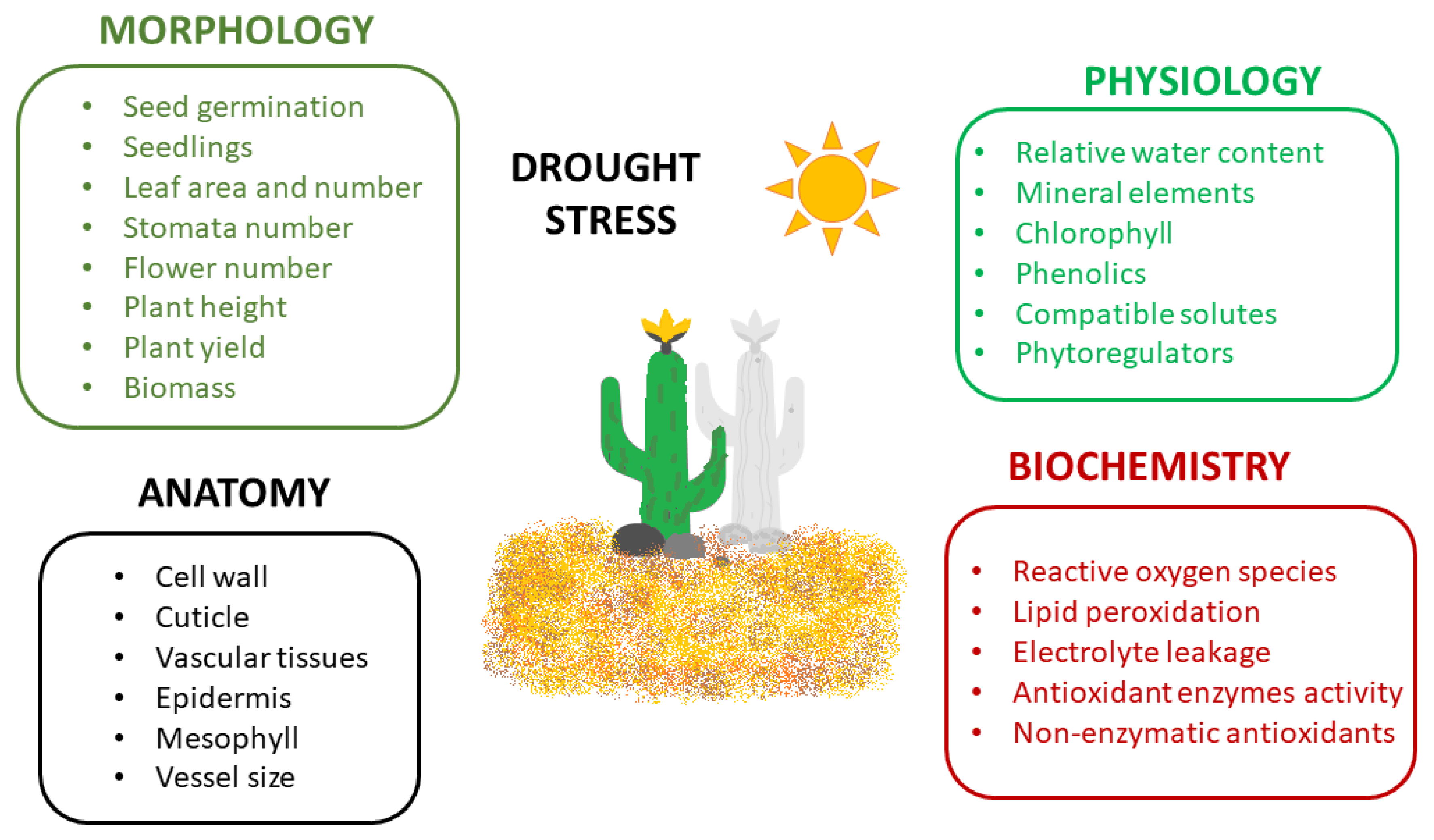
Plant Tolerance to Drought Stress in the Presence of Supporting Bacteria and Fungi: An Efficient Strategy in Horticulture
Abstract
1. Introduction
2. Climate Change
3. Plants under Drought Stress
4. Mechanisms of Resistance in Plants
5. Bacteria Supporting Horticultural Crops
5.1. Bacillus Species in Drought Stress
5.2. Actinomycetes Species in Drought Stress
6. Plant Growth-Promoting Fungi in Horticultural Crops
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Moretti, C.L.; Mattos, L.M.; Calbo, A.G.; Sargent, S.A. Climate changes and potential impacts on postharvest quality of fruit and vegetable crops: A review. Food Res. Int. 2010, 43, 1824–1832. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Sousa, C.S.; Soares, A.C.F.; Garrido, M.S. Characterization of Streptomycetes with potential to promote plant growth and biocontrol. Sci. Agric. 2008, 65, 50–55. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in agriculture: A sustainable approach to increasing climate change resilience. Front. Sustain. Food Syst. 2021, 5, 1–22. [Google Scholar] [CrossRef]
- International Society for Horticultural Science. Available online: https://www.ishs.org/defining-horticulture (accessed on 27 September 2021).
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Mohapatra, S.; Bhayana, S.; Varma, A. Revisiting plant-microbe interactions and microbial consortia application for enhancing sustainable agriculture: A review. Front. Microbiol. 2020, 11, 560406. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Shahid, M.A.; Mustafa, A.; Sayyed, R.Z.; Curá, J.A. Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: A review. Cells 2021, 10, 1551. [Google Scholar] [CrossRef]
- Ullah, A.; Nisar, M.; Ali, H.; Hazrat, A.; Hayat, K.; Keerio, A.A.; Ihsan, M.; Laiq, M.; Ullah, S.; Fahad, S.; et al. Drought tolerance improvement in plants: An endophytic bacterial approach. Appl. Microbiol. Biotechnol. 2019, 103, 7385–7397. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Zhang, L.; He, S.Y. Plant-microbe interactions facing environmental challenge. Cell Host Microbe 2019, 26, 183–192. [Google Scholar] [CrossRef]
- Kavadia, A.; Omirou, M.; Fasoula, D.; Ioannides, I.M. The importance of microbial inoculants in a climate-changing agriculture in Eastern Mediterranean region. Atmosphere 2020, 11, 1136. [Google Scholar] [CrossRef]
- Malik, A.; Mor, V.S.; Tokas, J.; Punia, H.; Malik, S.; Malik, K.; Sangwan, S.; Tomar, S.; Singh, P.; Singh, N.; et al. Biostimulant-treated seedlings under sustainable agriculture: A global perspective facing climate change. Agronomy 2021, 11, 14. [Google Scholar] [CrossRef]
- Porter, S.S.; Bantay, R.; Friel, C.A.; Garoutte, A.; Gdanetz, K.; Ibarreta, K.; Moore, B.M.; Shetty, P.; Siler, E.; Friesen, M.L. Beneficial microbes ameliorate abiotic and biotic sources of stress on plants. Funct. Ecol. 2020, 34, 2075–2086. [Google Scholar] [CrossRef]
- Fincheira, P.; Quiroz, A.; Tortella, G.; Diez, M.C.; Rubilar, O. Current advances in plant-microbe communication via volatile organic compounds as an innovative strategy to improve plant growth. Microbiol. Res. 2021, 247, 126726. [Google Scholar] [CrossRef] [PubMed]
- Hanaka, A.; Ozimek, E.; Majewska, M.; Rysiak, A.; Jaroszuk-Ściseł, J. Physiological diversity of Spitsbergen soil microbial communities suggests their potential as plant growth-promoting bacteria. Int. J. Mol. Sci. 2019, 20, 1207. [Google Scholar] [CrossRef] [PubMed]
- Hanaka, A.; Nowak, A.; Plak, A.; Dresler, S.; Ozimek, E.; Jaroszuk-Ściseł, J.; Wójciak-Kosior, M.; Sowa, I. Bacterial isolate inhabiting Spitsbergen soil modifies the physiological response of Phaseolus coccineus in control conditions and under exogenous application of methyl jasmonate and copper excess. Int. J. Mol. Sci. 2019, 20, 1909. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Van Der Heijden, M.G.A.; Sessitsch, A. Climate change effects on beneficial plant-microorganism interactions. FEMS Microbiol. Ecol. 2010, 73, 197–214. [Google Scholar] [CrossRef]
- Ma, Y.; Vosátka, M.; Freitas, H. Editorial: Beneficial microbes alleviate climatic stresses in plants. Front. Plant Sci. 2019, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Vimal, S.R.; Singh, J.S.; Arora, N.K.; Singh, S. Soil-plant-microbe interactions in stressed agriculture management: A review. Pedosphere 2017, 27, 177–192. [Google Scholar] [CrossRef]
- Gupta, S.; Seth, R.; Sharma, A. Plant Growth-Promoting Rhizobacteria Play a Role as Phytostimulators for Sustainable Agriculture; Choudhary, D., Varma, A., Tuteja, N., Eds.; Springer: Singapore, 2016. [Google Scholar]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Front. Plant Sci. 2016, 7, 918. [Google Scholar] [CrossRef]
- Pirttilä, A.M.; Tabas, H.M.P.; Baruah, N.; Koskimäki, J.J. Biofertilizers and biocontrol agents for agriculture: How to identify and develop new potent microbial strains and traits. Microorganisms 2021, 9, 817. [Google Scholar] [CrossRef]
- Vílchez, J.I.; García-Fontana, C.; Román-Naranjo, D.; González-López, J.; Manzanera, M. Plant drought tolerance enhancement by trehalose production of desiccation-tolerant microorganisms. Front. Microbiol. 2016, 7, 1577. [Google Scholar] [CrossRef]
- Finkel, O.M.; Castrillo, G.; Herrera Paredes, S.; Salas González, I.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Growth and developmental responses of crop plants under drought stress: A review. Zemdirb. Agric. 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Sharma, M.; Sudheer, S.; Usmani, Z.; Rani, R.; Gupta, P. Deciphering the omics of plant-microbe interaction: Perspectives and new insights. Curr. Genom. 2020, 21, 343–362. [Google Scholar] [CrossRef]
- Vasseur-Coronado, M.; du Boulois, H.D.; Pertot, I.; Puopolo, G. Selection of plant growth promoting rhizobacteria sharing suitable features to be commercially developed as biostimulant products. Microbiol. Res. 2021, 245, 1–10. [Google Scholar] [CrossRef]
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Biotechnol. 2019, 18, 597–616. [Google Scholar] [CrossRef]
- Kränzlein, M.; Geilfus, C.-M.; Franzisky, B.L.; Zhang, X.; Wimmer, M.A.; Zörb, C. Physiological responses of contrasting maize (Zea mays L.) hybrids to repeated drought. J. Plant Growth Regul. 2021. [Google Scholar] [CrossRef]
- Jamil, M.; Ahamd, M.; Anwar, F.; Zahir, Z.A.; Kharal, M.A.; Nazli, F. Inducing drought tolerance in wheat through combined use of L-tryptophan and Pseudomonas fluorescens. Pak. J. Agric. Sci. 2018, 55, 331–337. [Google Scholar] [CrossRef]
- Yin, B.; Wang, Y.; Liu, P.; Hu, J.; Zhen, W. Effects of vesicular-arbuscular mycorrhiza on the protective system in strawberry leaves under drought stress. Front. Agric. China 2010, 4, 165–169. [Google Scholar] [CrossRef]
- Hone, H.; Mann, R.; Yang, G.; Kaur, J.; Tannenbaum, I.; Li, T.; Spangenberg, G.; Sawbridge, T. Profiling, isolation and characterisation of beneficial microbes from the seed microbiomes of drought tolerant wheat. Sci. Rep. 2021, 11, 11916. [Google Scholar] [CrossRef]
- Zapata, T.; Galindo, D.M.; Corrales-Ducuara, A.R.; Ocampo-Ibáñez, I.D. The diversity of culture-dependent gram-negative Rhizobacteria associated with manihot esculenta crantz plants subjected to water-deficit stress. Diversity 2021, 13, 366. [Google Scholar] [CrossRef]
- Mustafa, S.; Kabir, S.; Shabbir, U.; Batool, R. Plant growth promoting rhizobacteria in sustainable agriculture: From theoretical to pragmatic approach. Symbiosis 2019, 78, 115–123. [Google Scholar] [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 2021, 11, 7. [Google Scholar] [CrossRef]
- IPCC Climate Change. The Physical Science Basis 2021; IPCC Climate Change: Geneva, Switzerland, 2021. [Google Scholar]
- Lelieveld, J.; Hadjinicolaou, P.; Kostopoulou, E.; Chenoweth, J.; El Maayar, M.; Giannakopoulos, C.; Hannides, C.; Lange, M.A.; Tanarhte, M.; Tyrlis, E.; et al. Climate change and impacts in the Eastern Mediterranean and the Middle East. Clim. Chang. 2012, 114, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, M.; Nisar, M.; Khan, N.; Hazrat, A.; Khan, A.H.; Hayat, K.; Fahad, S.; Khan, A.; Ullah, A. Drought tolerance strategies in plants: A mechanistic approach. J. Plant Growth Regul. 2021, 40, 926–944. [Google Scholar] [CrossRef]
- Hanaka, A.; Plak, A.; Zagórski, P.; Ozimek, E.; Rysiak, A.; Majewska, M.; Jaroszuk-Ściseł, J. Relationships between the properties of Spitsbergen soil, number and biodiversity of rhizosphere microorganisms, and heavy metal concentration in selected plant species. Plant Soil 2019, 436, 49–69. [Google Scholar] [CrossRef]
- Mędrek, K.; Gluza, A.; Siwek, K.; Zagórski, P. The meteorological conditions on the Calypsobyen in summer 2014 on the background of multiyear 1986–2011. Probl. Klim. Polar. 2014, 24, 37–50. (In Polish) [Google Scholar]
- Franczak, Ł.; Kociuba, W.; Gajek, G. Runoff variability in the Scott River (SW Spitsbergen) in summer seasons 2012–2013 in comparison with the period 1986–2009. QuaGeo 2016, 35, 39–50. [Google Scholar] [CrossRef][Green Version]
- Hu, Y.; Xie, G.; Jiang, X.; Shao, K.; Tang, X.; Gao, G. The relationships between the free-living and particle-attached bacterial communities in response to elevated eutrophication. Front. Microbiol. 2020, 11, 423. [Google Scholar] [CrossRef]
- Schimel, J.P. Life in dry soils: Effects of drought on soil microbial communities and processes. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 409–432. [Google Scholar] [CrossRef]
- Manzanera, M. Dealing with water stress and microbial preservation. Environ. Microbiol. 2021, 23, 3351–3359. [Google Scholar] [CrossRef]
- Brás, T.A.; Seixas, J.; Carvalhais, N.; Jagermeyr, J. Severity of drought and heatwave crop losses tripled over the last five decades in Europe. Environ. Res. Lett. 2021, 16, 065012. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef]
- Camaille, M.; Fabre, N.; Clément, C.; Barka, E.A. Advances in wheat physiology in response to drought and the role of plant growth promoting rhizobacteria to trigger drought tolerance. Microorganisms 2021, 9, 687. [Google Scholar] [CrossRef]
- Rysiak, A.; Dresler, S.; Hanaka, A.; Hawrylak-Nowak, B.; Strzemski, M.; Kováčik, J.; Sowa, I.; Latalski, M.; Wójciak, M. High temperature alters secondary metabolites and photosynthetic efficiency in Heracleum sosnowskyi. Int. J. Mol. Sci. 2021, 22, 4756. [Google Scholar] [CrossRef]
- Abdelaal, K.; Alkahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Xu, C.; Ma, W.; Yue, X.; Liang, X.; Zuo, X.; Knapp, A.K.; Smith, M.D.; Sardans, J.; Dijkstra, F.A.; et al. Effects of extreme drought on plant nutrient uptake and resorption in rhizomatous vs bunchgrass-dominated grasslands. Oecologia 2018, 188, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, L.P.; Guttikonda, S.K.; Phan Tran, L.S.; Nguyen, H.T. Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 2009, 50, 1260–1276. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.B.; Chu, L.Y.; Jaleel, C.A.; Zhao, C.X. Water-deficit stress-induced anatomical changes in higher plants. Comptes Rendus Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Hafez, Y.M.; El-Afry, M.M.; Tantawy, D.S.; Alshaal, T. Effect of some osmoregulators on photosynthesis, lipid peroxidation, antioxidative capacity, and productivity of barley (Hordeum vulgare L.) under water deficit stress. Environ. Sci. Pollut. Res. 2018, 25, 30199–30211. [Google Scholar] [CrossRef] [PubMed]
- Hafez, Y.; Attia, K.; Alamery, S.; Ghazy, A.; Al-Doss, A.; Ibrahim, E.; Rashwan, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K. Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy 2020, 10, 630. [Google Scholar] [CrossRef]
- Hargravei, K.R.; Kolb, K.J.; Ewers, F.W.; Davis, S.D. Conduit diameter and drought-induced embolism in Salvia mellifera Greene (Labiatae). New Phytol. 1994, 126, 695–705. [Google Scholar] [CrossRef]
- Liu, F.; Jensen, C.R.; Shahanzari, A.; Andersen, M.N.; Jacobsen, S.E. ABA regulated stomatal control and photosynthetic water use efficiency of potato (Solanum tuberosum L.) during progressive soil drying. Plant Sci. 2005, 168, 831–836. [Google Scholar] [CrossRef]
- Ullah, A.; Mushtaq, H.; Fahad, S.; Hakim; Shah, A.; Chaudhary, H.J. Plant growth promoting potential of bacterial endophytes in novel association with Olea ferruginea and Withania coagulans. Microbiology 2017, 86, 119–127. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ nutrition in plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol. Open 2018, 7. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Yang, S.L.; Dao, J.M.; Deng, J.; Shahzad, A.N.; Fan, X.; Li, R.D.; Quan, Y.J.; Bukhari, S.A.H.; Zeng, Z.H. Drought-induced alterations in photosynthetic, ultrastructural and biochemical traits of contrasting sugarcane genotypes. PLoS ONE 2020, 15, e0235845. [Google Scholar] [CrossRef]
- Cruz de Calvadio, M.H. Drought stress and reactive oxygen species. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Verma, G.; Srivastava, D.; Tiwari, P.; Chakrabarty, D. Reactive oxygen, nitrogen and sulfur species in plants: Production, metabolism, signaling and defense mechanisms. ROS modulation in crop plants under drought stress. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 311–336. [Google Scholar]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 1–16. [Google Scholar] [CrossRef]
- Thakur, M.; Sohal, B.S. Role of elicitors in inducing resistance in plants against pathogen infection: A review. ISRN Biochem. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Ali, S.; Khan, N. Delineation of mechanistic approaches employed by plant growth promoting microorganisms for improving drought stress tolerance in plants. Microbiol. Res. 2021, 249, 126771. [Google Scholar] [CrossRef] [PubMed]
- Vardharajula, S.; Ali, S.Z.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Jamiołkowska, A. Natural compounds as elicitors of plant resistance against diseases and new biocontrol strategies. Agronomy 2020, 10, 173. [Google Scholar] [CrossRef]
- Walters, D.; Newton, A.; Lyon, G. (Eds.) Induced Resistance for Plant Defence. A Sustainable Approach to Crop Protection; Blackwell Publishing: Oxford, UK, 2007; ISBN 978-1-4051-3447-7. [Google Scholar]
- Conrath, U.; Beckers, G.J.; Flors, V.; Garcia-Augustin, P.; Jakab, G.; Mauch, F.; Newman, M.-A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.; Pozo, M.J.; Ton, J. Recognizing plant defense priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef]
- Koziara, W.; Sulewska, H.; Panasiewicz, K. Effect of resistance stimulator application to some agricultural crops. J. Res. Appl. Agric. Eng. 2006, 51, 82–86. [Google Scholar]
- Babosha, A.V. Changes in lectin activity in plants treated with resistance inducers. Plant Physiol. 2004, 31, 51–55. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–407. [Google Scholar] [CrossRef] [PubMed]
- Henry, G.; Thonart, P.; Ongena, M. PAMPs, MAMPs, DAMPs and others: An update on the diversity of plant immunity elicitors. Biotechnol. Agron. Soc. Environ. 2012, 16, 257–268. [Google Scholar]
- Schwessinger, B.; Ronald, P.C. Plant innate immunity: Perception of conserved microbial signatures. Annu. Rev. Plant Biol. 2012, 63, 451–482. [Google Scholar] [CrossRef]
- Ranf, S. Sensing of molecular patterns through cell surface immune receptors. Curr. Opin. Plant Biol. 2017, 38, 68–77. [Google Scholar] [CrossRef]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef]
- Wang, C.J.; Yang, W.; Wang, C.; Gu, C.; Niu, D.D.; Liu, H.X.; Wang, Y.P.; Guo, J.H. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting Rhizobacterium strains. PLoS ONE 2012, 7, e52565. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Singh, H.B.; Keswani, C.; Reddy, M.S.; Sansinenea, E.; García-Estrada, C. Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms: Discovery and Applications; Springer: Singapore, 2019; ISBN 9789811358623. [Google Scholar]
- Oszust, K.; Pylak, M.; Frąc, M. Trichoderma-based biopreparation with prebiotics supplementation for the naturalization of raspberry plant rhizosphere. J. Mol. Sci. 2021, 22, 6356. [Google Scholar] [CrossRef]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.C. Perspectives and challenges of microbial application for crop improvement. Front. Plant Sci. 2017, 8, 49. [Google Scholar] [CrossRef]
- Sokolova, M.G.; Akimova, G.; Vaishlya, O.; Vedernikova, A. Physiological research of efficiency of biologically safe bacterial fertilizers. J. Manuf. Technol. Manag. 2010, 21, 956–970. [Google Scholar] [CrossRef]
- Abd El-Gleel Mosa, W.F.; Paszt, L.S.; Frąc, M.; Trzciński, P.; Treder, W.; Klamkowski, K. The role of biofertilizers in improving vegetative growth, yield and fruit quality of apple. Hortic. Sci. 2018, 45, 173–180. [Google Scholar] [CrossRef]
- Anli, M.; Baslam, M.; Tahiri, A.; Raklami, A.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Toubali, S.; Ait Rahou, Y.; et al. Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front. Plant Sci. 2020, 11, 516818. [Google Scholar] [CrossRef] [PubMed]
- Aseri, G.K.; Jain, N.; Panwar, J.; Rao, A.V.; Meghwal, P.R. Biofertilizers improve plant growth, fruit yield, nutrition, metabolism and rhizosphere enzyme activities of pomegranate (Punica granatum L.) in Indian Thar Desert. Sci. Hortic. 2008, 117, 130–135. [Google Scholar] [CrossRef]
- Cipriano, M.A.P.; Lupatini, M.; Lopes-Santos, L.; da Silva, M.J.; Roesch, L.F.W.; Destéfano, S.A.L.; Freitas, S.S.; Kuramae, E.E. Lettuce and rhizosphere microbiome responses to growth promoting Pseudomonas species under field conditions. FEMS Microbiol. Ecol. 2016, 92, fiw197. [Google Scholar] [CrossRef]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid and their potential in alleviating drought stress. Front. Microbiol. 2018, 8, 2580. [Google Scholar] [CrossRef] [PubMed]
- Astorga-Eló, M.; Gonzalez, S.; Acuña, J.J.; Sadowsky, M.J.; Jorquera, M.A. Rhizobacteria from ‘flowering desert’ events contribute to the mitigation of water scarcity stress during tomato seedling germination and growth. Sci. Rep. 2021, 11, 13745. [Google Scholar] [CrossRef]
- Milet, A.; Chaouche, N.K.; Dehimat, L.; Kaki, A.A.; Mounira, K.A.; Philippe, T. Flow cytometry approach for studying the interaction between Bacillus mojavensis and Alternaria alternata. Afr. J. Biotechnol. 2016, 15, 1417–1428. [Google Scholar] [CrossRef]
- Xie, Z.; Chu, Y.; Zhang, W.; Lang, D.; Zhang, X. Bacillus pumilus alleviates drought stress and increases metabolite accumulation in Glycyrrhiza uralensis Fisch. Environ. Exp. Bot. 2019, 158, 99–106. [Google Scholar] [CrossRef]
- Li, Y.; Shi, H.; Zhang, H.; Chen, S. Amelioration of drought effects in wheat and cucumber by the combined application of super absorbent polymer and potential biofertilizer. PeerJ 2019, 7, e6073. [Google Scholar] [CrossRef]
- Asghari, B.; Khademian, R.; Sedaghati, B. Plant growth promoting rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L.) under water shortage condition. Sci. Hortic. 2020, 263, 109132. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Sánchez-Romera, B.; Aroca, R.; Asins, M.J.; Declerck, S.; Dodd, I.C.; Martínez-Andújar, C.; Albacete, A.; Ruiz-Lozano, J.M. Exploring the use of recombinant inbred lines in combination with beneficial microbial inoculants (AM fungus and PGPR) to improve drought stress tolerance in tomato. Environ. Exp. Bot. 2016, 131, 47–57. [Google Scholar] [CrossRef]
- Behrooz, A.; Vahdati, K.; Rejali, F.; Lotfi, M.; Sarikhani, S.; Leslie, C. Arbuscular mycorrhiza and plant growth-promoting bacteria alleviate drought stress in walnut. HortScience 2019, 54, 1087–1092. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, S.; Dixit, V.; Kumar, M.; Agarwal, L.; Chauhan, P.S.; Nautiyal, C.S. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.). Plant Signal. Behav. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, V.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010, 62, 21–30. [Google Scholar] [CrossRef]
- Sarma, R.K.; Saikia, R. Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil 2014, 377, 111–126. [Google Scholar] [CrossRef]
- Roberson, E.B.; Firestone, M.K. Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl. Environ. Microbiol. 1992, 58, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, L.; Scartazza, A.; Curadi, M.; Picciarelli, P.; Toffanin, A. Azospirillum baldaniorum sp245 induces physiological responses to alleviate the adverse effects of drought stress in purple basil. Plants 2021, 10, 1141. [Google Scholar] [CrossRef]
- Vettori, L.; Russo, A.; Felici, C.; Fiaschi, G.; Morini, S.; Toffanin, A. Improving micropropagation: Effect of Azospirillum brasilense Sp245 on acclimatization of rootstocks of fruit tree. J. Plant Interact. 2010, 5, 249–259. [Google Scholar] [CrossRef]
- Heidari, M.; Mousavinik, S.M.; Golpayegani, A. Plant growth promoting Rhizobacteria (PGPR) effect on physiological parameters and mineral uptake in basil (Ociumum basilicum L.) under water stress. ARPN J. Agric. Biol. Sci. 2011, 6, 6–11. [Google Scholar]
- Jones, P.D.; Lister, D.H.; Jaggard, K.W.; Pidgeon, J.D. Future climate impact on the productivity of sugar beet (Beta vulgaris L.) in Europe. Clim. Chang. 2003, 58, 98–103. [Google Scholar] [CrossRef]
- Gauvry, E.; Mathot, A.-G.; Leguérinel, I.; Couvert, O.; Postollec, F.; Broussolle, V.; Coroller, L. Knowledge of the physiology of spore-forming bacteria can explain the origin of spores in the food environment. Res. Microbiol. 2016, 168, 369–378. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef] [PubMed]
- Prajakta, B.M.; Suvarna, P.P.; Raghvendra, S.P.; Alok, R.R. Potential biocontrol and superlative plant growth promoting activity of indigenous Bacillus mojavensis PB-35 (R11) of soybean (Glycine max) rhizosphere. SN Appl. Sci. 2019, 1, 1143. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Y.; Wang, C.; Liu, H.; Niu, D.; Wang, Y.; Guo, J. Enhancement of tomato (Lycopersicon esculentum) tolerance to drought stress by plant-growth-promoting rhizobacterium (PGPR) Bacillus cereus AR. J. Agric. Biotechnol. 2012, 20, 1097–1105. [Google Scholar]
- Lim, J.H.; Kim, S.D. Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis K11 in pepper. Plant Pathol. J. 2013, 29, 201–208. [Google Scholar] [CrossRef]
- Kaushal, M.; Wani, S.P. Plant-growth-promoting rhizobacteria: Drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 2016, 66, 35–42. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Kramer, S.; Green, D.M. Acid and alkaline phosphatase dynamics and their relationship to soil microclimate in a semiarid woodland. Soil Biol. Biochem. 2000, 32, 179–188. [Google Scholar] [CrossRef]
- Omer, A.M. Bioformulations of Bacillus spores for using as biofertilizer biovalorization of olive mill waste water for the production of natural biofertilizers and antioxidants view project isolation and identification of fungi and bacteria from different Egyptian e. Life Sci. J. 2010, 7, 1097–8135. [Google Scholar]
- O’Hara, G.W.; Boonkerd, N.; Dilworth, M.J. Mineral constraints to nitrogen fixation. Plant Soil 1988, 108, 93–110. [Google Scholar] [CrossRef]
- Xu, X.; Du, X.; Wang, F.; Sha, J.; Chen, Q.; Tian, G.; Zhu, Z.; Ge, S.; Jiang, Y. Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple Dwarf Rootstock seedlings. Front. Plant Sci. 2020, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Avakyan, Z.A. Silicon compounds in solution bacteria quartz degradation. Mikrobiologiya 1984, 54, 301–307. [Google Scholar]
- Malinovskaya, I.M.; Kosenko, L.V.; Votselko, S.K.; Podgorsky, V.S. The role of Bacillus mucilaginosus polysaccharide in the destruction of silicate minerals. Mikrobiologiya 1990, 59, 70–78. [Google Scholar]
- Barnawal, D.; Maji, D.; Bharti, N.; Chanotiya, C.S.; Kalra, A. ACC deaminase-containing Bacillus subtilis reduces stress ethylene-induced damage and improves mycorrhizal colonization and rhizobial nodulation in Trigonella foenum-graecum under drought stress. J. Plant Growth Regul. 2013, 32, 809–822. [Google Scholar] [CrossRef]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for beneficial microorganisms inocula used as biofertilizers. Sci. World J. 2012, 1–12. [Google Scholar] [CrossRef]
- Roberts, M.S.; Nakamura, L.K.; Cohan, F.M. Bacillus mojavensis sp. nov., distinguishable from Bacillus subtilis by sexual isolation, divergence in DNA sequence, and differences in fatty acid composition. Int. J. Syst. Bacteriol. 1994, 44, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Qiu, Z.; You, J.; Tan, H.; Zhou, S. Isolation and characterization of endophytic Streptomyces strains from surface-sterilized tomato (Lycopersicon esculentum) roots. Lett. Appl. Microbiol. 2004, 39, 425–430. [Google Scholar] [CrossRef]
- Moussa, T.A. Studies on biological control of sugar beet pathogen Rhizoctonia solani Kühn. J. Biol. Sci. 2002, 2, 800–804. [Google Scholar]
- Snook, M.E.; Mitchell, T.; Hinton, D.M.; Bacon, C.W. Isolation and characterization of Leu 7-surfactin from the endophytic bacterium Bacillus mojavensis RRC 101, a biocontrol agent for Fusarium verticillioides. J. Agric. Food Chem. 2009, 57, 4287–4292. [Google Scholar] [CrossRef]
- Romano-Armada, N.; Yañez-Yazlle, M.F.; Irazusta, V.P.; Rajal, V.B.; Moraga, N.B. Potential of bioremediation and PGP traits in Streptomyces as strategies for bio-reclamation of salt-affected soils for agriculture. Pathogens 2020, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, J.; Mohammadipanah, F. Biotechnological application and taxonomical distribution of plant growth promoting actinobacteria. J. Ind. Microbiol. Biotechnol. 2015, 42, 157–171. [Google Scholar] [CrossRef]
- Chitraselvi, R.P.E. Actinomycetes: Dependable tool for sustainable agriculture. Curr. Investig. Agric. Curr. Res. 2018, 1, 128–130. [Google Scholar] [CrossRef]
- Lawlor, K.; Knight, B.P.; Barbosa-Jefferson, V.L.; Lane, P.W.; Lilley, A.K.; Paton, G.I.; McGrath, S.P.; O’Flaherty, S.M.; Hirsch, P.R. Comparison of methods to investigate microbial populations in soils under different agricultural management. FEMS Microbiol. Ecol. 2000, 33, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Pierzynski, G.M.; Vance, G.F.; Sims, J.T. Soils and Environmental Quality; Taylor and Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Khamna, S.; Yokota, A.; Peberdy, J.F.; Lumyong, S. Indole-3-acetic acid production by Streptomyces sp. isolated from some Thai medicinal plant rhizosphere soils. Eur. Asian J. Biosci. 2010, 4, 23–32. [Google Scholar] [CrossRef]
- El-Tarabily, K.A. Promotion of tomato (Lycopersicon esculentum Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase-producing Streptomycete Actinomycetes. Plant Soil 2008, 308, 161–174. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Srinivas, V.; Vidya, M.S.; Rathore, A. Plant growth-promoting activities of Streptomyces spp. in sorghum and rice. Springerplus 2013, 2, 1–8. [Google Scholar] [CrossRef]
- El-Sayed, S.F.; Hassan, H.A.; El-Mogy, M.M. Impact of bio- and organic fertilizers on potato yield, quality and tuber weight loss after harvest. Potato Res. 2015, 58, 67–81. [Google Scholar] [CrossRef]
- Wahyudi, A.T.; Priyanto, J.A.; Afrista, R.; Kurniati, D.; Astuti, R.I.; Akhdiya, A. Plant growth promoting activity of Actinomycetes isolated from soybean rhizosphere. Online J. Biol. Sci. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Tredici, P. Del Nitrogen fixation: The story of the Frankia symbiosis. Arnold Arbor. 1995, 55, 26–31. [Google Scholar]
- Treseder, K.K.; Berlemont, R.; Allison, S.D.; Martiny, A.C. Drought increases the frequencies of fungal functional genes related to carbon and nitrogen acquisition. PLoS ONE 2018, 13, e0206441. [Google Scholar] [CrossRef] [PubMed]
- Treseder, K.K.; Lennon, J.T. Fungal traits that drive ecosystem dynamics on land. Microbiol. Mol. Biol. Rev. 2015, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Arias, D.; García-Machado, F.J.; Morales-Sierra, S.; García-García, A.L.; Herrera, A.J.; Valdés, F.; Luis, J.C.; Borges, A.A. A beginner’s guide to osmoprotection by biostimulants. Plants 2021, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Verma, H.; Kumar, D.; Kumar, V.; Kumari, M.; Singh, S.K.; Sharma, V.K.; Droby, S.; Santoyo, G.; White, J.F.; Kumar, A. The potential application of endophytes in management of stress from drought and salinity in crop plants. Microorganisms 2021, 9, 1729. [Google Scholar] [CrossRef]
- Bizos, G.; Papatheodorou, E.M.; Chatzistathis, T.; Ntalli, N.; Aschonitis, V.G.; Monokrousos, N. The role of microbial inoculants on plant protection, growth stimulation, and crop productivity of the olive tree (Olea europea L.). Plants 2020, 9, 743. [Google Scholar] [CrossRef]
- Bona, E.; Lingua, G.; Manassero, P.; Cantamessa, S.; Marsano, F.; Todeschini, V.; Copetta, A.; D’Agostino, G.; Massa, N.; Avidano, L.; et al. AM fungi and PGP Pseudomonads increase flowering, fruit production, and vitamin content in strawberry grown at low nitrogen and phosphorus levels. Mycorrhiza 2015, 25, 181–193. [Google Scholar] [CrossRef]
- Adamec, S.; Andrejiová, A. Mycorrhiza and stress tolerance of vegetables: A review. Acta Hortic. Regiotect. 2018, 21, 30–35. [Google Scholar] [CrossRef]
- Marulanda, A.; Barea, J.M.; Azcón, R. Stimulation of plant growth and drought tolerance by native microorganisms (AM fungi and bacteria) from dry environments: Mechanisms related to bacterial effectiveness. J. Plant Growth Regul. 2009, 28, 115–124. [Google Scholar] [CrossRef]
- Matias, S.R.; Pagano, M.C.; Muzzi, F.C.; Oliveira, C.A.; Carneiro, A.A.; Horta, S.N.; Scotti, M.R. Effect of rhizobia, mycorrhizal fungi and phosphate-solubilizing microorganisms in the rhizosphere of native plants used to recover an iron ore area in Brazil. Eur. J. Soil Biol. 2009, 45, 259–266. [Google Scholar] [CrossRef]
- Dreischhoff, S.; Das, I.S.; Jakobi, M.; Kasper, K.; Polle, A. Local responses and systemic induced resistance mediated by ectomycorrhizal fungi. Front. Plant Sci. 2020, 11, 590063. [Google Scholar] [CrossRef] [PubMed]
- De Battista, J.P.; Bacon, C.W.; Severson, R.; Plattner, R.D. Indole acetic acid production by the fungal endophyte of Tall Fescue. Agron. J. 1990, 82, 878–880. [Google Scholar] [CrossRef]
- Khan, A.L.; Waqas, M.; Khan, A.R.; Hussain, J.; Kang, S.M.; Gilani, S.A.; Hamayun, M.; Shin, J.H.; Kamran, M.; Al-Harrasi, A.; et al. Fungal endophyte Penicillium janthinellum LK5 improves growth of ABA-deficient tomato under salinity. World J. Microbiol. Biotechnol. 2013, 29, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Clayy, K. Impact of a horizontally transmitted endophyte, Balansia henningsiana, on growth and drought tolerance of Panicum rigidulum. Int. J. Plant Sci. 2009, 170, 599–608. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Li, H.; Sivasithamparam, K.; Jones, M.G.K.; Wylie, S.J. Fungal endophytes and a virus confer drought tolerance to Nicotiana benthamiana plants through modulating osmolytes, antioxidant enzymes and expression of host drought responsive genes. Environ. Exp. Bot. 2018, 149, 95–108. [Google Scholar] [CrossRef]
- Davies, F.T.; Potter, J.R.; Linderman Davies, R.G.; Ft, R.; Linderman, R. Drought resistance of mycorrhizal pepper plants independent of leaf P concentration-response in gas exchange and water relations. Physiol. Plant. 1993, 87, 45–53. [Google Scholar] [CrossRef]
- Liao, X.; Chen, J.; Guan, R.; Liu, J.; Sun, Q. Two arbuscular mycorrhizal fungi alleviates drought stress and improves plant growth in Cinnamomum migao seedlings. Mycobiology 2021, 49, 396–405. [Google Scholar] [CrossRef]
- Azad, K.; Kaminskyj, S. A fungal endophyte strategy for mitigating the effect of salt and drought stress on plant growth. Symbiosis 2016, 68, 73–78. [Google Scholar] [CrossRef]
- Sun, C.; Johnson, J.M.; Cai, D.; Sherameti, I.; Oelmüller, R.; Lou, B. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J. Plant Physiol. 2010, 167, 1009–1017. [Google Scholar] [CrossRef]
- Morsy, M.; Cleckler, B.; Armuelles-Millican, H. Fungal endophytes promote tomato growth and enhance drought and salt tolerance. Plants 2020, 9, 877. [Google Scholar] [CrossRef]
- Subramanian, K.S.; Santhanakrishnan, P.; Balasubramanian, P. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci. Hortic. 2006, 107, 245–253. [Google Scholar] [CrossRef]
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef]
- Wu, Q.S.; Xia, R.X.; Zou, Y.N. Improved soil structure and citrus growth after inoculation with three arbuscular mycorrhizal fungi under drought stress. Eur. J. Soil Biol. 2008, 44, 122–128. [Google Scholar] [CrossRef]
- Thirumalai, E.; Venkatachalam, A.; Suryanarayanan, T.S. Fungal endophytes of betel leaves: The need to study mycotoxin-producing endophytes in leafy vegetables. Sydowia 2020, 73, 83–88. [Google Scholar] [CrossRef]
- Carbone, M.J.; Alaniz, S.; Mondino, P.; Gelabert, M.; Eichmeier, A.; Tekielska, D.; Bujanda, R.; Gramaje, D. Drought influences fungal community dynamics in the grapevine rhizosphere and root microbiome. J. Fungi 2021, 7, 686. [Google Scholar] [CrossRef]
- Piri, R.; Moradi, A.; Balouchi, H.; Salehi, A. Improvement of cumin (Cuminum cyminum) seed performance under drought stress by seed coating and biopriming. Sci. Hortic. 2019, 257, 108667. [Google Scholar] [CrossRef]

| Bacteria | Changes in Plants | Plants | Ref. |
|---|---|---|---|
| Bacillus pumilus | - H total biomass (to 34.9%) - H antioxidant enzyme activities - H flavonoids, polysaccharide and glycyrrhizic acid contents | Glycyrrhiza uralensis | [96] |
| Bacillus sp. | - H roots and shoots fresh and dry weight - H shoot length | Cucumis sativus | [97] |
| Azotobacter chroococcum | - L GPX activity (to 12.5%) | Mentha pulegium | [98] |
| Pseudomonas putida KT2440 (pUCP22:otsAB) | - H trehalose [11-fold vs. P. putida KT2440 (pUCP) without the gen] - H fresh and dry weight - H fully turgid weight - H RWC | Capsicum annuum cv. Maor | [23] |
| Azospirillum brasilense | - L GPX activity (to 14.7%) - H CAT activity (2.6-fold vs. control) | Mentha pulegium | [98] |
| Variovorax paradoxus 5C-2 | - H shoot dry weight - H net photosynthesis - H relative Chl content - L proline content | Solanum lycopresicum cv. Boludo F1 | [99] |
| Rhodococcus sp. 4J2A2 | xeroprotectant effect of trehalose in preventing the biomolecules | Solanum esculentum cv. F144 | [23] |
| Azotobacter chroococcum with Azospirilum lipofrum | - H fresh weight - H root growth and length - H total phenolics content in leaves - H peroxidase activity | Juglans regia | [100] |
| Azotobacter chroococcum with Azospirillum brasilense | - H RWC (from 64.6% to 72.1%) - H Fv/Fm (from 0.56 to 0.75) - H SOD activity (from 34.7% to 57.2%) - L GPX activity (to 26.9%) | Mentha pulegium | [98] |
| Microbacterium sp. 3J1 or Arthrobacter koreensis 5J12A or Arthrobacter piechaudii 366-5 | - H fresh and dry weight - H turgid weight - H RWC - H roots and stems length | Capsicum annuum cv. Maor | [23] |
| Bacillus cereus AR156 with Bacillus subtilis SM21 with Serratia sp. XY21 | - H proline content in leaves - L MDA content in leaves - L peroxidation in plasmalemma | Cucumis sativus | [82] |
| Fungi | Changes in Plants | Plants | Ref. |
|---|---|---|---|
| Glomus intraradices | - H content of N in roots and shoots - H flower and fruit production - H fruit yield - H dry root mass | Lycopersicum esculentum | [159] |
| Glomus mosesseae | - slow down the reduction of Chl a+b - inhibit the decomposition of carotenoids | Fragaria x ananassa | [32] |
| Glomus etunicatum | - H fresh weight - H number of leaves - H content of N, P, Zn in leaves | Juglans regia | [100] |
| Ampelomyces sp. | - H dry weight of root and shoot - H fruit weight - H stress tolerance | Solanum lycopersicum var. Better Boy | [158] |
| Phoma spp. | - H proline content, CAT and SOD activities - H chlorophyll content - H MDA content - H water content in leaves | Pinus tablaeformis | [160] |
| Piriformospora indica | - H root and shoot growth - H lateral root development - H peroxidase, CAT and SOD activities in leaves | Brassica rapa subsp. pekinensis | [157] |
| Trichodermaharzianum | - H fresh and dry weight of roots - H osmolyte concentration | Theobroma cacao | [160] |
| Glomus mossae with Glomus etunicatum | - H height - H content of N, P, Zn in leaves - L leaves abscission | Juglans regia | [100] |
| Glomus lamellosum or Glomus etunicatum | - H fresh and dry weight - H stem fresh weight - H water content in leaves - L MDA content - H CAT activity | Cinnamomum migao | [155] |
| Glomus mosseae or G. versiforme or G. diaphanum | - H enzyme activity in soil - L CAT activity in soil - H hyphal density | Poncirus trifoliata | [161] |
| Chaetomium globosum or Penicillium resedanum | - H shoot dry weight - H shoot length - H photosynthesis rate | Capsicum annum | [151] |
| Alternaria sp. or Trichoderma harzianum | - H root and shoot dry weight | Solanum lycopersicum var. Rutger | [156] |
| Bacteria with Fungi | Changes in Plants | Plants | Ref. |
|---|---|---|---|
| Pseudomonas fluorescence with Trichoderma harzianum | - H growth parameters - H seedling emergency - H root and shoot length - H CAT and APX activities | Cuminum cyminum | [164] |
| Variovorax paradoxus 5C-2 with Rhizophorus irregularis MULC | - H shoot dry weight - H net photosynthesis - no change: relative Chl content vs. control - H oxidative damage - L proline content | Solanum lycopersicum cv. Boludo F1 | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanaka, A.; Ozimek, E.; Reszczyńska, E.; Jaroszuk-Ściseł, J.; Stolarz, M. Plant Tolerance to Drought Stress in the Presence of Supporting Bacteria and Fungi: An Efficient Strategy in Horticulture. Horticulturae 2021, 7, 390. https://doi.org/10.3390/horticulturae7100390
Hanaka A, Ozimek E, Reszczyńska E, Jaroszuk-Ściseł J, Stolarz M. Plant Tolerance to Drought Stress in the Presence of Supporting Bacteria and Fungi: An Efficient Strategy in Horticulture. Horticulturae. 2021; 7(10):390. https://doi.org/10.3390/horticulturae7100390
Chicago/Turabian StyleHanaka, Agnieszka, Ewa Ozimek, Emilia Reszczyńska, Jolanta Jaroszuk-Ściseł, and Maria Stolarz. 2021. "Plant Tolerance to Drought Stress in the Presence of Supporting Bacteria and Fungi: An Efficient Strategy in Horticulture" Horticulturae 7, no. 10: 390. https://doi.org/10.3390/horticulturae7100390
APA StyleHanaka, A., Ozimek, E., Reszczyńska, E., Jaroszuk-Ściseł, J., & Stolarz, M. (2021). Plant Tolerance to Drought Stress in the Presence of Supporting Bacteria and Fungi: An Efficient Strategy in Horticulture. Horticulturae, 7(10), 390. https://doi.org/10.3390/horticulturae7100390