Phytochemical Traits and Biological Activity of Eryngium amethystinum and E. alpinum (Apiaceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Herbal Material
2.2. Microorganisms
2.3. Gas Chromatography and Mass Spectrometry (GC and GC–MS)
2.3.1. Sample Extract Preparation
2.3.2. GC, GC–MS Conditions
2.4. HPLC Analysis
2.4.1. Sample Extract Preparation
2.4.2. Preparation of Standard Solutions
2.4.3. HPLC Conditions
2.5. Total Phenol and Total Flavonoid Content
2.6. Antioxidant Capacity
2.6.1. DPPH Radical-Scavenging Activity
2.6.2. β-Carotene-Linoleic Acid Assay
2.6.3. Fe2+ Chelating Activity (ChA)
2.6.4. The Reducing Power of the Extracts
2.7. Antimicrobial Susceptibility Assay
2.7.1. Micro-Dilution Assay
2.7.2. Inhibition of Germination of Candida Albicans Blastospores
2.8. Statistical Analysis
3. Results and Discussion
3.1. Gas Chromatography and Mass Spectrometry (GC and GC–MS)
3.2. HPLC Analysis
3.3. Total Phenol and Total Flavonoid Content
3.4. Antioxidant Capacity
3.4.1. DPPH Radical-Scavenging Activity
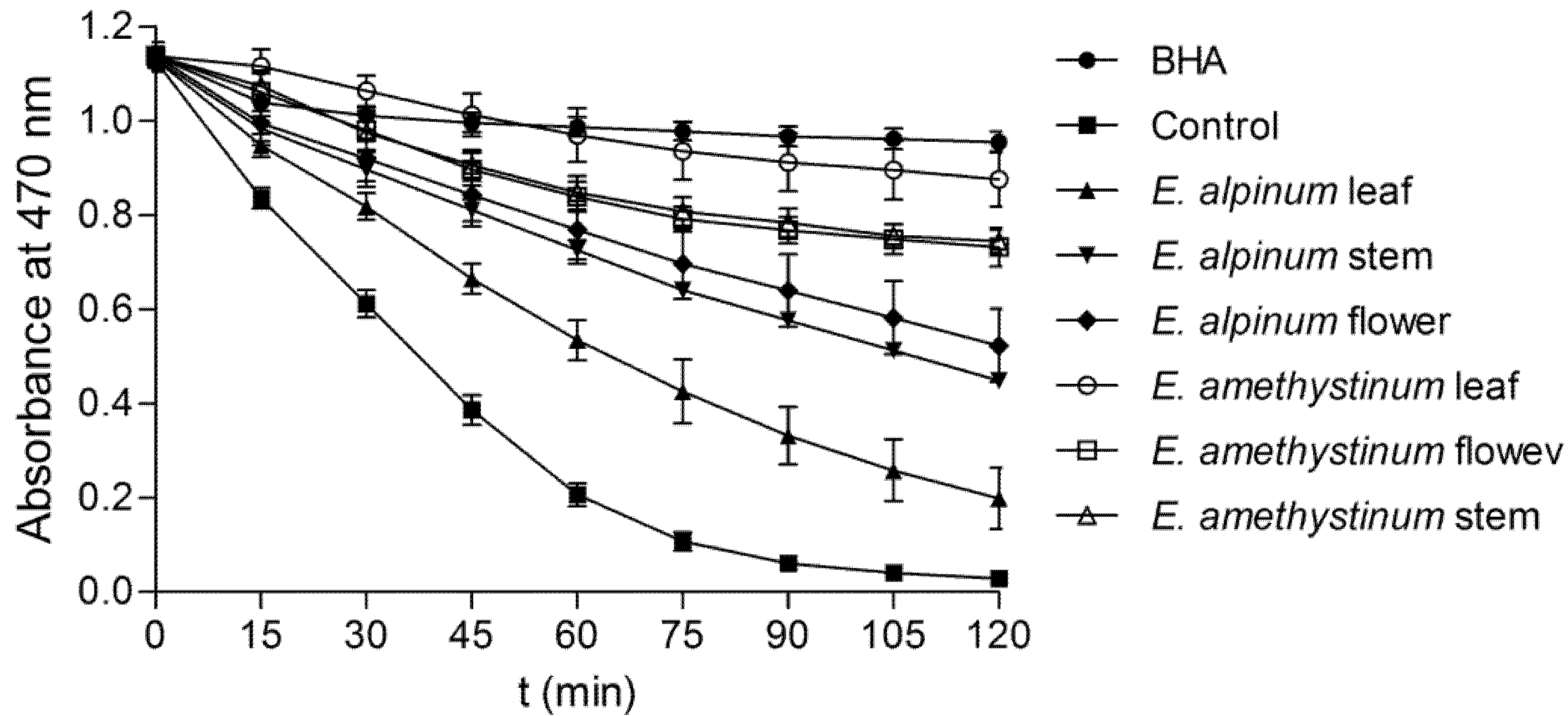
3.4.2. β-Carotene-Linoleic Acid Assay
3.4.3. Chelating Activity
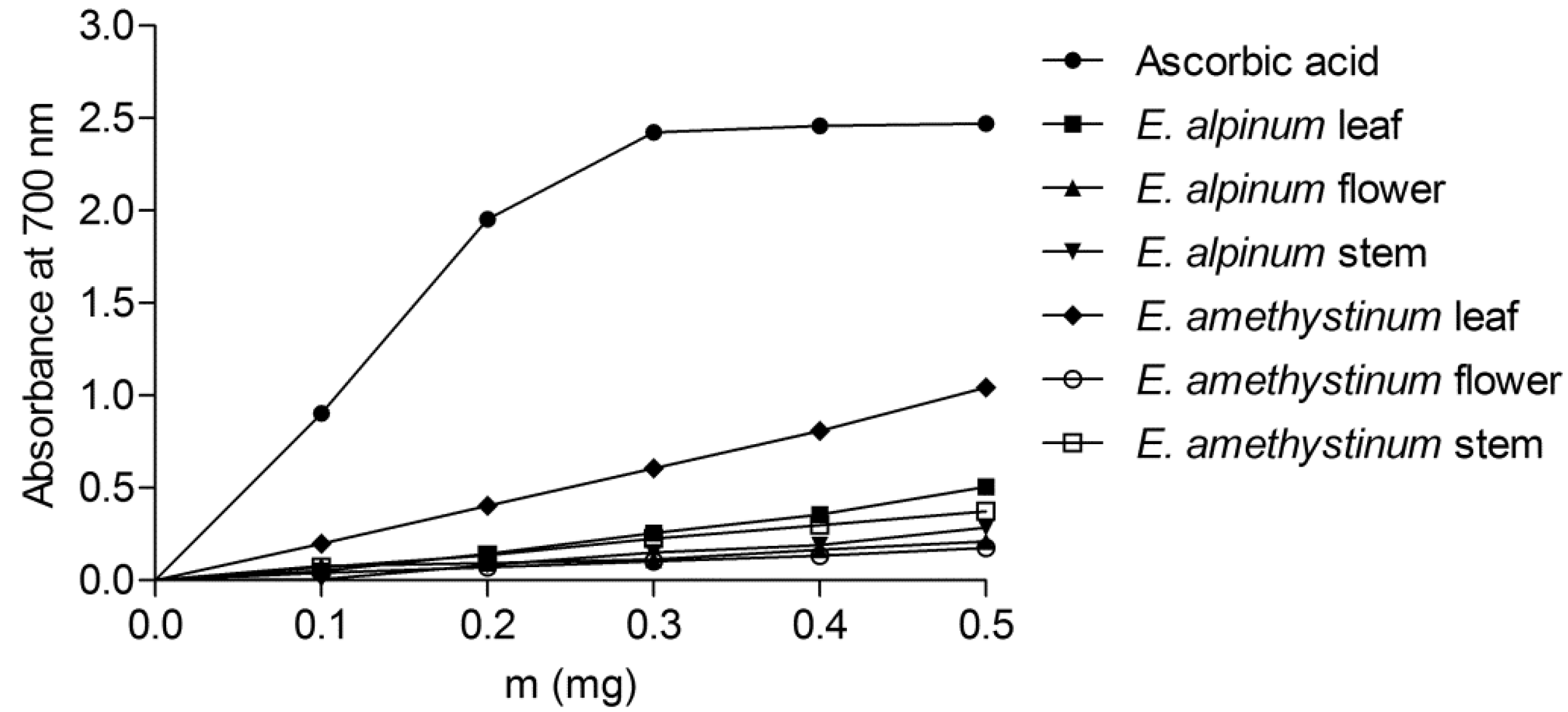
3.4.4. The Reducing Power of the Extracts
3.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wielgorskaya, T. Dictionary of Generic Names of Seed Plants; Columbia University Press: New York, NY, USA, 1995; p. 254. [Google Scholar]
- Chater, A.O. Eryngium L. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1978; Volume 2, pp. 320–324. [Google Scholar]
- Domac, R. Croatian Flora; Školska knjiga: Zagreb, Croatia, 1994; pp. 231–232. [Google Scholar]
- Abu-Rabia, A. Herbs as a food and medicine source in Palestine. Asian Pac. J. Cancer Prev. 2005, 6, 404–407. [Google Scholar] [PubMed]
- Martinis, A.P.; Salgueiro, L.G.; de Proenca Cunha, A.; Vila, R.; Casigueral, F.; Casanova, J. Essential oil composition of Eryngium foetidum from S. Tome e Principe. J. Essent. Oil Res. 2003, 15, 93–95. [Google Scholar] [CrossRef]
- Yesilada, E.; Tanaka, S.; Tabata, M.; Sezik, E. The antiinflammatory activity of the fractions from Eryngium billardieri in mice. Phytother. Res. 1989, 3, 38–40. [Google Scholar] [CrossRef]
- Pahlow, M. Great Book of Medicinal Plants; Cankarjeva založba: Ljubljana, Slovenia; Zagreb, Croatia, 1989; pp. 153–154. [Google Scholar]
- Redžić, S.S. The ecological aspect of ethnobotany and ethnopharmacology of population in Bosnia and Herzegovina. Coll. Antropol. 2007, 31, 869–890. [Google Scholar]
- Grlić, L.J. Encyclopaedia of Wild Edible Plants; August Cesarec: Zagreb, Croatia, 1986; pp. 245–246. [Google Scholar]
- Palá-Paúl, J.; Copeland, L.M.; Brophy, J.J.; Goldsack, R.J. Essential oil of Eryngium rosulatum P. W. Michael: A new undescribed species from eastern Australia. Biochem. Syst. Ecol. 2006, 34, 796–801. [Google Scholar] [CrossRef]
- Capetanos, C.; Saroglou, V.; Marin, P.D.; Simic, A.; Skaltsa, H.D. Essential oil analysis of two endemic Eryngium species from Serbia. J. Serb. Chem. Soc. 2007, 72, 961–965. [Google Scholar] [CrossRef]
- Flamini, G.; Tebano, M.; Cioni, L. Composition of the essential oils from leafy parts of the shoots, flowers and fruits of Eryngium amethystinum from Amiata Mount (Tuscany, Italy). Food Chem. 2008, 107, 671–674. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Ownby, S.; Wang, P.; Yuan, W.; Zhang, W.; Beasley, R.S. Phenolic compounds and rare polyhydroxylated triterpenoid saponins from Eryngium yuccifolium. Phytochemistry 2008, 69, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Damianaki, A.; Bakogeorgou, E.; Kampa, M.; Notas, G.; Hatzoglou, A.; Panagiotou, S.; Gemetzi, C.; Kouroumalis, E.; Martin, P.M.; Castanas, E. Potent inhibitory action of red wine polyphenols on human breast cancer cells. J. Cell. Biochem. 2000, 78, 429–441. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- NIST WebBook. Available online: https://webbook.nist.gov/ (accessed on 14 August 2021).
- Kremer, D.; Košir, I.J.; Kosalec, I.; Zovko Končić, M.; Potočnik, T.; Čerenak, A.; Bezić, N.; Srečec, S.; Dunkić, V. Investigation of chemical compounds, antioxidant and antimicrobial properties of Teucrium arduini L. (Lamiaceae). Curr. Drug Targets 2013, 14, 1006–1014. [Google Scholar] [CrossRef]
- Čeh, B.; Kač, M.; Košir, I.J.; Abram, V. Relationships between xanthohumol and polyphenol content in hop leaves and hop cones with regard to water supply and cultivar. Int. J. Mol. Sci. 2007, 8, 989–1000. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteau reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Zovko Končić, M.; Kremer, D.; Karlović, K.; Kosalec, I. Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L. and Berberis croatica Horvat. Food Chem. Toxicol. 2010, 48, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Pegg, R.B.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J.A. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of ferritin as lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D.; Chuang, D.-Y. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000, 70, 437–441. [Google Scholar] [CrossRef]
- National Committee for Laboratory Standards (NCCLS). Reference Method for Broth Dilution Antifungal Susceptibility of Yeasts, Approved Standards M27-A; National Committee for Laboratory Standards: Wayne, PA, USA, 1997. [Google Scholar]
- National Committee for Laboratory Standards (NCCLS). Reference Method for Dilution Antimicrobial Susceptibility Testing of Bacteria that Growth Aerobically, Approved Standards M07-A8; National Committee for Laboratory Standards: Wayne, PA, USA, 2009. [Google Scholar]
- Ishida, K.; de Mello, J.C.; Cortez, D.A.; Filho, B.P.; Ueda-Nakamura, T.; Nakamura, C.V. Influence of tannins from Stryphnodendron adstringens on growth and virulence factors of Candida albicans. J. Antimicrob. Chemother. 2006, 58, 942–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- JMP; Version 6; SAS Institute Inc.: Cary, NC, USA, 2005–2021.
- STATISTICA; Version 7; StatSoft Inc.: Tulsa, OK, USA, 2005.
- Dunkić, V.; Vuko, E.; Bezić, N.; Kremer, D.; Ruščić, M. Composition and antiviral activity of the essential oils of Eryngium alpinum and E. amethystinum. Chem. Biodivers. 2013, 10, 1894–1902. [Google Scholar] [CrossRef]
- Cianfaglione, K.; Blomme, E.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Dall’Acqua, S.; Maggi, F. Cytotoxic essential oils from Eryngium campestre and Eryngium amethystinum (Apiaceae) growing in central Italy. Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Medbouhi, A.; Benbelaïd, F.; Djabou, N.; Beaufay, C.; Bendahou, M.; Quetin-Leclercq, J.; Tintaru, A.; Costa, J.; Muselli, A. Essential oil of Algerian Eryngium campestre: Chemical variability and evaluation of biological activities. Molecules 2019, 24, 2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landoulsi, A.; Hennebelle, T.; Bero, J.; Rivière, C.; Sahpaz, S.; Quetin-Leclercq, J.; Neut, C.; Benhamida, J.; Roumy, V. Antimicrobial and light-enhanced antimicrobial activities, cytotoxicity and chemical variability of all Tunisian Eryngium species. Chem. Biodivers. 2020, 14. [Google Scholar] [CrossRef]
- Casiglia, S.; Brunoa, M.; Rossellia, S.; Senatore, F. Chemical composition and antimicrobial activity of the essential oil from flowers of Eryngium triquetrum (Apiaceae) collected wild in Sicily. Nat. Prod. Commun. 2016, 11, 1019–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikowska, M.; Kalemba, D.; Dlugaszewska, J.; Thiem, B. Chemical composition of essential oils from rare and endangered species—Eryngium maritimum L. and E. alpinum L. Plants 2020, 9, 417. [Google Scholar] [CrossRef] [Green Version]
- Kikowska, M.; Thiem, B.; Szopa, A.; Klimek-Szczykutowicz, M.; Rewers, M.; Sliwinska, E.; Ekiert, H. Comparative analysis of phenolic acids and flavonoids in shoot cultures of Eryngium alpinum L.: An endangered and protected species with medicinal value. Plant Cell Essent. Organ Cult. 2019, 139, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Ikramov, M.T.; Bandyukova, V.A.; Khalmatov, K.K. Flavonoids of some Eryngium species. Khim. Prir. Soedin. 1971, 7, 117–118. [Google Scholar] [CrossRef]
- Kartnig, T.; Wolf, J. Flavonoids from the aerial parts of Eryngium campestre. Planta Med. 1993, 59, 285–286. [Google Scholar] [CrossRef]
- Suleiman, A.K. Phytochemistry of Eryngium creticum. Alexandria J. Pharm. Sci. 1994, 8, 73–75. [Google Scholar]
- Zarnack, J.; Hildebrandt, B.; Hiller, K.; Otto, A. To the knowledge of the compounds contained in some Saniculoideae. part 33. isolation of flavonol glycosides from Eryngium giganteum M.B. Z. Chem. 1979, 19, 214–215. [Google Scholar] [CrossRef]
- Hiller, K.; Pohl, B.; Franke, P. Flavonoid spectrum of Eryngium maritimum L. Part 35. Components of some Saniculoideae. Pharmazie 1981, 36, 451–452. [Google Scholar]
- Vukic, M.D.; Vukovic, N.L.; Djelic, G.T.; Obradovic, A.; Kacaniova, M.M.; Markovic, S.; Popović, S.; Baskić, D. Phytochemical analysis, antioxidant, antibacterial and cytotoxic activity of different plant organs of Eryngium serbicum L. Ind. Crops Prod. 2018, 115, 88–97. [Google Scholar] [CrossRef]
- Le Claire, E.; Schwaiger, S.; Banaigs, B.; Stuppner, H.; Gafner, F. Distribution of a new rosmarinic acid derivative in Eryngium alpinum L. and other Apiaceae. J. Agric. Food Chem. 2005, 53, 4367–4372. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Nabavi, S.F.; Alinezhad, H.; Zare, M.; Azimi, R. Biological activities of flavonoid-rich fraction of Eryngium caucasicum Trautv. Riv. Eur. Sci. Med. Farmacol. 2012, 16 (Suppl. 3), 81–87. [Google Scholar]
- Daneshzadeh, S.M.; Abbaspour, H.; Amjad, L.; Nafchi, A.M. An investigation on phytochemical, antioxidant and antibacterial properties of extract from Eryngium billardieri F. Delaroche. J. Food Meas. Charact. 2020, 14, 708–715. [Google Scholar] [CrossRef]
- Nejati, M.; Masoudi, S.; Dastan, D.; Masnabadi, N. Phytochemical analysis and antibacterial activity of E. pyramidale Boiss. & Hausskn. J. Chil. Chem. Soc. 2021, 66, 5230–5236. [Google Scholar] [CrossRef]
- Kholkhal, W.; Ilias, F.; Bekhechi, C.; Bekkara, F.A. Eryngium maritimum: A rich medicinal plant of polyphenols and flavonoids compounds with antioxidant, antibacterial and antifungal activities. Curr. Res. J. Biol. Sci. 2012, 4, 437–443. [Google Scholar]
- Meot-Duros, L.; Le Floch, G.; Magné, C. Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J. Ethnopharmacol. 2008, 116, 258–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marčetić, M.D.; Petrović, S.D.; Milenković, M.T.; Niketić, M.S. Composition, antimicrobial and antioxidant activity of the extracts of Eryngium palmatum Pančić and Vis. (Apiaceae). Cent. Eur. J. Biol. 2014, 9, 149–155. [Google Scholar] [CrossRef]
- Minatel, I.O.; Vanz Borges, C.; Ferreira, M.I.; Gomez Gomez, H.A.; Chen, C.-Y.O.; Pace Pereira Lima, G. Phenolic compounds: Functional properties, impact of processing and dioavailability. In Phenolic Compunds—Biological Activit; Soto Hernández, M., Ed.; Intech: London, UK, 2017; pp. 1–24. [Google Scholar] [CrossRef] [Green Version]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food. Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Guo, Y.; Baschieri, A.; Amorati, R.; Valgimigli, L. Synergic antioxidant activity of γ-terpinene with phenols and polyphenols enabled by hydroperoxyl radicals. Food Chem. 2021, 345, 128468. [Google Scholar] [CrossRef] [PubMed]
- Johari, M.A.; Khong, H.Y. Total phenolic content and antioxidant and antibacterial activities of Pereskia bleo. Adv. Pharmacol. Pharm. Sci. 2019, 428593. [Google Scholar] [CrossRef] [Green Version]
- Thiem, B.; Goślińska, O.; Kikowska, M.; Budzianowski, J. Antimicrobial activity of three Eryngium L. species (Apiaceae). Herba Pol. 2010, 56, 52–59. [Google Scholar]
- Hołderna-Kędzia, E.; Kędzia, B. Estimation of antibiotic activity of plant extracts. Postępy Fitoter. 2010, 2, 59–70. [Google Scholar]
- Karimi, S.; Lotfipour, L.; Asnaashari, S.; Asgharian, P.; Sarvari, Y.; Hazrati, S. Phytochemical analysis and anti-microbial activity of some Important medicinal plants from north-west of Iran. Iran. J. Pharm. Res. 2019, 18, 1871–1883. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Valérian Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (quantitative structure–activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Ashida, H.; Danno, G. Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Biosci. Biotechnol. Biochem. 2002, 66, 1009–1014. [Google Scholar] [CrossRef] [Green Version]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Bouhdid, S.; Abrini, J.; Amensour, M.; Zhiri, A.; Espuny, M.J.; Manresa, A. Functional and ultrastructural changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Cinnamomum verum essential oil. J. Appl. Microbiol. 2010, 109, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Lin, Y.; Chai, X.; Duan, X.; Zhao, X.; Chun, C. Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci. Nutr. 2019, 7, 2546–2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α- and β-Pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- De Lucca, A.J.; Pauli, A.; Schilcher, H.; Sien, T.; Bhatnagar, D.; Walsh, T.J. Fungicidal and bactericidal properties of bisabolol and dragosantol. J. Essent. Oil Res. 2011, 23, 47–54. [Google Scholar] [CrossRef]
- Marcel Forrer, F.; Kulika, E.M.; Filippi, A.; Waltimo, T. The antimicrobial activity of alpha-bisabolol and tea tree oil against Solobacterium moorei, a Gram-positive bacterium associated with halitosis. Arch. Oral Biol. 2013, 58, 10–16. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Schmidt, E.; Bail, S.; Friedl, S.M.; Jirovetz, L.; Gerhard Buchbauer, G.; Wanner, J.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Geissler, M. Antimicrobial activities of single aroma compounds. Nat. Prod. Commun. 2009, 5, 1365–1368. [Google Scholar] [CrossRef] [Green Version]
- Celik, A.; Aydınlık, N.; Arslan, I. Phytochemical constituents and inhibitory activity towards methicillin-resistant Staphylococcus aureus strains of Eryngium species (Apiaceae). Chem. Biodivers. 2011, 8, 454–459. [Google Scholar] [CrossRef]
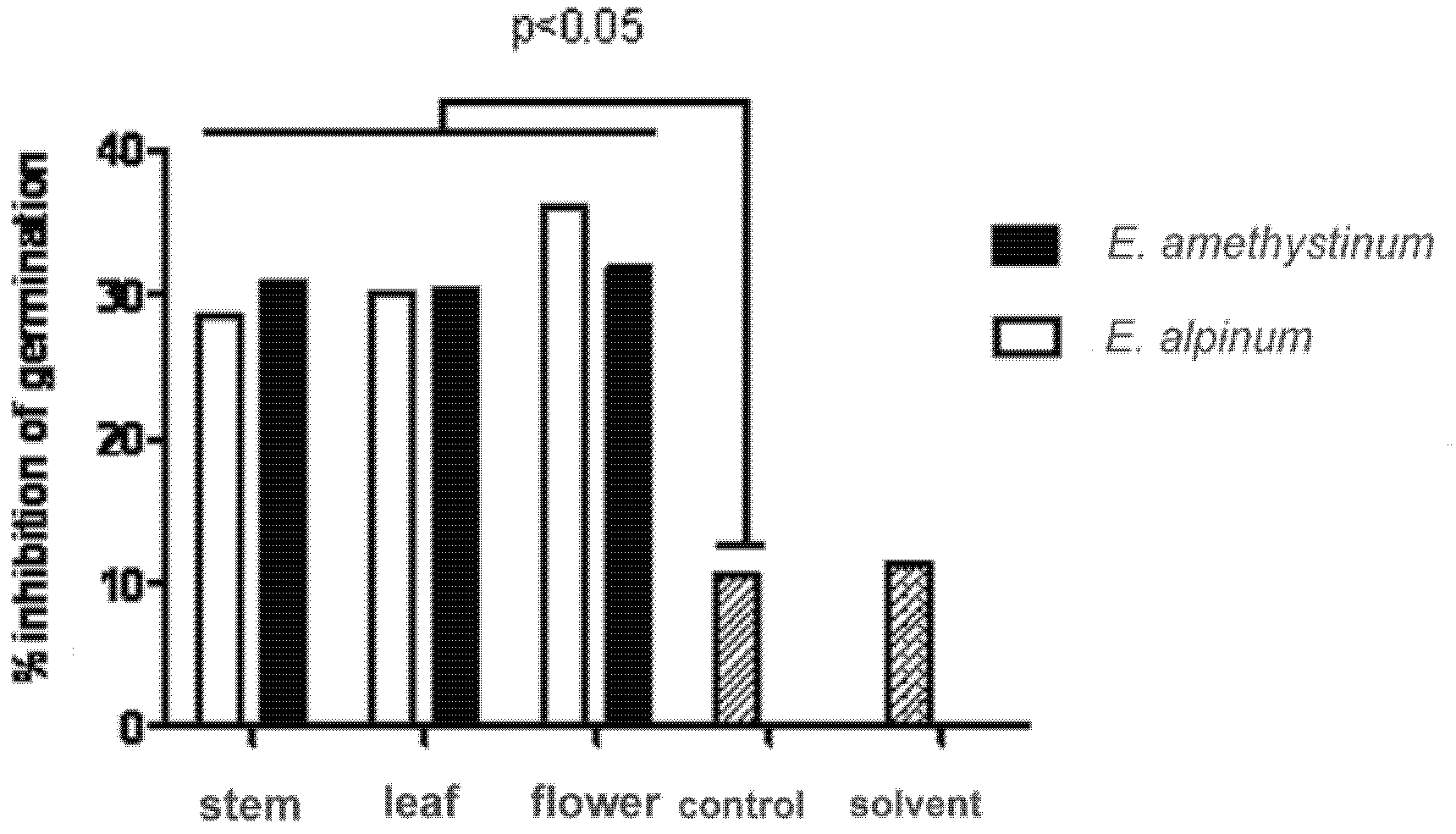
| Component | R | E. amethystinum | E. alpinum | Identification | CAS No. |
|---|---|---|---|---|---|
| Monoterpene hydrocarbons | 14.5 | 12.0 | |||
| α-Pinene | 938 | 10.2 ± 0.01 | 1.9 ± 0.01 | RI, MS, Co-GC | 80-56-8 |
| Camphene | 962 | – | 0.8 ± 0.01 | RI, MS | 79-92-5 |
| β-Pinene | 982 | 0.3 ± 0.1 | 3.3 ± 0.07 | RI, MS, Co-GC | 127-91-3 |
| Myrcene | 992 | 2.3 ± 0.01 | 5.1 ± 0.01 | RI, MS | 123-35-3. |
| Limonene | 1032 | 0.9 ± 0.05 | – | RI, MS, Co-GC | 5989-27-5 |
| (Z)-β-Ocimene | 1052 | – | 0.6 ± 0.07 | RI, MS | 3338-55-4 |
| Terpinolene | 1089 | 0.8 ± 0.07 | 0.3 ± 0.02 | RI, MS | 586-62-9 |
| Oxygenated monoterpenes | 8.9 | 4.7 | |||
| β-Thujone | 1121 | 0.3 ± 0.01 | - | RI, MS | 1125-12-8 |
| trans-Pinocarveol | 1147 | 0.7 ± 0.01 | – | RI, MS | 547-61-5 |
| Camphor | 1151 | 4.1 ± 0.03 | 2.8 ± 0.01 | RI, MS, Co-GC | 76-22-2 |
| Borneol | 1176 | – | 0.1 ± 0.02 | RI, MS | 507-70-0 |
| Terpinen-4-ol | 1184 | 0.7 ± 0.01 | 0.3 ± 0.01 | RI, MS | 562-74-3 |
| β-Thujone | 1121 | 0.3 ± 0.01 | - | RI, MS | 471-15-8 |
| Myrtenol | 1197 | 0.9 ± 0.01 | 0.6 ± 0.01 | RI, MS | 515-00-4 |
| Linalyl acetate | 1252 | 0.8 ± 0.02 | 0.3 ± 0.02 | RI, MS | 115-95-7 |
| Bornyl acetate | 1285 | 0.4 ± 0.07 | – | RI, MS | 76-49-3 |
| α-Terpenyl acetate | 1349 | 0.1 ± 0.02 | 0.4 ± 0.03 | RI, MS | 80-26-2 |
| Sesquiterpene hydrocarbons | 35.2 | 28.9 | |||
| α-Copaene | 1377 | 0.9 ± 0.01 | – | RI, MS | 3856-25-5 |
| β-Bourbonene | 1383 | 1.7 ± 0.01 | 0.6 ± 0.07 | RI, MS | 5208-59-3 |
| α-Gurjunene | 1407 | 3.6 ± 0.01 | tr | RI, MS | 489-40-7 |
| β-Caryophyllene | 1424 | 15.2 ± 0.01 | 2.1 ± 0.01 | RI, MS, Co-GC | 87-44-5 |
| β -Copaene | 1429 | 0.8 ± 0.02 | 0.5 ± 0.07 | RI, MS | 18252-44-3 |
| trans-α-Bergamotene | 1433 | 0.4 ± 0.01 | – | RI, MS | 13474-59-4 |
| (Z)-β-Farnesene | 1454 | 0.3 ± 0.01 | 0.2 ± 0.03 | RI, MS | 28973-97-9 |
| α-Humulene | 1456 | 1.1 ± 0.01 | – | RI, MS | 6753-98-6 |
| allo-Aromadendrene | 1465 | 0.6 ± 0.01 | – | RI, MS | 25246-27-9 |
| Germacrene D | 1481 | 5.9 ± 0.01 | 8.2 ± 0.01 | RI, MS | 23986-74-5 |
| β-Bisabolene | 1494 | – | 1.3 ± 0.01 | RI, MS | 495-61-4 |
| Bicyclogermacrene | 1500 | 4.7 ± 0.01 | 13.2 ± 0.01 | RI, MS | 24703-35-3 |
| δ-Cadinene | 1517 | – | 2.8 ± 0.01 | RI, MS | 483-76-1 |
| Oxygenated sesquiterpenes | 19.7 | 41.4 | |||
| Spathulenol | 1577 | 0.3 ± 0.02 | – | RI, MS | 6750-60-3 |
| Caryophyllene oxide | 1581 | 4.2 ± 0.01 | 27.9 ± 0.01 | RI, MS, Co-GC | 1139-30-6 |
| γ-Eudesmol | 1632 | 6.4 ± 0.01 | 5.7 ± 0.01 | RI, MS | 1209-71-8 |
| α-Cadinol | 1655 | 0.5 ± 0.01 | – | RI, MS | 481-34-5 |
| α-Bisabolol | 1688 | 8.3 ± 0.01 | 7.8 ± 0.01 | RI, MS | 515-69-5 |
| Phenolic compounds | 2.0 | 1.7 | |||
| Thymol | 1290 | 1.2 ± 0.01 | 0.9 ± 0.01 | RI, MS, Co-GC | 89-83-8 |
| Carvacrol | 1299 | 0.5 ± 0.01 | 0.6 ± 0.01 | RI, MS, Co-GC | 499-75-2 |
| Eugenol | 1370 | 0.3 ± 0.07 | 0.2 ± 0.01 | RI, MS, Co-GC | 97-53-0 |
| Carbonylic compounds | 10.0 | 0.2 | |||
| 3-Octanol acetate | 1125 | 0.4 ± 0.02 | 0.2 ± 0.01 | RI, MS | 4864-61-3 |
| Butylhexanoate | 1193 | 0.3 ± 0.01 | – | RI, MS | 626-82-4 |
| 2,3,6-Trimethylbenzaldehyde | 1340 | 9.3 ± 0.01 | – | RI, MS | 34341-29-2 |
| Hydrocarbons | 1.0 | 1.5 | |||
| Eicosane | 2000 | – | 0.2 ± 0.03 | RI, MS, Co-GC | 112-95-8 |
| Docosane | 2200 | 0.5 ± 0.01 | 0.3 ± 0.01 | RI, MS, Co-GC | 629-97-0 |
| Tricosane | 2300 | – | 0.2 ± 0.01 | RI, MS, Co-GC | 638-67-5 |
| Tetracosane | 2400 | 0.2 ± 0.01 | – | RI, MS, Co-GC | 646-31-1 |
| Pentacosane | 2500 | – | 0.3 ± 0.01 | RI, MS, Co-GC | 629-99-2 |
| Hexacosane | 2600 | – | 0.1 ± 0.02 | RI, MS, Co-GC | 630-01-3 |
| Octacosane | 2800 | – | 0.3 ± 0.02 | RI, MS, Co-GC | 630-02-4 |
| Nonacosane | 2900 | 0.3 ± 0.02 | 0.1 ± 0.1 | RI, MS, Co-GC | 630-03-5 |
| Total identified (%) | 91.3 | 90.4 | |||
| Yield (%) | 0.1 | 0.1 |
| Compound | E. amethystinum | E. alpinum |
| Chrysin | – | – |
| Rutin | 0.002 ± 0.000 | 0.001 ± 0.000 |
| Quercetin | – | – |
| Quercitrin | 0.026 ± 0.005 | – |
| Cichoric acid | – | – |
| Coumaric acid | – | tr |
| Ferulic acid | – | – |
| Protocatehuic acid | 0.015 ± 0.003 | – |
| Rosmarinic acid | 0.005 ± 0.001 | – |
| Syringic acid | – | – |
| Tannic acid | – | – |
| Species | Plant Part | TP (mg/g) | TF (mg/g) | EC50 (μg/mL) | ANT (%) | ChEC50 (μg/mL) | SRP (mg−1) |
|---|---|---|---|---|---|---|---|
| leaf | 34.48 ± 0.33 A | 17.24 ± 1.16 A | 30.73 ± 0.29 A | 90.94 ± 1.58 A | 682.58 ± 4.56 A | 1.05 ± 0.02 A | |
| E. amethystinum | flower | 11.77 ± 0.45 B | 9.65 ± 0.52 B | 169.78 ± 2.86 B | 84.71 ± 0.19 B | 484.12 ± 17.44 B | 0.17 ± 0.01 B |
| stem | 23.8 ± 0.71 C | 16.21 ± 0.97 A | 100.25 ± 2.24 C | 85.26 ± 1.42 B | 684.14 ± 39.95 A | 0.38 ± 0.00 C | |
| leaf | 73.42 ± 1.05 D | 1.78 ± 0.24 C | 268.47 ± 17.19 D | 48.81 ± 5.24 C | 1049.68 ± 21.59 C | 1.12 ± 0.08 A | |
| E. alpinum | flower | 29.39 ± 2.92 E | 11.34 ± 0.30 D | 1510.17 ± 29.74 E | 75.89 ± 3.09 D | 331.2 ± 6.88 D | 0.33 ± 0.04 C |
| stem | 33.77 ± 1.93 A | 13.50 ± 0.27 E | 241.08 ± 4.66 F | 71.59 ± 1.45 D | 951.14 ± 6.20 E | 0.67 ± 0.06 D | |
| Standard | - | - | a 2.83 ± 0.02 A | a 95.39 ± 0.21 A | b 219.16 ± 4.70 Fc 5.49 ± 0.25 G | d 7.59 ± 0.08 E |
| Species | Plant | MIC ± SD (mg/mL) | |||
|---|---|---|---|---|---|
| Part | S. aureus ATCC 6538 | E. coli ATCC 10536 | C. albicans ATCC 10231 | M. gypseum MFBF S2 | |
| stem | 0.39 ± 0.02 | 1.94 ± 0.48 | 0.16 ± 0.12 | 0.24 ± 0.07 | |
| E. amethystinum | leaf | 0.39 ± 0.020 | 1.53 ± 0.24 | 0.06 ± 0.018 * | 0.16 ± 0.12 |
| flower | 1.32 ± 0.60 | 1.53 ± 0.24 | 0.43 ± 0.334 | 0.06 ± 0.02 * | |
| stem | 0.24 ± 0.07 | 1.94 ± 0.48 | 0.39 ± 0.20 | 0.21 ± 0.14 | |
| E. alpinum | leaf | 0.29 ± 0.02 | 1.94 ± 0.48 | 0.39 ± 0.20 | 0.08 ± 0.06 * |
| flower | 0.24 ± 0.07 * | 1.53 ± 0.24 | 0.32 ± 0.29 | 0.13 ± 0.13 | |
| Species | Plant | MIC ± SD (mg/mL) | |||
|---|---|---|---|---|---|
| Part | C. albicans MFBF 40630/2 | C. parapsilosis MFBF 4800 | C. krusei MFBF 429 | C. glabrata MFBF 3309 | |
| stem | 0.37 ± 0.05 | 0.37 ± 0.05 | 0.37 ± 0.05 | 0.37 ± 0.05 | |
| E. amethystinum | leaf | 0.37 ± 0.05 | 0.37 ± 0.05 | 0.37 ± 0.05 | 0.37 ± 0.05 |
| flower | 0.37 ± 0.05 | 0.37 ± 0.05 | 0.37 ± 0.05 | 0.37 ± 0.05 | |
| stem | 0.37 ± 0.05 | 1.11 ± 0.05 | 0.37 ± 0.05 | 0.37 ± 0.05 | |
| E. alpinum | leaf | 0.37 ± 0.05 | 1.11 ± 0.05 | 0.37 ± 0.05 | 0.37 ± 0.05 |
| flower | 0.37 ± 0.05 | 1.11 ± 0.05 | 0.12 ± 0.01 | 0.37 ± 0.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kremer, D.; Zovko Končić, M.; Kosalec, I.; Košir, I.J.; Potočnik, T.; Čerenak, A.; Srečec, S.; Dunkić, V.; Vuko, E. Phytochemical Traits and Biological Activity of Eryngium amethystinum and E. alpinum (Apiaceae). Horticulturae 2021, 7, 364. https://doi.org/10.3390/horticulturae7100364
Kremer D, Zovko Končić M, Kosalec I, Košir IJ, Potočnik T, Čerenak A, Srečec S, Dunkić V, Vuko E. Phytochemical Traits and Biological Activity of Eryngium amethystinum and E. alpinum (Apiaceae). Horticulturae. 2021; 7(10):364. https://doi.org/10.3390/horticulturae7100364
Chicago/Turabian StyleKremer, Dario, Marijana Zovko Končić, Ivan Kosalec, Iztok Jože Košir, Tanja Potočnik, Andreja Čerenak, Siniša Srečec, Valerija Dunkić, and Elma Vuko. 2021. "Phytochemical Traits and Biological Activity of Eryngium amethystinum and E. alpinum (Apiaceae)" Horticulturae 7, no. 10: 364. https://doi.org/10.3390/horticulturae7100364
APA StyleKremer, D., Zovko Končić, M., Kosalec, I., Košir, I. J., Potočnik, T., Čerenak, A., Srečec, S., Dunkić, V., & Vuko, E. (2021). Phytochemical Traits and Biological Activity of Eryngium amethystinum and E. alpinum (Apiaceae). Horticulturae, 7(10), 364. https://doi.org/10.3390/horticulturae7100364












