Effects of Water Stress on Vegetative Growth and ‘Merlot’ Grapevine Yield in a Semi-Arid Mediterranean Climate
Abstract
1. Introduction
2. Materials and Methods
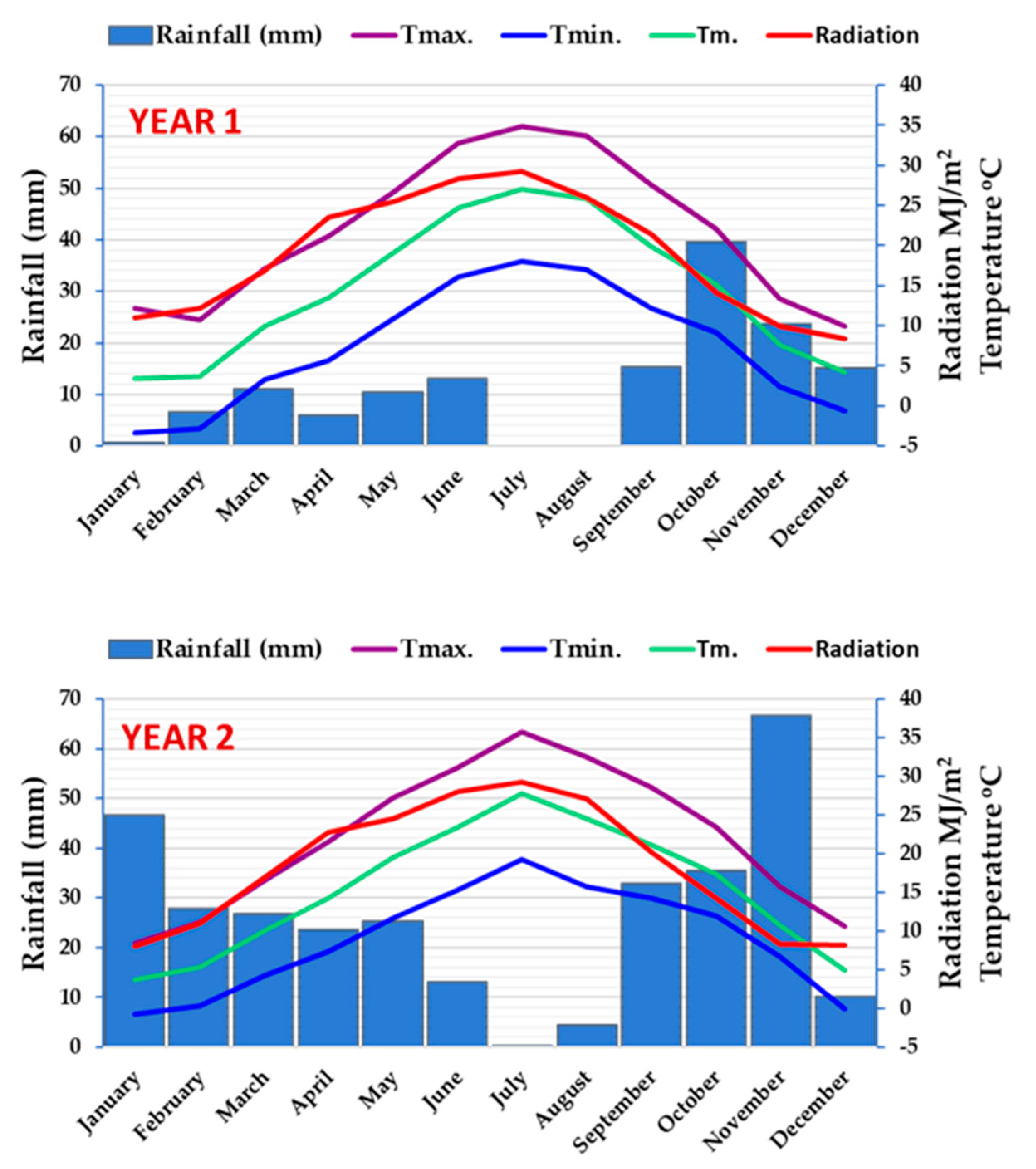
2.1. Study Area
2.2. Plant Material
2.3. Experimental Design
2.4. Water Regime
2.5. Water Potential and Leaf Development
2.6. Yield and Its Components
2.7. Statistical Analysis
3. Results
3.1. Effects on Leaf Development
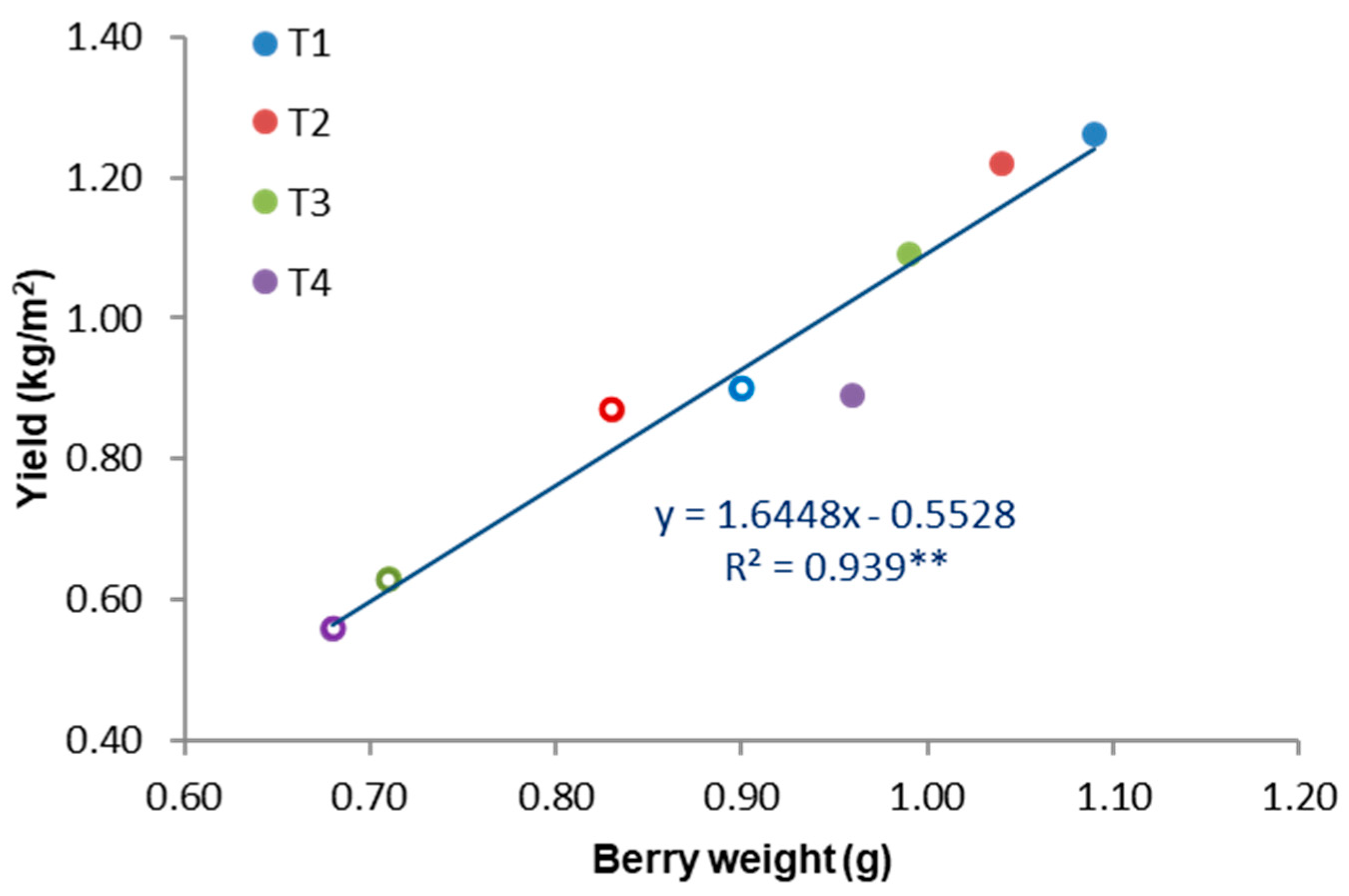
3.2. Effects on Yield
4. Discussion
4.1. Effects on Leaf Development
4.2. Effects on Yield
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tester, M.; Bacic, A. Abiotic Stress Tolerance in Grasses. From Model Plants to Crop Plants. Plant Physiol. 2005, 137, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Huglin, P.; Schneider, C. Cap. 6.2. Relations entre les facteurs du milieu naturel et la vigne. In Biologie et Écologie de la Vigne, 2nd ed.; Lavoisier-Technique & Documentation: París, France, 1998; pp. 283–286. [Google Scholar]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.-T.; Machado, N.; Moutinho-Pereira, J.M. Grapevine abiotic stress assessment and search for sustainable adaptation strategies in Mediterranean-like climates. A review. Agron. Sustain. Dev. 2018, 38, 66. [Google Scholar] [CrossRef]
- Chaves, M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef]
- Zarrouk, O.; Costa, J.; Francisco, R.D.B.; Lopes, C.; Chaves, M.M. Drought and water management in Mediterranean vineyards. In Grapevine in a Changing Environment: A Molecular and Ecophysiological Perspective; GeróS, H., Chaves, M.M., Medrano, H., Delrot, S., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 38–67. [Google Scholar]
- Rodrigues, M.L.; Chaves, M.M.; Wendler, R.; David, M.M.; Quick, W.P.; Leegood, R.C.; Stitt, M.; Pereira, J.S. Osmotic Adjustment in Water Stressed Grapevine Leaves in Relation to Carbon Assimilation. Funct. Plant Biol. 1993, 20, 309–321. [Google Scholar] [CrossRef]
- Chaves, M.; Santos, T.; Souza, C.; Ortuño, M.; Rodrigues, M.; Lopes, C.; Maroco, J.; Pereira, J. Deficit irrigation in grapevine improves water-use efficiency while controlling vigour and production quality. Ann. Appl. Biol. 2007, 150, 237–252. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Hidalgo, L. Tratado de Viticultura General, 2nd ed.; Mundi-Prensa, S.A., Ed.; Artes Gráficas Cuesta: Madrid, Spain, 1999; p. 743. [Google Scholar]
- Lissarrague, J.R. Necesidades de agua de la vid. Consecuencias del estrés hídrico y del riego en el viñedo. Agricultura 1997, 785, 943–950. [Google Scholar]
- Gómez del Campo, M.V. Mecanismos de Adaptación a la Sequía en la Vid. Evaluación del Consumo de Agua, Crecimiento y Desarrollo Vegetativo, Productividad y Eficiencia en el Uso del Agua de Cuatro Genotipos de Vid (Vitis vinifera L.). Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 1998. [Google Scholar]
- Escalona, J.M.; Flexas, J.; Medrano, H. Stomatal y non-stomatal limitations of photosynthesis under water stress in field-grown grapevines. Aust. J. Plant Physiol. 1999, 26, 421–433. [Google Scholar] [CrossRef]
- FlexasA, J.; Bota, J.; Escalona, J.M.; Sampol, B.; Medrano, H. Effects of drought on photosynthesis in grapevines under field conditions: An evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 2002, 29, 461–471. [Google Scholar] [CrossRef]
- Cuevas, E.; Baeza, P.; Lissarrague, J.R. Variation in stomatal behaviour and gas exchange between mid-morning and mid-afternoon of north–south oriented grapevines. (Vitis vinifera L. cv. Tempranillo) at different levels of soil water availability. Sci. Hortic. 2006, 108, 173–180. [Google Scholar] [CrossRef]
- Moutinho-Pereira, J.; Magalhaes, N.; Gonçalves, B.; Bacelar, E.A.; Brito, M.E.B.; Correia, C. Gas exchange and water relations of three Vitis vinifera L. cultivars growing under Mediterranean climate. Photosynthetica 2007, 45, 202–207. [Google Scholar] [CrossRef]
- Martínez, J.; Chacón, J.L. Variability of stomatal conductance with leaf water potential in 5 grapevine cultivars: Consequences for water efficiency. In Proceedings of the XVI International GiESCO Symposium, Davis, CA, USA, 14–16 July 2009; pp. 333–336. [Google Scholar]
- Pinheiro, C.; Chaves, M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2010, 62, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Dinis, L.-T.; Ferreira, H.; Pinto, G.; Bernardo, S.; Correia, C.M.; Moutinho-Pereira, J.M. Kaolin-based, foliar reflective film protects photosystem II structure and function in grapevine leaves exposed to heat and high solar radiation. Photosynthetica 2016, 54, 47–55. [Google Scholar] [CrossRef]
- Conesa, M.R.; De La Rosa, J.; Domingo, R.; Banon, S.; Perez-Pastor, A. Changes induced by water stress on water relations, stomatal behaviour and morphology of table grapes (cv. Crimson Seedless) grown in pots. Sci. Hortic. 2016, 202, 9–16. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A. Modified grape composition under climate change conditions requires adaptations in the vineyard. OENO One 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Bota, B.J.; Flexas, J.; Medrano, H. Genetic variability of photosynthesis and water use in Balearic grapevine cultivars. Ann. Appl. Biol. 2001, 138, 353–361. [Google Scholar] [CrossRef]
- Ojeda, H.; Lebon, E.; Deis, L.; Vita, F.; Carbonneau, A. Régulation stomatique de cépages méditerranéens en situation de déficit hydrique dans le sud de La France. Resultats preliminaires. In Proceedings of the XIV International Symposium of GESCO, Geisenheim, Germany, 23–27 August 2005; pp. 581–587. [Google Scholar]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Mérillon, J.M.; Cushman, J.C.; Cramer, G.R. Water deficit alters differentially metabolic pathways affecting important flavour and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 212. [Google Scholar] [CrossRef]
- Tomás, M.; Medrano, H.; Brugnoli, E.; Escalona, J.; Martorell, S.; Pou, A.; Ribas-Carbó, M.; Flexas, J. Variability of mesophyll conductance in grapevine cultivars under water stress conditions in relation to leaf anatomy and water use efficiency. Aust. J. Grape Wine Res. 2014, 20, 272–280. [Google Scholar] [CrossRef]
- Martorell, S.; Díaz-Espejo, A.; Tomàs, M.; Pou, A.; El Aou-Ouad, H.; Escalona, J.M.; Vadell, J.; Ribas-Carbó, M.; Flexas, J.; Medrano, H. Differences in water-use-efficiency between two Vitis vinifera cultivars (Grenache and Tempranillo) explained by the combined response of stomata to hydraulic and chemical signals during water stress. Agric. Water Manag. 2015, 156, 1–9. [Google Scholar] [CrossRef]
- Carbonneau, A. Aspects qualitatifs. In Traité D’irrigation; Tiercelin, J.R., Ed.; Tec & Doc: Paris, France, 1998; pp. 257–276. [Google Scholar]
- Choné, X.; Van Leeuwen, C.; Dubordieu, D.; Gaudillère, J.P. Stem water potential is a sensitive indicator of grapevine water status. Ann. Bot. 2001, 87, 477–483. [Google Scholar] [CrossRef]
- Williams, L.; Araujo, F. Correlations among Predawn Leaf, Midday Leaf, and Midday Stem Water Potential and their Correlations with other Measures of Soil and Plant Water Status in Vitis vinifera. J. Am. Soc. Hortic. Sci. 2002, 127, 448–454. [Google Scholar] [CrossRef]
- Deloire, A.; Carbonneau, A.; Wang, Z.; Ojeda, H. Vine and water: A short review. OENO One 2004, 38, 1–13. [Google Scholar] [CrossRef]
- Ojeda, H. Irrigation qualitative de précisión de la vigne. Progrès Agric. Vitic. 2007, 124, 133–141. [Google Scholar]
- Medrano, H.; Escalona, J.M.; Flexas, J. Indicadores integradores del estado hídrico de la planta. In Fundamentos, Aplicación y Consecuencias del Riego en la Vid; Editorial Agrícola Española; Baeza, P., Lisarrague, J.R., de Miguel, P.S., Eds.; AMV Ediciones: Madrid, Spain, 2007; 264p. [Google Scholar]
- Salón, J.L.; Chirivella, C.; Castel, J.R. Response of cv. Bobal to timing of deficit irrigation in Requena, Spain: Water relations, yield and wine quality. Am. J. Enol. Vitic. 2005, 56, 1–8. [Google Scholar]
- Reynolds, A.G.; Lowrey, V.D.; Tomec, L.; Hakimi, J.; de Savigny, C. Influence of irrigation on vine performance, fruit composition and wine quality of Chardonnay in a cool, humid climate. Am. J. Enol. Vitic. 2007, 58, 217–228. [Google Scholar]
- Intrigliolo, D.; Castel, J.R. Response of grapevine cv. “Tempranillo” to timing and amount of irrigation: Water relations, vine growth, yield and berry and wine composition. Irrig. Sci. 2010, 28, 113–125. [Google Scholar] [CrossRef]
- Romero, P.; Gil-Muñoz, R.; Amor, F.M.; Valdés, E.; Fernández, J.I.; Martínez-Cutillas, A. Regulated deficit irrigation based upon optimun water status improves phenolic composition in Monastrell grapes and wines. Agric. Water Manag. 2013, 121, 85–101. [Google Scholar] [CrossRef]
- Herrera, J.C.; Bucchetti, B.; Sabbatini, P.; Comuzzo, P.; Zulini, L.; Vecchione, A.; Peterlunger, E.; Castellarin, S.D. Effect of water deficit and severe shoot trimming on the composition ofVitis vinifera L. Merlot grapes and wines. Aust. J. Grape Wine Res. 2015, 21, 254–265. [Google Scholar] [CrossRef]
- Casassa, L.F.; Larsen, R.C.; Harbertson, J.F. Effects of Vineyard and Winemaking Practices Impacting Berry Size on Evolution of Phenolics during Winemaking. Am. J. Enol. Vitic. 2016, 67, 257–268. [Google Scholar] [CrossRef]
- Keller, M.; Romero, P.; Gohil, H.; Smithyman, R.P.; Riley, W.R.; Casassa, L.F.; Harbertson, J.F. Deficit Irrigation Alters Grapevine Growth, Physiology, and Fruit Microclimate. Am. J. Enol. Vitic. 2016, 67, 426–435. [Google Scholar] [CrossRef]
- Williams, L.E.; Dokoozlian, N.K.; Wample, R. Grape. In Handbook of Environmental Physiology of Fruit Crops. Vol 1. Temperate Crops; Schaffer, B., Anderson, P.C., Eds.; CRC Press: Boca Ratón, FL, USA, 1994; pp. 85–133. [Google Scholar]
- Ginestar, C.; Eastham, J.; Gray, S.; Lland, P. Use of sap flow sensors to schedule vineyard irrigation. I. Effects of post-veraison water deficit on water relations, vine growth and yield of Shiraz grapevine. Am. J. Enol. Vitic. 1998, 49, 413–420. [Google Scholar]
- Cuevas, E. Estudio de Mecanismos de Adaptación Ecofísiológica de la Vid (Vitis vinifera L., cv. Tempranillo) al Déficit Hídrico. Evaluación del Consumo de Agua y de las Respuestas Agronómicas en Diferentes Regímenes Hídricos. Ph.D. Thesis, Departamento de Producción Vegetal: Fitotecnia, Universidad Politécnica de Madrid, Madrid, Spain, 2001; 219p. [Google Scholar]
- Fernández, O. Análisis de las Diferencias en el Crecimiento Vegetativo, en los Componentes del Rendimiento y en la Composición de las Uvas y Vinos de cv. Cabernet Sauvignon (Vitis vinífera L.) Producidos por el Déficit Hídrico Durante Pre-Envero y Post-Envero. Ph.D. Thesis, Departamento de Producción Vegetal: Fitotecnia, Universidad Politécnica de Madrid, Madrid, Spain, 2013; 381p. [Google Scholar]
- García-Escudero, E.; López, R.; Santamaría, P.; Zaballa, O. Control del rendimiento en viñedos conducidos en régimen de riego localizado. Vitic. Enol. Prof. 2000, 69, 12–24. [Google Scholar]
- Hardie, W.D.; Martin, S.R. Shoot growth on de-fruited grapevines: A physiological indicator for irrigation scheduling. Aust. J. Grape Wine Res. 2000, 6, 52–58. [Google Scholar] [CrossRef]
- Paranychianakis, N.V.; Aggelides, S.; Angelakis, A.N. Influence of rootstock, irrigation level and recycled water on growth and yield of Soultanina grapevines. Agric. Water Manag. 2004, 69, 13–27. [Google Scholar] [CrossRef]
- Shellie, K.C. Vine and berry response of Merlot (Vitis vinifera L.) to differential water stress. Am. J. Enol. Vitic. 2006, 57, 514–518. [Google Scholar]
- Basile, B.; Marsal, J.; Mata, M.; Vallverdú, X.; Bellvert, J.; Girona, J. Phenological Sensitivity of Cabernet Sauvignon to Water Stress: Vine Physiology and Berry Composition. Am. J. Enol. Vitic. 2011, 62, 452–461. [Google Scholar] [CrossRef]
- Munitz, S.; Netzer, Y.; Schwartz, A. Sustained and regulated deficit irrigation of field-grown Merlot grapevines. Aust. J. Grape Wine Res. 2016, 23, 87–94. [Google Scholar] [CrossRef]
- Matthews, M.A.; Anderson, M.M.; Schultz, H.R. Phenolic and growth responses to early and late season water deficits in Cabernet Franc. Vitis 1987, 26, 147–160. [Google Scholar]
- Poni, S.; Lakso, A.N.; Turner, J.R.; Melious, R.E. The effects of pre- and post-veraison water stress on growth and physiology of potted Pinot Noir grapevines at varying crop levels. Vitis 1994, 32, 207–214. [Google Scholar]
- Kliewer, W.M.; Wolpert, J.A.; Benz, M. Trellis and vine spacing effects on growth, canopy microclimate, yield and fruit composition of cabernet sauvignon. Acta Hortic. 2000, 526, 21–32. [Google Scholar] [CrossRef]
- Gómez-del-Campo, M.; Ruiz, C.; Lissarrague, J.R. Effect of Water Stress on Leaf Area Development, Photosynthesis, and Productivity in Chardonnay and Airén Grapevines. Am. J. Enol. Vitic. 2002, 53, 138–143. [Google Scholar]
- Ojeda, H.; Andary, C.; Creaba, E.; Carbonneau, A.; Deloire, A. Influence of pre-and postveraison water deficit on synthesis and concentration of skin phenolic compounds during berry growth of Vitis vinifera var. Shiraz. Am. J. Enol. Vitic. 2002, 53, 261–267. [Google Scholar]
- Bellvert, J.; Marsal, J.; Mata, M.; Girona, J. Yield, Must Composition, and Wine Quality Responses to Preveraison Water Deficits in Sparkling Base Wines of Chardonnay. Am. J. Enol. Vitic. 2015, 67, 1–12. [Google Scholar] [CrossRef]
- Buesa, I.; Pérez, D.; Castel, J.; Intrigliolo, D.S.; Castel, J.R. Effect of deficit irrigation on vine performance and grape composition of Vitis vinífera L. cv. Muscat of Alexandria. Aust. J. Grape Wine Res. 2017, 23, 251–259. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef]
- Poni, S.; Intrieri, C. Grapevine photosynthesis: Effects linked to light radiation and leaf age. Adv. Hortic. Sci. 2001, 15, 5–15. [Google Scholar]
- Kliewer, W.M.; Dokoozlian, N. Leaf area/crop weight ratios of grapevines: Influence on fruit composition and wine quality. Am. J. Enol. Vitic. 2005, 56, 170–181. [Google Scholar]
- FAO-ISRIC-ISSS. World Reference Base for Soil Resources; FAO: Rome, Italy, 2006; p. 142. [Google Scholar]
- Soil Survey Staff. Key to Soil Taxonomy, 12th ed.; USDA-Natural Resources, United States Department of Agriculture, Conservation Service: Washington, DC, USA, 2014; p. 379. [Google Scholar]
- Pavloušek, P. Preliminary results of tests of grapevine rootstocks resistance to lime-induced chlorosis. Acta Univ. Agric. Silvic. Mendel. Brun. 2008, 56, 299–302. [Google Scholar] [CrossRef]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap Pressure in Vascular Plants: Negative hydrostatic pressure can be measured in plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Johnson, L.F.; Pierce, L.L. Indirect Measurement of Leaf Area Index in California North Coast Vineyards. HortScience 2004, 39, 236–238. [Google Scholar] [CrossRef]
- Stewart, A.M.; Edmisten, K.L.; Wells, R.; Collins, G.D. Measuring Canopy Coverage with Digital Imaging. Commun. Soil Sci. Plant Anal. 2007, 38, 895–902. [Google Scholar] [CrossRef]
- Hsiao, T.C. Plant Responses to Water Stress. Annu. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Sommer, K.J.; Clingeleffer, P.R. Vine canopy development and carbohydrate partitioning as influenced by pruning. In Proceedings of the Ninth Australian Wine Industry Technical Conference, Adelaide, Australia, 16–19 July 1995; pp. 123–127. [Google Scholar]
- Rubio, J.A. Riego y Aclareo de Racimos: Efectos en la Actividad Fisiológica, en el Control del Rendimiento y en la Calidad de la Uva del cv. Tempranillo (Vitis vinifera L.). Ph.D. Thesis, Departamento de Producción Vegetal: Fitotecnia, Universidad Politécnica de Madrid, Madrid, Spain, 2002; 218p. [Google Scholar]
- Williams, L.E.; Grimes, D.W. Modelling vine growth development of a data set for a water balance subroutine. In Proceedings of the VI Australian Wine Industry Technical Conference, Adelaide, Australia, 14–17 July 1986; pp. 169–174. [Google Scholar]
- Acevedo-Opazo, C.; Ortega-Farias, S.; Fuentes, S. Effects of grapevine (Vitis vinifera L.) water status on water consumption, vegetative growth and grape quality: An irrigation scheduling application to achieve regulated deficit irrigation. Agric. Water Manag. 2010, 97, 956–964. [Google Scholar] [CrossRef]
- Smart, R.E.; Turkington, C.R.; Evans, J.C. Grapevine response to furrow and trickle irrigation. Am. J. Enol. Vitic. 1974, 25, 62–66. [Google Scholar]
- Pellegrino, A.; Gozé, E.; Lebon, E.; Wery, J. A model-based diagnosis tool to evaluate the water stress experienced by grapevine in field sites. Eur. J. Agron. 2006, 25, 49–59. [Google Scholar] [CrossRef]
- Hidalgo, L. La Calidad del Vino Desde el Viñedo, 1st ed.; Mundi-Prensa, S.A., Ed.; Artes Gráficas Cuesta: Madrid, Spain, 2006; 390p. [Google Scholar]
- Kizildeniz, T.; Mekni, I.; Santesteban, H.; Pascual, I.; Morales, F.; Irigoyen, J.J. Effects of climate change including elevated CO2 concentration, temperature and water deficit on growth, water status, and yield quality of grapevine (Vitis vinifera L.) cultivars. Agric. Water Manag. 2015, 159, 155–164. [Google Scholar] [CrossRef]
- Shellie, K. Water Productivity, Yield, and Berry Composition in Sustained versus Regulated Deficit Irrigation of Merlot Grapevines. Am. J. Enol. Vitic. 2014, 65, 197–205. [Google Scholar] [CrossRef]
- Baeza, P.; Sánchez-De-Miguel, P.; Centeno, A.; Junquera, P.; Linares, R.; Lissarrague, J.R. Water relations between leaf water potential, photosynthesis and agronomic vine response as a tool for establishing thresholds in irrigation scheduling. Sci. Hortic. 2007, 114, 151–158. [Google Scholar] [CrossRef]
- Martínez, J.; Romero, R.; Chacón, J.L. Efectos del rigor del deficit hídrico de las plantas sobre el peso unitario de la uva madura en cv. Merlot. XI Congreso de la Sociedad Española de Ciencias Hortícolas (SECH). Actas Hortic. 2007, 48, 150–153. [Google Scholar]
- Martínez, E.M.; Rey, B.J.; Fandiño, M.; Cancela, J.J. Impact of water stress and nutrition on Vitis vinifera cv. “Albariño”: Soil-plant water relationships, cumulative effects and productivity. Span. J. Agric. Res. 2016, 14, e1202. [Google Scholar] [CrossRef]
- Camps, J.O.; Ramos, M.C. Grape harvest and yield responses to inter-annual changes in temperature and precipitation in an area of north-east Spain with a Mediterranean climate. Int. J. Biometeorol. 2011, 56, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Cooley, N.; Clingeleffer, P.; Walker, R. Effect of water deficits and season on berry development and composition of Cabernet Sauvignon (Vitis vinifera L.) grown in a hot climate. Aust. J. Grape Wine Res. 2017, 23, 260–272. [Google Scholar] [CrossRef]
- Freeman, B.M.; Lee, T.H.; Turkington, C.R. Interaction of irrigation and pruning level on growth and yield of Shiraz vines. Am. J. Enol. Vitic. 1979, 30, 218–223. [Google Scholar]
- Girona, J.; Mata, M.; Del Campo, J.; Arbones, A.; Bartra, E.; Marsal, J. The use of midday leaf water potential for scheduling deficit irrigation in vineyards. Irrig. Sci. 2005, 24, 115–127. [Google Scholar] [CrossRef]
- Santesteban, L.; Miranda, C.; Royo, J. Regulated deficit irrigation effects on growth, yield, grape quality and individual anthocyanin composition in Vitis vinifera L. cv. “Tempranillo”. Agric. Water Manag. 2011, 98, 1171–1179. [Google Scholar] [CrossRef]
- Ferreyra, R.; Sellés, G.; Ruíz, R.; Sellés, I. Effect of water stress applied at different development periods of grapevine cv. Chardonnay on production and wine quality. Agric. Técnica 2003, 63, 277–286. [Google Scholar]
- Williams, L.E.; Matthews, M.A. Grapevine. In Irrigation of Agricultural Crops; Stewart, B.A., Nielsen, D.R., Eds.; UC Davis Publications: Madison, WI, USA, 1990; pp. 1019–1055. [Google Scholar]
- Nadal, M.; Arola, L. Effects of limited irrigation on the composition of must and wine of Cabernet Sauvignon under semi-arid conditions. Vitis 1995, 34, 151–154. [Google Scholar]
- García-Escudero, E.; López, R.; Santamaría, P.; Zaballa, O.; Arroyo, M.C. Ensayos de riego localizado en viñedos productivos de cv. Tempranillo. Vitic. Enol. Prof. 1997, 50, 35–47. [Google Scholar]
- Esteban, M.A.; Villanueva, M.J.; Lissarrague, J.R. Effects of irrigation on changes in berry composition of Tempranillo during maturation. Sugars, organic acids and mineral elements. Am. J. Enol. Vitic. 1999, 50, 418–434. [Google Scholar]
- Tandonnet, J.; Ollat, N.; Neveux, M.; Renoux, J. Effect of three levels of water supply on the vegetative and reproductive development of merlot and cabernet sauvignon grapevines. Acta Hortic. 1999, 493, 301–308. [Google Scholar] [CrossRef]
- Ojeda, H.; Deloire, A.; Carbonneau, A. Influence of water deficits on grape berry growth. Vitis 2001, 40, 141–145. [Google Scholar]
- Castellarin, S.D.; Degan, M.; Di Gaspero, G.; Peterlunger, E. Impact of water deficit on the synthesis of phenolic compounds during berry ripening of Vitis vinifera L. cv. Merlot. In Proceedings of the XIV International Symposium of GESCO, Geisenheim, Germany, 23–27 August 2005; pp. 173–179. [Google Scholar]
- Niculcea, M.; Martínez-Lapuente, L.; Guadalupe, Z.; Sánchez-Díaz, M.; Ayestarán, B.; Antolín, M.C. Characterization of phenolic composition of Vitis vinifera L. ‘Tempranillo’ and ‘Graciano’ subjected to deficit irrigation during berry development. Vitis 2015, 54, 9–16. [Google Scholar]
- Ferlito, F.; Nicolosi, E.; Gentile, A.; Lo Piero, A.R.; Squadrito, M.; Continella, A. Responses of four winegrape varieties to managed water stress and partial defoliation in an arid environment. Vitis 2014, 53, 73–80. [Google Scholar]

| Treatment | Period | Water Status of the Vine | |
|---|---|---|---|
| Flowering-Veraison | Veraison-Maturity | Type of Stress | |
| T1(0–0.2; −0.2) | 0 Mpa ≥ ΨPD ≥ −0.2 Mpa | ΨPD ≥ −0.2 Mpa | None—Light |
| T2(−0.2–0.4; −0.4) | −0.2 Mpa > ΨPD ≥ −0.4 Mpa | ΨPD ≥ −0.4 Mpa | Light—Moderate |
| T3(−0.4–0.6; −0.6) | −0.4 Mpa > ΨPD ≥ −0.6 Mpa | ΨPD ≥ −0.6 Mpa | Moderate—Intense |
| T4(−0.6; −0.8) | −0.6 Mpa > ΨPD | ΨPD ≥ −0.8 Mpa | Intense |
| Treatment | Year | Repetition | Irrigation Period | Volume (mm) | ||
|---|---|---|---|---|---|---|
| Start | Final | Total | Average | |||
| T1(0–0.2;−0.2) | 1 | T1-1 | 17-June | 17-August | 131.01 | 132.27 |
| T1-2 | 133.54 | |||||
| 2 | T1-1 | 25-May | 11-August | 132.69 | 131.71 | |
| T1-2 | 130.72 | |||||
| T2(−0.2–0.4; −0.4) | 1 | T2-1 | 17-June | 16-August | 114.95 | 117.04 |
| T2-2 | 119.14 | |||||
| 2 | T2-1 | 5-June | 11-August | 110.37 | 110.12 | |
| T2-2 | 109.86 | |||||
| T3(−0.4–0.6; −0.6) | 1 | T3-1 | 17-June | 12-August | 93.82 | 93.07 |
| T3-2 | 92.33 | |||||
| 2 | T3-1 | 27-June | 11-August | 66.12 | 67.36 | |
| T3-2 | 68.61 | |||||
| T4(−0.6; −0.8) | 1 | T4-1 | 17-June | 12-August | 70.95 | 70.63 |
| T4-2 | 70.31 | |||||
| 2 | T4-1 | 28-June | 11-August | 55.22 | 56.46 | |
| T4-2 | 57.70 | |||||
| Year | Treatment | Samples | TLA | ELA | LI |
|---|---|---|---|---|---|
| (ΨPD) | (n) | Mean ± SD | Mean ± SD | Mean ± SD | |
| 1 | T1(0–0.2; −0.2) | 20 | 1.62ab z ± 0.08 | 0.83b ± 0.02 | 0.56 ± 0.07 |
| T2(−0.2–0.4; −0.4) | 20 | 1.64b ± 0.18 | 0.83ab ± 0.00 | 0.51 ± 0.06 | |
| T3(−0.4–0.6; −0.6) | 20 | 1.52ab ± 0.18 | 0.81ab ± 0.06 | 0.52 ± 0.06 | |
| T4(−0.6; −0.8) | 20 | 1.37a ± 0.08 | 0.75a ± 0.03 | 0.55 ± 0.08 | |
| Sig y | ** | * | ns | ||
| 2 | T1(0–0.2; −0.2) | 20 | 1.51c ± 0.03 | 0.85b ± 0.03 | 0.57a ± 0.05 |
| T2(−0.2–0.4; −0.4) | 20 | 1.36b ± 0.17 | 0.86b ± 0.02 | 0.64b ± 0.10 | |
| T3(−0.4–0.6; −0.6) | 20 | 1.30b ± 0.08 | 0.76a ± 0.04 | 0.60ab ± 0.06 | |
| T4(−0.6; −0.8) | 20 | 1.16a ± 0.00 | 0.72a ± 0.04 | 0.62ab ± 0.04 | |
| Sig | *** | *** | * | ||
| Year 1 | 80 | 1.54b ± 0.12 | 0.81 ± 0.04 | 0.54a ± 0.02 | |
| Year 2 | 80 | 1.33a ± 0.15 | 0.80 ± 0.07 | 0.61b ± 0.03 | |
| Sig | *** | ns | *** |
| Year | Treatment | Samples | Yield | Bunches | Bunch Weight | Berry Weight z |
|---|---|---|---|---|---|---|
| (ΨPD) | (n) | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| 1 | T1(0–0.2; −0.2) | 20 | 1.26b y ± 0.03 | 25.63 ± 4.72 | 177.01b ± 28.71 | 1.09 ± 0.02 |
| T2(−0.2–0.4; −0.4) | 20 | 1.22b ± 0.01 | 25.05 ± 4.49 | 176.21b ± 25.23 | 1.04 ± 0.07 | |
| T3(−0.4–0.6; −0.6) | 20 | 1.09ab ± 0.07 | 25.60 ± 3.97 | 155.25ab ± 23.61 | 0.99 ± 0.08 | |
| T4(−0.6; −0.8) | 20 | 0.89a ± 0.03 | 24.90 ± 4.16 | 131.79a ± 20.11 | 0.96 ± 0.07 | |
| Sig x | *** | ns | * | ns | ||
| 2 | T1(0–0.2; −0.2) | 20 | 0.90b ± 0.02 | 24.10 ± 1.54 | 136.07b ± 18.68 | 0.90d ± 0.03 |
| T2(−0.2–0.4; −0.4) | 20 | 0.87b ± 0.11 | 24.80 ± 2.36 | 126.59b ± 15.92 | 0.83c ± 0.04 | |
| T3(−0.4–0.6; −0.6) | 20 | 0.63ab ± 0.02 | 24.65 ± 1.64 | 93.08a ± 12.99 | 0.71b ± 0.03 | |
| T4(−0.6; −0.8) | 20 | 0.56a ± 0.14 | 24.65 ± 3.47 | 83.00a ± 14.65 | 0.68a ± 0.03 | |
| Sig | * | ns | ** | *** | ||
| Year 1 | 80 | 1.12 ± 0.17 | 25.30 ± 0.37 | 160.07 ± 21.37 | 1.02 ± 0.06 | |
| Year 2 | 80 | 0.74 ± 0.17 | 24.55 ± 0.31 | 109.69 ± 25.62 | 0.78 ± 0.10 | |
| Sig | *** | ns | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chacón-Vozmediano, J.L.; Martínez-Gascueña, J.; García-Navarro, F.J.; Jiménez-Ballesta, R. Effects of Water Stress on Vegetative Growth and ‘Merlot’ Grapevine Yield in a Semi-Arid Mediterranean Climate. Horticulturae 2020, 6, 95. https://doi.org/10.3390/horticulturae6040095
Chacón-Vozmediano JL, Martínez-Gascueña J, García-Navarro FJ, Jiménez-Ballesta R. Effects of Water Stress on Vegetative Growth and ‘Merlot’ Grapevine Yield in a Semi-Arid Mediterranean Climate. Horticulturae. 2020; 6(4):95. https://doi.org/10.3390/horticulturae6040095
Chicago/Turabian StyleChacón-Vozmediano, Juan L., Jesús Martínez-Gascueña, Francisco J. García-Navarro, and Raimundo Jiménez-Ballesta. 2020. "Effects of Water Stress on Vegetative Growth and ‘Merlot’ Grapevine Yield in a Semi-Arid Mediterranean Climate" Horticulturae 6, no. 4: 95. https://doi.org/10.3390/horticulturae6040095
APA StyleChacón-Vozmediano, J. L., Martínez-Gascueña, J., García-Navarro, F. J., & Jiménez-Ballesta, R. (2020). Effects of Water Stress on Vegetative Growth and ‘Merlot’ Grapevine Yield in a Semi-Arid Mediterranean Climate. Horticulturae, 6(4), 95. https://doi.org/10.3390/horticulturae6040095






