Effects of Foliar Application of Gibberellic Acid on the Salt Tolerance of Tomato and Sweet Pepper Transplants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Transplant Production
2.2. Statistics and Principal Component Analysis
3. Results
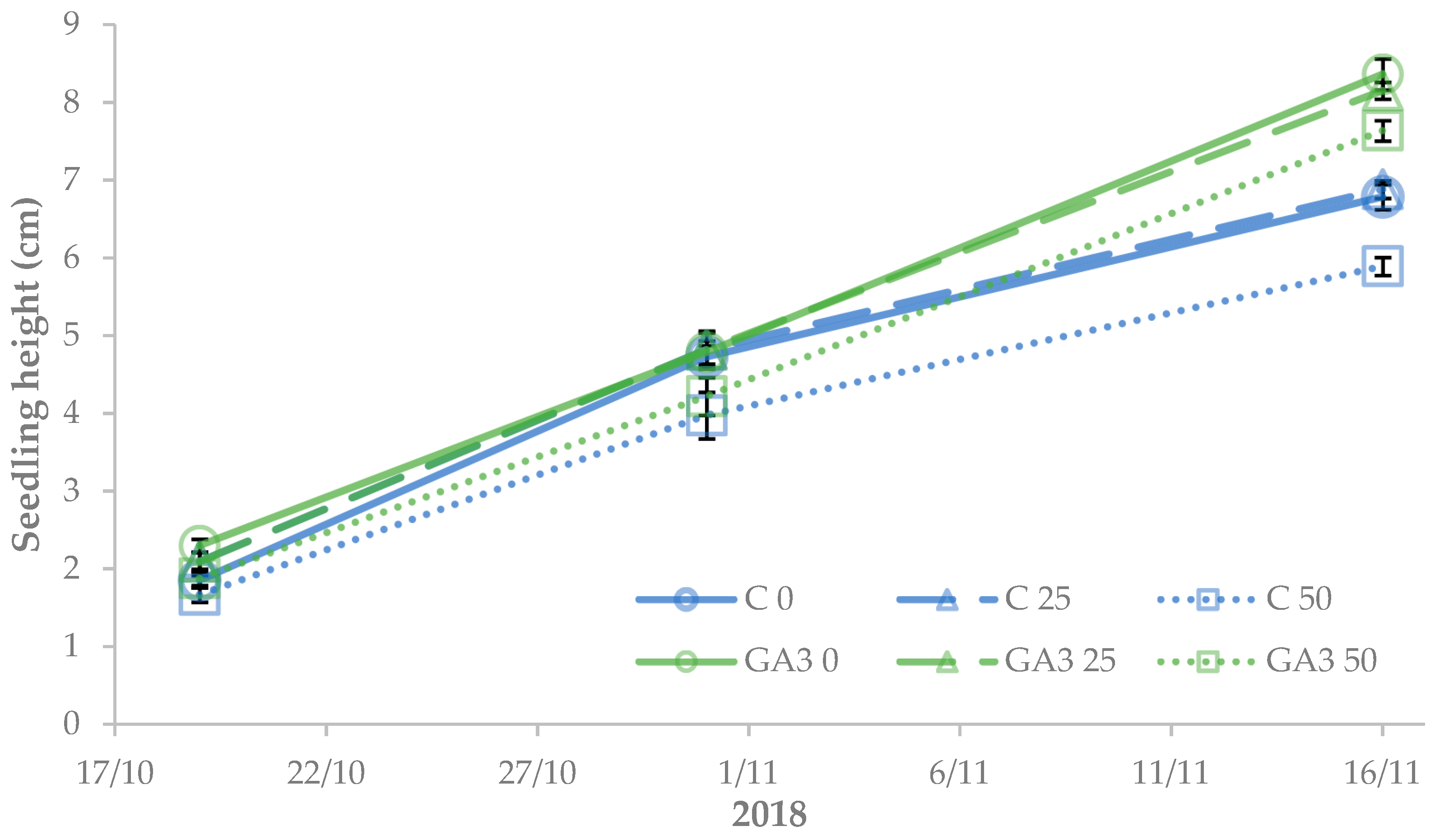
3.1. Morphophysiological Parameters of Tomato Seedlings
3.2. Morphophysiological Parameters of Sweet Pepper Seedlings
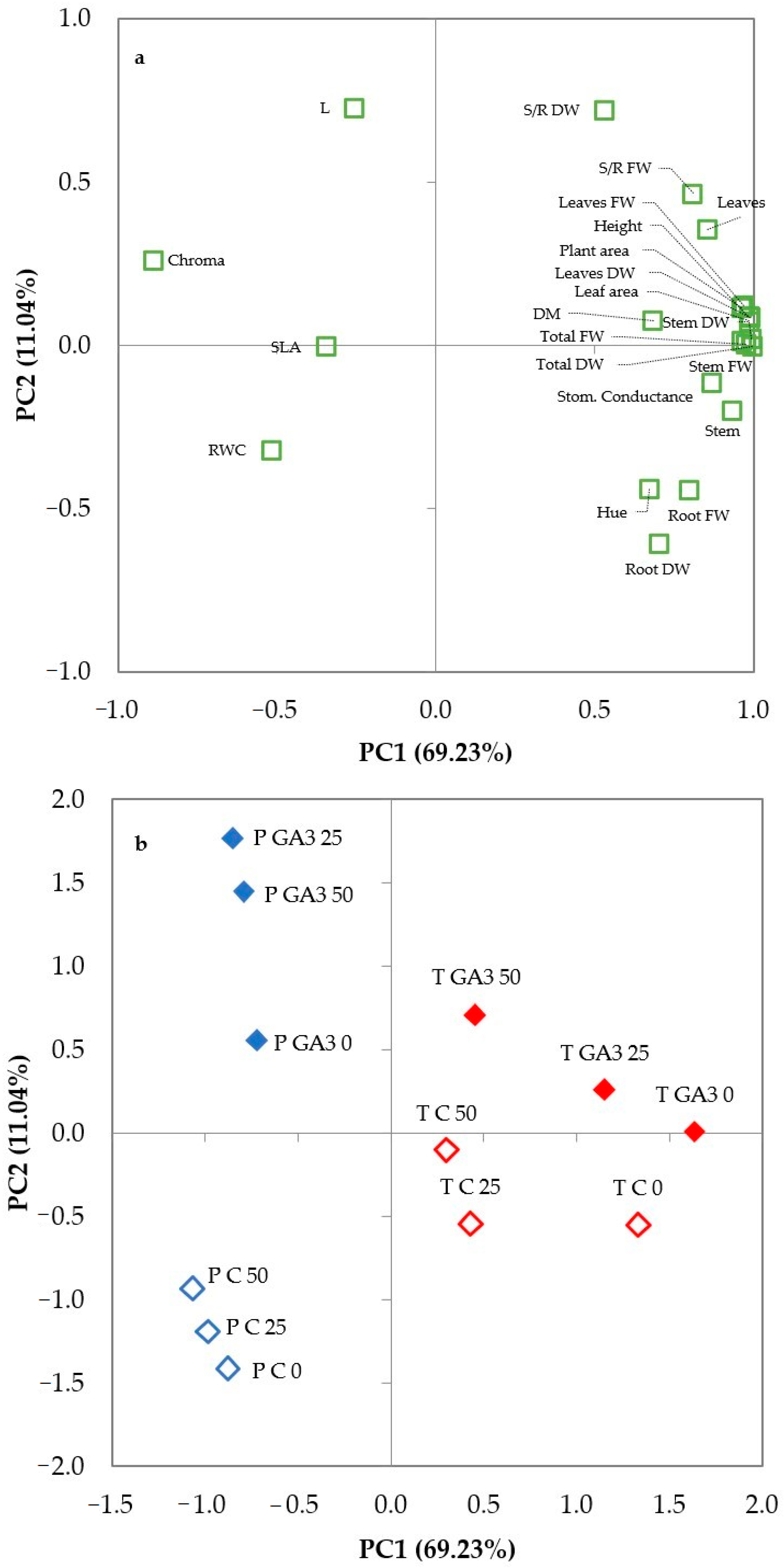
3.3. Principal Components Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicola, S.; Cantliffe, D.J. Increasing cell size and reducing medium compression enhance lettuce transplant quality and field production. HortScience 1996, 31, 184–189. [Google Scholar] [CrossRef]
- Russo, V.M. Organic vegetable transplant production. HortScience 2005, 40, 623–628. [Google Scholar] [CrossRef]
- Caracciolo, G.; Moncada, A.; Prinzivalli, C.; D’Anna, F. Effects of planting dates on strawberry plug plant performance in Sicily. Acta Hortic. 2009, 842, 155–158. [Google Scholar] [CrossRef]
- Swiader, J.M.; Ware, G.W.; McCollum, J.P. Producing Vegetable Crops: Teacher’s Manual; Interstate Publishers: Crete, IL, USA, 1992; ISBN 0813429048. [Google Scholar]
- Kloepper, J.W.; Reddy, M.S.; Rodriguez-Kabana, R.; Kenney, D.S.; Kokalis-Burelle, N.; Martinez-Ochoa, N.; Vavrina, C.S. Application for rhizobacteria in transplant production and yield enhancement. Acta Hortic. 2004, 631, 217–229. [Google Scholar] [CrossRef]
- Mariani, L.; Ferrante, A. Agronomic Management for Enhancing Plant Tolerance to Abiotic Stresses—Drought, Salinity, Hypoxia, and Lodging. Horticulturae 2017, 3, 52. [Google Scholar] [CrossRef]
- Foolad, M.R. Recent Advances in Genetics of Salt Tolerance in Tomato. Plant Cell. Tissue Organ Cult. 2004, 76, 101–119. [Google Scholar] [CrossRef]
- Maggio, A.; Barbieri, G.; Raimondi, G.; de Pascale, S. Contrasting Effects of GA 3 Treatments on Tomato Plants Exposed to Increasing Salinity. J. Plant Growth Regul. 2010, 29, 63–72. [Google Scholar] [CrossRef]
- Wang, Y.; Mopper, S.; Hasenstein, K.H. Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. J. Chem. Ecol. 2001, 27, 327–342. [Google Scholar] [CrossRef]
- Egamberdieva, D. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 2009, 31, 861–864. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B.; Weber, D.J. Action of plant growth regulators and salinity on seed germination of Ceratoides lanata. Can. J. Bot. 2004, 82, 37–42. [Google Scholar] [CrossRef]
- Afzal, I.; Basra, S.A.; Iqbal, A. The effects of seed soaking with plant growth regulators on seedling vigor of wheat under salinity stress. J. Stress Physiol. Biochem. 2005, 1, 6–14. [Google Scholar]
- Vetrano, F.; Moncada, A.; Miceli, A. Use of Gibberellic Acid to Increase the Salt Tolerance of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2020, 10, 505. [Google Scholar] [CrossRef]
- Khan, M.M.A.; Gautam, C.; Mohammad, F.; Siddiqui, M.H.; Naeem, M.; Khan, M.N. Effect of gibberellic acid spray on performance of tomato. Turk. J. Biol. 2006, 30, 11–16. [Google Scholar]
- Khan, N.A.; Ansari, H.R. Effect of gibberellic acid spray during ontogeny of mustard on growth, nutrient uptake and yield characteristics. J. Agron. Crop Sci. 1998, 181, 61–63. [Google Scholar] [CrossRef]
- Pal, P.; Yadav, K.; Kumar, K.; Singh, N. Effect of gibberellic acid and potassium foliar sprays on productivity and physiological and biochemical parameters of parthenocarpic cucumber cv.‘Seven Star F1’. J. Hortic. Res. 2016, 24, 93–100. [Google Scholar] [CrossRef]
- Shah, S.H. Effects of Salt Stress on Mustard As Affected By Gibberellic Acid Application. Gen. Appl. Plant Physiol. 2007, 33, 97–106. [Google Scholar]
- Gelmesa, D.; Abebie, B.; Desalegn, L. Effects of gibberellic acid and 2, 4-dichlorophenoxyacetic acid spray on fruit yield and quality of tomato (Lycopersicon esculentum Mill.). J. Plant Breed. Crop Sci. 2010, 2, 316–324. [Google Scholar]
- Khan, N.A.; Mir, R.; Khan, M.; Javid, S. Effects of gibberellic acid spray on nitrogen yield efficiency of mustard grown with different nitrogen levels. Plant Growth Regul. 2002, 38, 243–247. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of Gibberellic Acid on Growth, Yield, and Quality of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef]
- Miceli, A.; Vetrano, F.; Sabatino, L.; D’Anna, F.; Moncada, A. Influence of preharvest gibberellic acid treatments on postharvest quality of minimally processed leaf lettuce and rocket. Horticulturae 2019, 5, 63. [Google Scholar] [CrossRef]
- Moncada, A.; Vetrano, F.; Esposito, A.; Miceli, A. Fertigation Management and Growth-Promoting Treatments Affect Tomato Transplant Production and Plant Growth after Transplant. Agronomy 2020, 10, 1504. [Google Scholar] [CrossRef]
- Feller, C.; Bleiholder, H.; Buhr, L.; Hack, H.; Hess, M.; Klose, R.; Meier, U.; Stauss, R.; van den Boom, T.; Weber, E. Phanologische Entwicklungsstadien von Gemusepflanzen II. Fruchtgemuse und Hulsenfruchte. Nachr. Dtsch. Pflanzenschutzd. 1995, 47, 217–232. [Google Scholar]
- Kazemi, M. Effect of gibberellic acid and potassium nitrate spray on vegetative growth and reproductive characteristics of tomato. J. Biol. Environ. Sci. 2014, 8, 1–9. [Google Scholar]
- Choudhury, S.; Islam, N.; Ali, M. Growth and Yield of Summer Tomato as Influenced by Plant Growth Regulators. Int. J. Sustain. Agric. 2013, 5, 25–28. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Blumwald, E. Developing salt-tolerant crop plants: Challenges and opportunities. Trends Plant Sci. 2005, 10, 615–620. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Gama, P.B.S.; Inanaga, S.; Tanaka, K.; Nakazawa, R. Physiological response of common bean (Phaseolus vulgaris L.) seedlings to salinity stress. Afr. J. Biotechnol. 2007, 6, 79–88. [Google Scholar]
- Bayuelo-Jiménez, J.S.; Debouck, D.G.; Lynch, J.P. Growth, gas exchange, water relations, and ion composition of Phaseolus species grown under saline conditions. Field Crops Res. 2003, 80, 207–222. [Google Scholar] [CrossRef]
- Shahbaz, M.; Ashraf, M.; Al-Qurainy, F.; Harris, P.J.C. Salt Tolerance in Selected Vegetable Crops. Crit. Rev. Plant Sci. 2012, 31, 303–320. [Google Scholar] [CrossRef]
- Moncada, A.; Vetrano, F.; Miceli, A. Alleviation of Salt Stress by Plant Growth-Promoting Bacteria in Hydroponic Leaf Lettuce. Agronomy 2020, 10, 1523. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R.; Maas, E. V Plant salt tolerance. Agric. Salin. Assess. Manag. 2012, 2, 405–459. [Google Scholar]
- FAO Drainage Paper 61, Agricultural Drainage Water Management in Arid and Semi-Arid Areas, Annex 1. Crop Salt Tolerance Data. Available online: http://www.fao.org/3/y4263e/y4263e0e.htm (accessed on 10 September 2020).
- Le Rudulier, D. Osmoregulation in rhizobia: The key role of compatible solutes. Grain Legum. 2005, 42, 18–19. [Google Scholar]
- Srivastava, J.P.; Gupta, S.C.; Lal, P.; Muralia, R.N.; Kumar, A. Effect of salt stress on physiological and biochemical parameters of wheat. Annu. Arid Zo. 1988, 27, 197–204. [Google Scholar]
- Shalhevet, J. Plants under salt and water stress. In Plant Adaptation to Environmental Stress; Fowden, L., Mansfield, T., Stoddart, J., Eds.; Chapman and Hall: London, UK, 1993; p. 133. [Google Scholar]
- Lee, I.-J. Practical application of plant growth regulator on horticultural crops. J. Hort. Sci 2003, 10, 211–217. [Google Scholar]
- Khan, N.A. Comparative effect of modes of gibberellic acid application on photosynthetic biomass distribution and productivity of rapeseed-mustard. Physiol. Mol. Biol. Plants 2003, 9, 141–145. [Google Scholar]
- Achard, P.; Gusti, A.; Cheminant, S.; Alioua, M.; Dhondt, S.; Coppens, F.; Beemster, G.T.S.; Genschik, P. Gibberellin Signaling Controls Cell Proliferation Rate in Arabidopsis. Curr. Biol. 2009, 19, 1188–1193. [Google Scholar] [CrossRef]
- Ubeda-Tomás, S.; Federici, F.; Casimiro, I.; Beemster, G.T.S.; Bhalerao, R.; Swarup, R.; Doerner, P.; Haseloff, J.; Bennett, M.J. Gibberellin Signaling in the Endodermis Controls Arabidopsis Root Meristem Size. Curr. Biol. 2009, 19, 1194–1199. [Google Scholar] [CrossRef]
- Emongor, V.E. Effect of benzyladenine and gibberellins on growth, yield and yield components of common bean (Phaseolus vulgaris). UNISWA Res. J. Agric. Sci. Technol 2002, 6, 65–72. [Google Scholar] [CrossRef]
- Gupta, V.N.; Datta, S.K. Influence of gibberellic acid (GA3) on growth and flowering in chrysanthemum (Chrysanthemummorifolium, Ramat) cv. Jayanti. Indian J. Plant Physiol. 2001, 6, 420–422. [Google Scholar]
- Huttly, A.K.; Phillips, A.L. Gibberellin-regulated plant genes. Physiol. Plant. 1995, 95, 310–317. [Google Scholar] [CrossRef]
- Van Huizen, R.; Ozga, J.A.; Reinecke, D.M. Influence of auxin and gibberellin on in vivo protein synthesis during early pea fruit growth. Plant Physiol. 1996, 112, 53–59. [Google Scholar] [CrossRef][Green Version]
- Cohn, N.S.; Zhang, L.; Mitchell, J.P.; Vierheller, C.-Z.J. Gibberellin-stimulated changes in abundance of two mRNAs in the developing shoot of dwarf peas (Pisum sativum L.). Int. J. Plant Sci. 1994, 155, 498–505. [Google Scholar] [CrossRef]
- Shiri, Y.; Solouki, M.; Ebrahimie, E.; Emamjomeh, A.; Zahiri, J. Gibberellin causes wide transcriptional modifications in the early stage of grape cluster development. Genomics 2020, 112, 820–830. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Khan, M.N.; Mohammad, F.; Khan, M.M.A. Role of nitrogen and gibberellin (GA3) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J. Agron. Crop Sci. 2008, 194, 214–224. [Google Scholar] [CrossRef]
- De Freitas, S.T.; Jiang, C.-Z.; Mitcham, E.J. Mechanisms involved in calcium deficiency development in tomato fruit in response to gibberellins. J. Plant Growth Regul. 2012, 31, 221–234. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef]
- Liptay, A.; Nicholls, S. Nitrogen Supply during Greenhouse Transplant Production Affects Subsequent Tomato Root Growth in the Field. J. Am. Soc. Hortic. Sci. 1993, 118, 339–342. [Google Scholar] [CrossRef]
- Javid, M.G.; Sorooshzadeh, A.; Moradi, F.; Ali, S.; Modarres, M. Review article The role of phytohormones in alleviating salt stress in crop plants. Annu. Rev. Plant Biol. 2011, 5, 726–734. [Google Scholar]
- Yuan, K.; Rashotte, A.M.; Wysocka-Diller, J.W. ABA and GA signaling pathways interact and regulate seed germination and seedling development under salt stress. Acta Physiol. Plant. 2011, 33, 261–271. [Google Scholar] [CrossRef]
- Sun, T. Gibberellin Metabolism, Perception and Signaling Pathways in Arabidopsis. Arab. Book 2008, 6, e0103. [Google Scholar] [CrossRef] [PubMed]
- Gonai, T.; Kawahara, S.; Tougou, M.; Satoh, S.; Hashiba, T.; Hirai, N.; Kawaide, H.; Kamiya, Y.; Yoshioka, T. Abscisic acid in the thermoinhibition of lettuce seed germination and enhancement of its catabolism by gibberellin. J. Exp. Bot. 2004, 55, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Evins, W.H.; Varner, J.E. Hormonal control of polyribosome formation in barley aleurone layers. Plant Physiol. 1972, 49, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Broughton, W.J.; McComb, A.J. Changes in the pattern of enzyme development in gibberellin-treated pea internodes. Ann. Bot. 1971, 35, 213–228. [Google Scholar] [CrossRef]
- Johri, M.; Varner, J.E. Enhancement of RNA synthesis in isolated pea nuclei by gibberellic acid. Proc. Natl. Acad. Sci. USA 1968, 59, 269. [Google Scholar] [CrossRef]
- Roth-Bejerano, N.; Lips, S.H. Hormonal regulation of nitrate reductase activity in leaves. New Phytol. 1970, 69, 165–169. [Google Scholar] [CrossRef]
- Wood, A.; Paleg, L.G. The influence of gibberellic acid on the permeability of model membrane systems. Plant Physiol. 1972, 50, 103–108. [Google Scholar] [CrossRef]
- Wood, A.; Paleg, L.G. Alteration of liposomal membrane fluidity by gibberellic acid. Funct. Plant Biol. 1974, 1, 31–40. [Google Scholar] [CrossRef]
- Al-Wakeel, S.A.M.; Dadoura, S.S.; Hamed, A.A. Interactive effects of water stress and gibberellic acid on mineral composition of fenugreek plant. Egypt. J. Physiol. Sci. 1994, 18, 269–272. [Google Scholar]
- Ansari, H. Effect of Some Phytohormones and NPK on Growth and Metabolism of Mustard. Ph.D. Thesis, Aligarh Muslim University, Aligarh, India, 1996. [Google Scholar]
- Daie, J.; Watts, M.; Aloni, B.; Wyse, R.E. In vitro and in vivo modification of sugar transport and translocation in celery by phytohormones. Plant Sci. 1986, 46, 35–41. [Google Scholar] [CrossRef]
- Estruch, J.J.; Peretó, J.G.; Vercher, Y.; Beltrán, J.P. Sucrose loading in isolated veins of Pisum sativum: Regulation by abscisic acid, gibberellic acid, and cell turgor. Plant Physiol. 1989, 91, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, D.R.; Patrick, J.W. Gibberellic-acid-promoted transport of assimilates in stems of Phaseolus vulgaris L. Planta 1979, 145, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Soundy, P.; Cantliffe, D.J.; Hochmuth, G.J.; Stoffella, P.J. Management of nitrogen and irrigation in lettuce transplant production affects transplant root and shoot development and subsequent crop yields. HortScience 2005, 40, 607–610. [Google Scholar] [CrossRef]
- Vetrano, F.; Miceli, C.; Angileri, V.; Frangipane, B.; Moncada, A.; Miceli, A. Effect of Bacterial Inoculum and Fertigation Management on Nursery and Field Production of Lettuce Plants. Agronomy 2020, 10, 1477. [Google Scholar] [CrossRef]
- Cleland, R.E. Introduction: Nature, occurrence and functioning of plant hormones. In Biochemistry and Molecular Biology of Plant Hormones; Hooykaas, P.J.J., Hall, M.A., Libbenga, K.R., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 33, pp. 3–22. [Google Scholar]
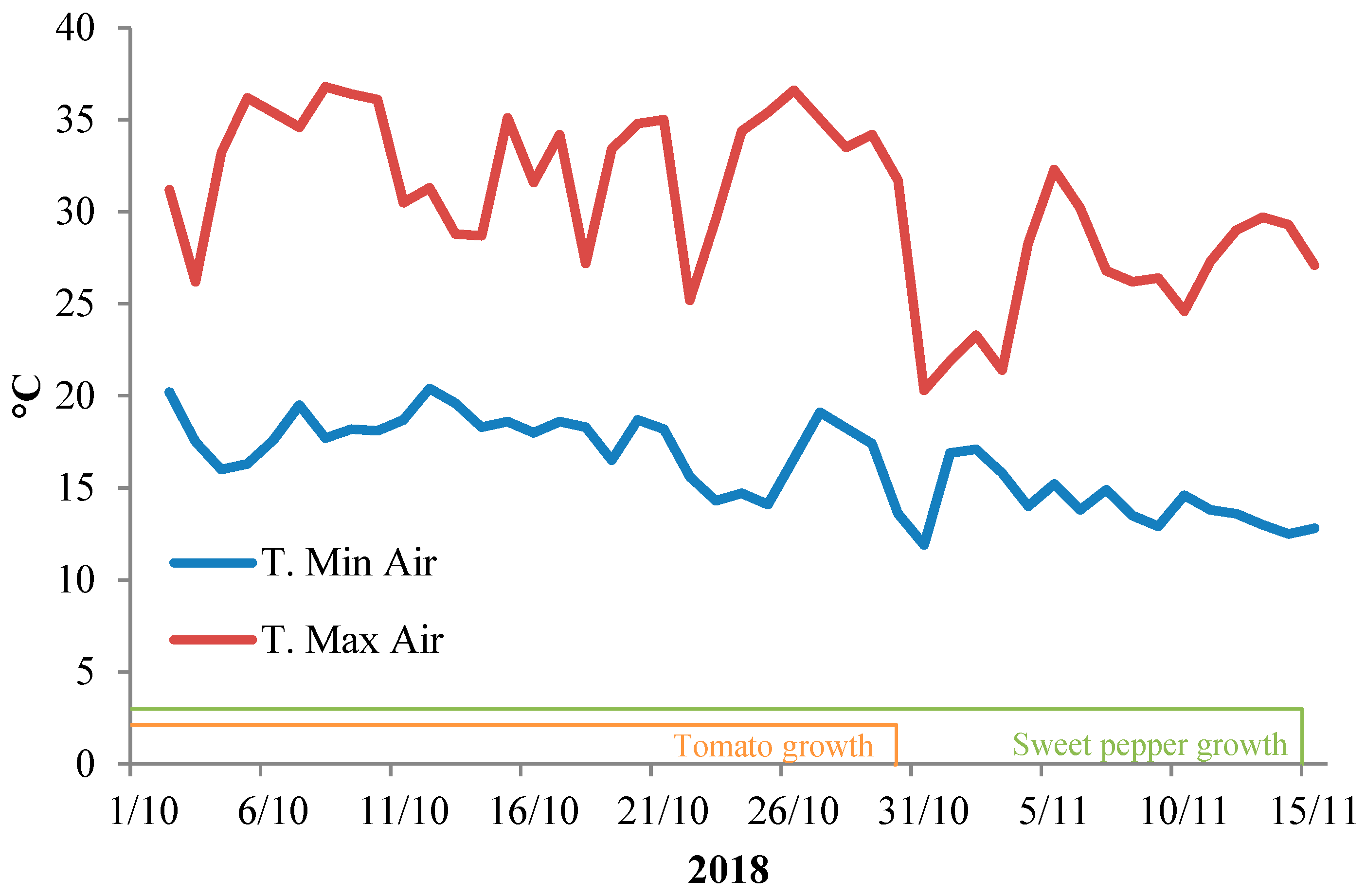
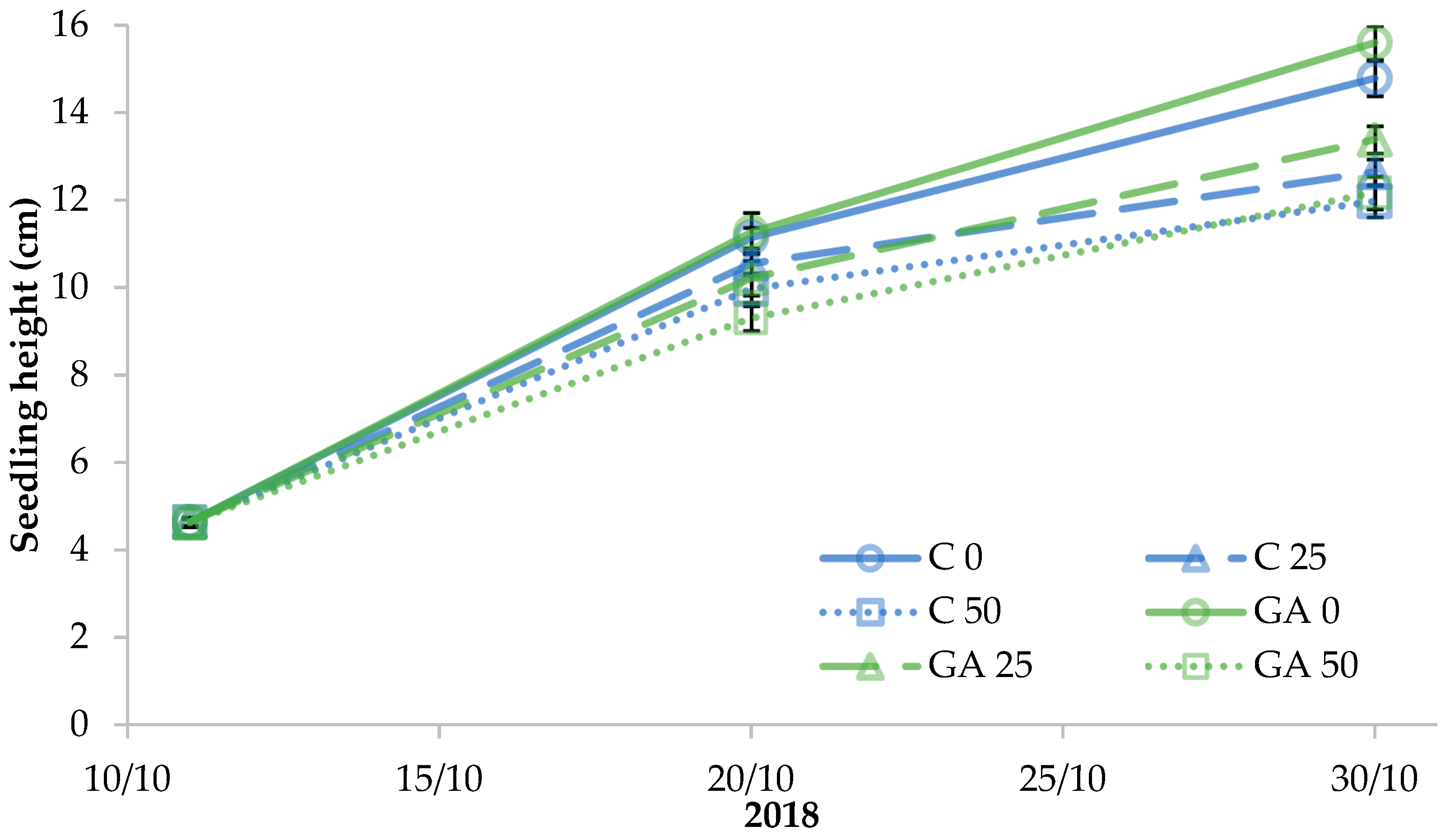

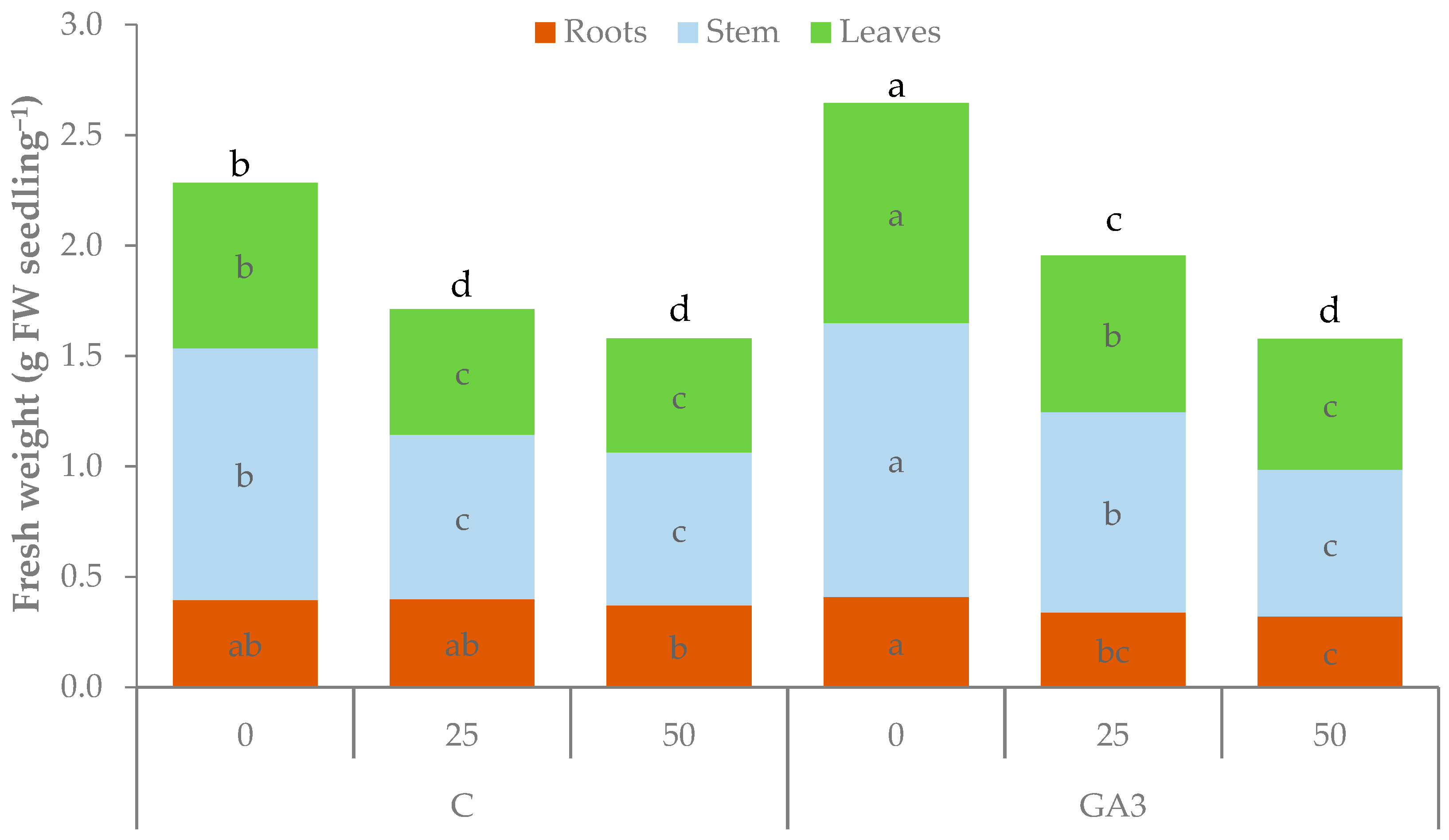
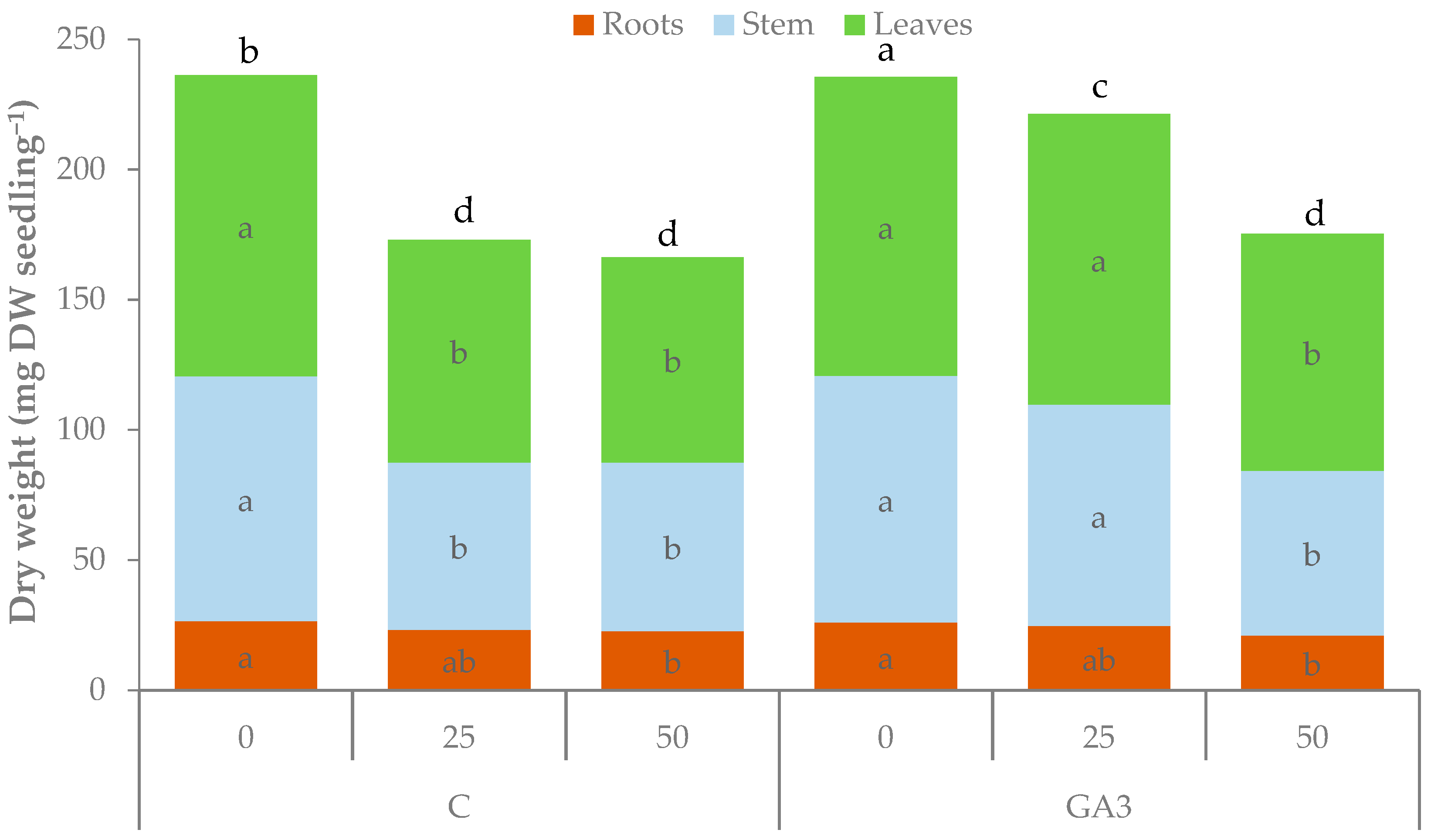

| Source of Variance | Seedling Fresh Weight (g FW) | Seedling Dry Weight (mg DW) | Dry Matter (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Roots | Stem | Leaves | Shoot/Root | Total | Roots | Stem | Leaves | Shoot/Root | |||
| Treatment | ||||||||||||
| C | z 1.86 | 0.39 | 0.86 | 0.61 | 3.8 | 191.8 | 24.2 | 74.3 | 93.3 | 7.0 | 11.9 | |
| GA3 | 2.06 | 0.36 | 0.94 | 0.77 | 4.7 | 210.7 | 23.9 | 81.0 | 105.8 | 7.8 | 11.7 | |
| NaCl (mM) | ||||||||||||
| 0 | 2.46 | 0.40 | 1.19 | 0.87 | 5.1 | 235.9 | 26.3 a | 94.4 | 115.3 | 8.2 | 10.5 | |
| 25 | 1.83 | 0.37 | 0.82 | 0.64 | 4.0 | 197.1 | 24.0 ab | 74.6 | 98.5 | 7.2 | 12.3 | |
| 50 | 1.58 | 0.35 | 0.68 | 0.55 | 3.6 | 170.8 | 21.9 b | 64.0 | 84.9 | 6.8 | 12.6 | |
| Treatment × NaCl | ||||||||||||
| C | 0 | 2.28 b | 0.40 ab | 1.14 b | 0.75 b | 4.8b | 236.3 a | 26.5 | 94.0 a | 115.8 a | 8.3a | 11.4 b |
| 25 | 1.71 d | 0.40 ab | 0.74 c | 0.57 c | 3.3c | 173.0 b | 23.3 | 64.3 b | 85.5 b | 6.5b | 11.9 ab | |
| 50 | 1.58 d | 0.37 b | 0.69 c | 0.52 c | 3.2c | 166.3 b | 22.8 | 64.8 b | 78.8 b | 6.3b | 12.4 ab | |
| GA3 | 0 | 2.64 a | 0.41 a | 1.24 a | 0.99 a | 5.4a | 235.5 a | 26.0 | 94.8 a | 114.8 a | 8.2a | 9.6 c |
| 25 | 1.95 c | 0.34 bc | 0.91 b | 0.71 b | 4.7b | 221.3 a | 24.8 | 85.0 a | 111.5 a | 7.9a | 12.7 a | |
| 50 | 1.58 d | 0.32 c | 0.67 c | 0.59 c | 3.9c | 175.3 b | 21.0 | 63.3 b | 91.0 b | 7.2ab | 12.8 a | |
| Significance x | ||||||||||||
| Treatment | ns | ns | ns | ** | ** | ** | ns | * | *** | * | ns | |
| NaCl | *** | *** | *** | *** | ** | *** | * | *** | *** | ** | *** | |
| Treatment × NaCl | ** | *** | *** | ** | * | ** | ns | ** | ** | * | ** | |
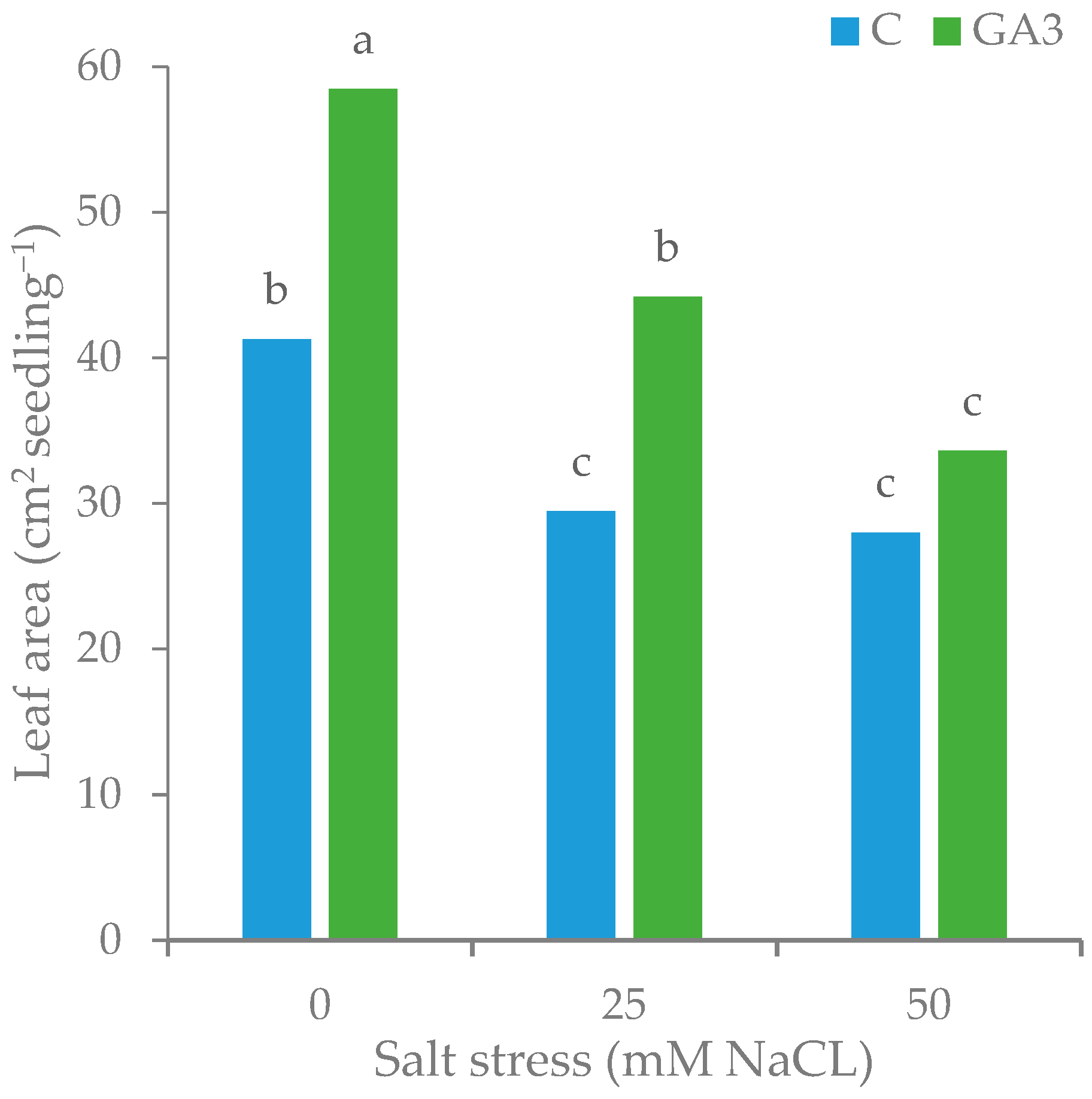
| Source of Variance | Number of Leaves | Leaf Area | SLA (cm2 g DW−1) | Stomatal Conductance (mmol m2 s−1) | RWC(%) | L * | Chroma | Hue° | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (cm2 Seedling−1) | (cm2 Leaf−1) | |||||||||
| Treatment | ||||||||||
| C | z 4.61 b | 32.90 | 7.11 | 353.4 | 188.1 | 86.8 a | 51.7 | 49.0 b | 120.5 b | |
| GA3 | 5.44 a | 45.41 | 8.37 | 425.3 | 219.5 | 80.0 b | 52.1 | 50.9 a | 121.3 a | |
| NaCl (mM) | ||||||||||
| 0 | 5.27 a | 49.86 | 9.39 | 435.1 | 371.8 | 87.5 a | 50.3 a | 48.4 b | 121.2 a | |
| 25 | 5.06 ab | 36.82 | 7.35 | 370.9 | 171.6 | 83.8 a | 52.1 ab | 50.7 a | 121.3 a | |
| 50 | 4.75 b | 30.80 | 6.47 | 362.1 | 67.8 | 78.8 b | 53.2 b | 50.7 a | 120.1 b | |
| Treatment × NaCl | ||||||||||
| C | 0 | 4.79 | 41.25 b | 8.62 b | 360.3 b | 369.9 a | 90.0 | 50.1 | 47.6 | 120.9 |
| 25 | 4.60 | 29.47 c | 6.42 c | 345.0 b | 123.0 c | 90.1 | 52.8 | 50.2 | 121.0 | |
| 50 | 4.45 | 27.99 c | 6.29 c | 354.9 b | 71.3 d | 80.2 | 52.3 | 49.1 | 119.5 | |
| GA3 | 0 | 5.75 | 58.47 a | 10.17 a | 509.9 a | 373.7 a | 85.0 | 50.6 | 49.2 | 121.5 |
| 25 | 5.53 | 44.17 b | 8.28 b | 396.7 b | 220.3 b | 77.6 | 51.5 | 51.2 | 121.6 | |
| 50 | 5.05 | 33.60 c | 6.65 c | 369.3 b | 64.4 d | 77.3 | 54.1 | 52.2 | 120.7 | |
| Significance x | ||||||||||
| Treatment | *** | *** | * | *** | ns | *** | ns | ** | * | |
| NaCl | ** | *** | *** | ** | *** | ** | ** | ** | ** | |
| Treatment × NaCl | ns | *** | *** | ** | * | ns | ns | ns | ns | |
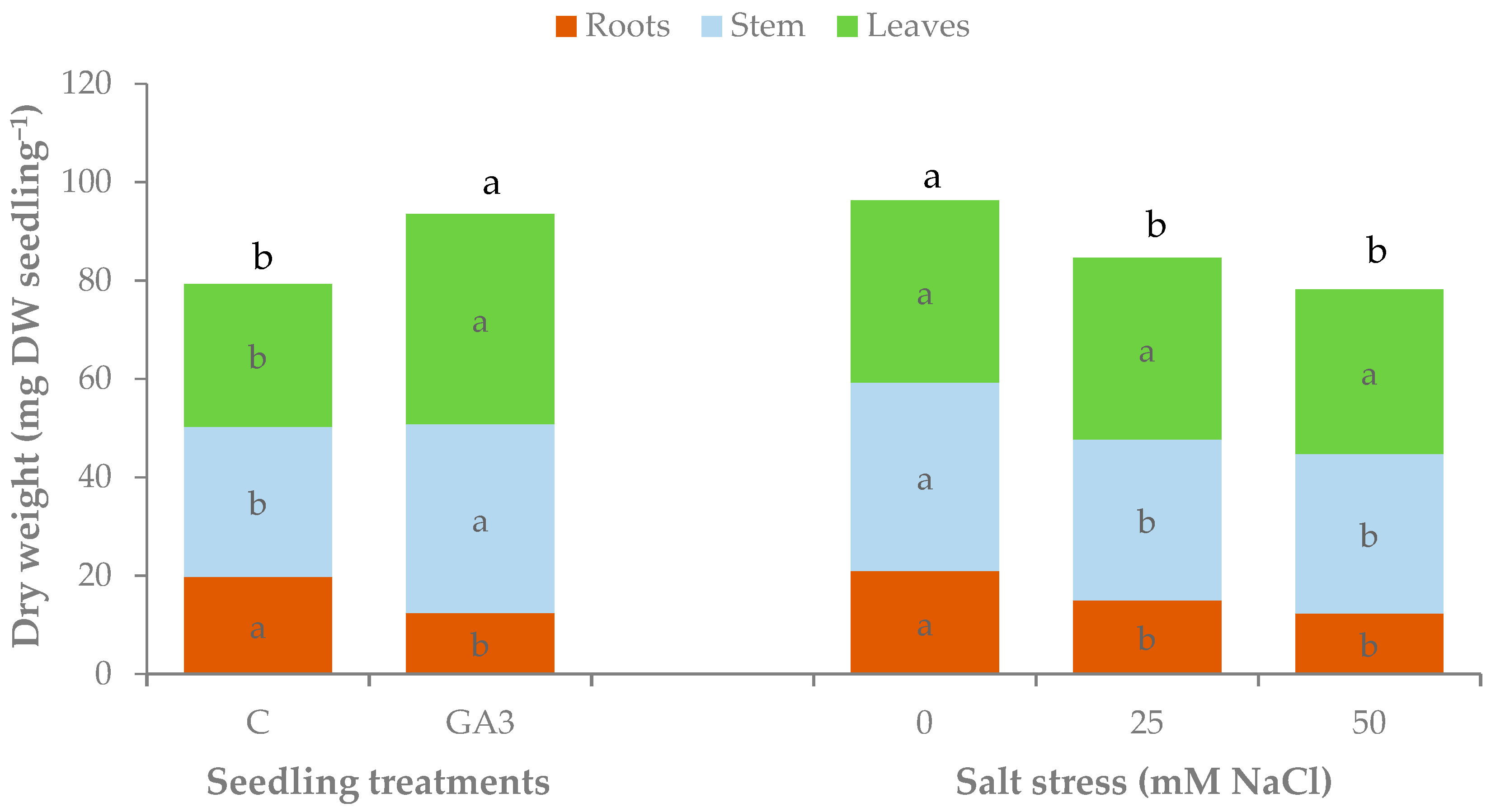
| Source of Variance | Seedling Fresh Weight (g FW) | Seedling Dry Weight (mg DW) | Dry Matter (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Roots | Stem | Leaves | Shoot/Root | Total | Roots | Stem | Leaves | Shoot/Root | |||
| Treatment | ||||||||||||
| C | z 0.97 b | 0.29 | 0.41 b | 0.27 b | 2.4 b | 79.3 b | 19.8 | 30.5 b | 29.0 b | 3.0 | 8.8 b | |
| GA3 | 1.14 a | 0.25 | 0.51 a | 0.38 a | 3.7 a | 93.5 a | 12.4 | 38.4 a | 42.7 a | 7.6 | 9.1 a | |
| NaCl (mM) | ||||||||||||
| 0 | 1.15 a | 0.31 | 0.50 a | 0.34 | 2.8 b | 96.3 a | 21.0 | 38.3 a | 37.0 | 3.6 | 8.9 | |
| 25 | 1.06 ab | 0.28 | 0.45 a | 0.33 | 3.0 ab | 84.6 b | 15.0 | 32.6 b | 37.0 | 5.5 | 8.8 | |
| 50 | 0.95 b | 0.22 | 0.42 b | 0.31 | 3.3 a | 78.2 b | 12.3 | 32.4 b | 33.5 | 6.8 | 9.0 | |
| Treatment × NaCl | ||||||||||||
| C | 0 | 1.06 | 0.32 a | 0.46 | 0.29 | 2.3 | 87.3 | 22.0 a | 34.3 | 31.0 | 3.0 d | 8.8 |
| 25 | 0.98 | 0.32 a | 0.39 | 0.27 | 2.1 | 77.2 | 20.0 a | 28.2 | 29.0 | 2.9 d | 8.7 | |
| 50 | 0.86 | 0.23 b | 0.38 | 0.25 | 2.8 | 73.2 | 17.3 a | 28.9 | 27.0 | 3.2 d | 8.9 | |
| GA3 | 0 | 1.24 | 0.29 a | 0.55 | 0.39 | 3.2 | 105.3 | 20.0 a | 42.3 | 43.0 | 4.3 c | 9.0 |
| 25 | 1.14 | 0.23 b | 0.52 | 0.39 | 3.9 | 92.1 | 10.0 b | 37.1 | 45.0 | 8.2 b | 9.0 | |
| 50 | 1.04 | 0.21 b | 0.46 | 0.37 | 3.9 | 83.2 | 7.3 b | 35.9 | 40.0 | 10.3 a | 9.2 | |
| Significance x | ||||||||||||
| Treatment | *** | *** | *** | *** | *** | ** | *** | *** | *** | *** | ** | |
| NaCl | * | *** | * | ns | * | ** | *** | ** | ns | *** | ns | |
| Treatment × NaCl | ns | ** | ns | ns | ns | ns | ** | ns | ns | *** | ns | |
| Source of Variance | Number of Leaves | Leaf Area | SLA (cm2 g DW−1) | Stomatal Conductance (mmol m2 s−1) | RWC (%) | L* | Chroma | Hue° | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (cm2 seedling−1) | (cm2 leaf−1) | |||||||||
| Treatment | ||||||||||
| C | z 3.75 b | 13.44 b | 3.57 b | 463.4 | 49.8 | 90.8 | 50.5 b | 58.7 b | 120.3 a | |
| GA3 | 4.44 a | 20.10 a | 4.51 a | 472.3 | 54.0 | 89.6 | 54.8 a | 61.8 a | 119.0 b | |
| NaCl (mM) | ||||||||||
| 0 | 4.35 a | 19.37 a | 4.41 a | 519.7 | 58.5 a | 93.1 a | 53.2 a | 60.8 a | 119.7 ab | |
| 25 | 4.01 b | 16.29 ab | 4.03 ab | 488.7 | 54.0 a | 89.5 b | 53.6 a | 60.8 a | 119.1 b | |
| 50 | 3.93 b | 14.64 b | 3.69 b | 395.0 | 43.1 b | 88.1 b | 51.0 b | 59.0 b | 120.1 a | |
| Treatment × NaCl | ||||||||||
| C | 0 | 3.93 | 15.41 | 3.93 | 497.1 b | 55.7 | 93.2 | 50.6 | 59.1 | 120.8 |
| 25 | 3.73 | 13.52 | 3.63 | 500.8 b | 52.6 | 89.8 | 51.2 | 59.1 | 119.9 | |
| 50 | 3.60 | 11.38 | 3.16 | 392.3 c | 40.7 | 89.5 | 49.6 | 57.7 | 120.2 | |
| GA3 | 0 | 4.78 | 23.32 | 4.88 | 542.4 a | 61.3 | 93.0 | 55.9 | 62.6 | 118.7 |
| 25 | 4.30 | 19.07 | 4.43 | 476.7 b | 55.3 | 89.2 | 55.9 | 62.5 | 118.3 | |
| 50 | 4.25 | 17.90 | 4.21 | 397.8 c | 45.5 | 86.6 | 52.5 | 60.3 | 120.0 | |
| Significance x | ||||||||||
| Treatment | *** | *** | *** | ns | ns | ns | *** | *** | *** | |
| NaCl | ** | ** | ** | *** | ** | *** | *** | *** | * | |
| Treatment × NaCl | ns | ns | ns | ** | ns | ns | ns | ns | ns | |
| Variable | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Height | 0.984 | 0.089 | −0.022 | 0.087 |
| Stem diameter | 0.934 | −0.201 | −0.113 | 0.101 |
| Total fresh weight | 0.976 | 0.003 | 0.203 | 0.002 |
| Root fresh weight | 0.795 | −0.443 | 0.052 | 0.294 |
| Stem fresh weight | 0.962 | 0.010 | 0.240 | −0.060 |
| Leaf fresh weight | 0.970 | 0.122 | 0.178 | −0.021 |
| Shoot/Root FW | 0.810 | 0.466 | 0.204 | −0.259 |
| Total dry weight | 0.995 | −0.003 | −0.025 | 0.056 |
| Root dry weight | 0.704 | −0.609 | 0.050 | 0.335 |
| Stem dry weight | 0.994 | 0.020 | 0.035 | 0.006 |
| Leaf dry weight | 0.989 | 0.084 | −0.079 | 0.042 |
| Shoot/Root DW | 0.530 | 0.720 | −0.080 | −0.364 |
| Dry matter % | 0.684 | 0.075 | −0.667 | 0.255 |
| Leaf number | 0.855 | 0.353 | 0.174 | 0.180 |
| Plant area | 0.962 | 0.114 | 0.202 | 0.028 |
| Leaf area | 0.986 | 0.074 | 0.137 | 0.027 |
| SLA | −0.346 | −0.005 | 0.839 | 0.254 |
| Stomatal conductance | 0.869 | −0.114 | 0.381 | −0.231 |
| RWC | −0.520 | −0.321 | 0.573 | −0.073 |
| L* | −0.256 | 0.726 | 0.042 | 0.599 |
| Chroma | −0.890 | 0.260 | 0.314 | 0.025 |
| Hue° | 0.672 | −0.442 | −0.118 | −0.342 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miceli, A.; Vetrano, F.; Moncada, A. Effects of Foliar Application of Gibberellic Acid on the Salt Tolerance of Tomato and Sweet Pepper Transplants. Horticulturae 2020, 6, 93. https://doi.org/10.3390/horticulturae6040093
Miceli A, Vetrano F, Moncada A. Effects of Foliar Application of Gibberellic Acid on the Salt Tolerance of Tomato and Sweet Pepper Transplants. Horticulturae. 2020; 6(4):93. https://doi.org/10.3390/horticulturae6040093
Chicago/Turabian StyleMiceli, Alessandro, Filippo Vetrano, and Alessandra Moncada. 2020. "Effects of Foliar Application of Gibberellic Acid on the Salt Tolerance of Tomato and Sweet Pepper Transplants" Horticulturae 6, no. 4: 93. https://doi.org/10.3390/horticulturae6040093
APA StyleMiceli, A., Vetrano, F., & Moncada, A. (2020). Effects of Foliar Application of Gibberellic Acid on the Salt Tolerance of Tomato and Sweet Pepper Transplants. Horticulturae, 6(4), 93. https://doi.org/10.3390/horticulturae6040093





