Abstract
Avocado byproducts are a rich source of health-promoting biomolecules. The purpose of this work is to study three groups of statistically different avocado fruit sizes (Persea americana Mill.) (small (S), medium (M), and large (L)), and their relationship with total phenolic and flavonoid contents (TPC and TFC, respectively), DPPH (2,2-diphenyl-1-picrylhydrazyl) scavenging capacity and individual phenolics, and the activities of phenylalanine ammonia-lyase (PAL), chalcone synthase (CHS), and polyphenol oxidase (PPO) in avocado peel extract (APE). The results indicated that TPC, TFC, and antioxidant and enzymatic activities were higher in the APE of the S group (p < 0.05). The flavonoids (flavanols and flavonols) and phenolic acids were also significatively concentrated in S group’s APE. Overall, the phenolic content was significantly lower in the L group. Positive correlations (p < 0.0001 and p < 0.05) were observed between TPC, TPF, DPPH, and enzymatic activity, and negative correlations resulted for avocado weight and volume. The outstanding phenolic content and enzymatic activity of avocado peels from low-cost avocado byproducts are ideal for biorefinery applications, thereby increasing the bioeconomy of the avocado industry.
Keywords:
polyphenols; avocado byproducts; abiotic stress; CHS; PAL; PPO; biorefinery; circular economies 1. Introduction
The avocado (Persea americana Mill.), a native of Mesoamerica, is a heavily consumed tropical fruit. Originating in California, the “Hass” avocado is a hybrid of two races (Guatemalan and Mexican) and is mainly harvested in Latin America. The “Hass” avocado leads in overall avocado production [1,2]. The yield of avocado orchards is quantified from the fruit size amount; the number of cells of a fruit determines avocado fruit size [1], although stressors such as water shortage affect the development of avocado fruit [2]. After harvesting, avocados are sorted according to size as large, medium, and small. Low-weight and -volume fruits are economically and environmentally unviable because they are poorly valorized and are eventually rejected as waste [1,3]. Thus, the use of undesired avocados and their byproducts in refinery applications may contribute to the sustainability of avocado production.
In avocado fruit development, cells multiply rapidly primarily during anthesis. Following that, the volume of the fruit increases until maturity. Thus, the final fruit size is principally influenced by the number of cells rather than the individual cell size [3]. Stressors such as cultivar age, fruit load, light, nutrition, pests, and water supply [1,4] are the main factors that influence fruit development. Water, in particular, has been shown to impact avocado production [5]. In drought periods, there is a considerable increase in the proportion of small-sized avocados [2]. In the central–north part of Chile, one of the world’s principal avocado-producing regions, the overexploitation of avocado production has led to a severe shortage in water supply, thus affecting avocado yield [6]. The resulting low-caliber fruits (5–40% of the total crop) are undesirable due to their lack of economic value and are usually discarded or used in processing that results in the segregation of byproducts, thus negatively affecting the avocado value chain and the environment [1,3,6].
Avocado byproducts are rich in phytochemicals that have industrial applications. Polyphenols are secondary plant metabolites created in response to environmental stressors for constant maintenance of redox homeostasis [4,7]. These metabolites are biosynthesized on the cytoplasmic face of the endoplasm through the malonic and shikimic acid pathways catalyzed by phenylalanine ammonia-lyase (PAL) and chalcone synthase (CHS), regulated by polyphenol oxidase (PPO) enzymes, and stored in the vacuole of the cell. The phenolic composition of avocado peel is remarkable compared to that of other tropical fruits. The study of the metabolism and physiology of fruits such as avocado has advanced due to the active link between the bioactive molecules and potential applications for wellness and bioindustrial purposes. Thus, a number of successful cases in biorefining undervalued byproducts have recently been reviewed, including when green methods of extraction are employed. Byproducts that are high in polyphenols and enzymes have been found to be a suitable raw material for extraction applications, reducing the negative impact on the environment and the economy and promoting the sustainability of the food industry [6,7,8,9,10,11,12,13,14].
There is a current lack of comparative studies that correlate the phenolic content with the physiology of the fruit. Thus, in this study, it is hypothesized that the phenolic and enzymatic activity composition of avocado peel extract (APE) varies according to the fruit size of “Hass” avocado. To investigate this hypothesis, total phenolic content (TPC), total flavonoid content (TFC), DPPH scavenging capacity, and the contents of individual phenolics, in addition to PAL, CHS, and PPO activities, were measured, analyzed, and correlated with the APE from three groups of avocados that statistically differed in size according to their weight and volume (small (S), medium (M), and large (L)). In conclusion, it is demonstrated that due to its high phenolic and enzymatic content, the peel byproduct of small, low-cost avocados is a rich source of raw material for industrial biorefinery applications.
2. Material and Methods
2.1. Chemicals
Methanol, sodium carbonate, phosphate, gallic acid, Folin–Ciocalteu reagent, acetic acid, acetonitrile, HPLC-grade distilled water, 2,2-diphenyl-1-picrylhydrazyl (DPPH), aluminum chloride, sodium acetate, boric acid, sodium tetraborate, hydrochloric acid, potassium cyanide, ethanol, glycerol tris(hydroxymethyl)aminomethane, L-phenylalanine acetate, and catechol were purchased from Merck (Darmstadt, Germany). Standards: (+)-catechin hydrant, (−)-epicatechin, quercetin, (±)-naringenin, naringin, hesperidin, rutin, kaempferol, and ferulic, caffeic, and coumaric acids were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
2.2. Sampling and Ripeness State Evaluation
Ninety fruit samples of “Hass” avocado from the north of Chile were harvested when their dry matter approached 23% [1]. Then, they were sorted by their volume and weight and classified as S-, M-, and L-sized avocados. Subsequently, fruits were equally ripened to a ready-to-eat state, according to procedures used by other authors [11]. Firmness and color are two maturity indexes that are commonly associated with avocado fruit ripeness [12,13]. Firmness and color were measured at 3 points on each sample (one central on the fruit base and two laterals). A TA-XT2 texture analyzer, coupled with a cylindrical plunger P6 (Stable Micro Systems, Ltd., Godalming, UK), was employed for firmness assessment at a 1 cm/min penetration speed (depth 0.7 cm). The color was measured according to International Standard based on the lightness (L*), green–red (a*), and blue–yellow (b*) relation (CIE L*, a*,b*), using a Chroma Meter CR-400 (Konica Minolta, Tokyo, Japan).
2.3. Extraction Procedure
Each group of avocados (S, M, and L) was washed and dried separately; then, peels were separated from the flesh, cut into small pieces (1 × 0.5 cm approx.), and immediately oven-dried (Memmert, Buechenbach, Germany; 60 °C for 24 h). After drying, avocado peels from L, M, and S fruit were separately powdered (particle size: 0.2 mm) in an electrical grinder (Oster BVSTBMH23-052, Shanghai, China). Each avocado powder was kept in sealed bags in the dark at 4 °C until extraction was performed (between 1 or 2 days).
A solid–liquid extraction with low-frequency sonication was carried out to obtain avocado peel extracts. Avocado peel powder (1 g) was mixed with 25 mL of a solvent comprising 80% methanol in distilled water in a 100 mL flask, covered, then immersed in an ultrasonic bath (Elmasonic S 10 H, Singen, Germany). The samples were sonicated at a constant frequency of 37 kHz and 90 W for 30 min at 40 °C, according to previous trials. The extracts were immediately vacuum-filtered using a cellulose filter 1 (90 mm), followed by centrifugation for 20 min at 10,000× g at 4 °C; supernatants were kept at −80 °C until analyses.
2.4. Phytochemical Characterization of APEs
Phytochemical characterization of the avocado peel extracts (APEs) from the L, M, and S groups was performed to determine TPC, TFC, and DPPH scavenging capacity. To perform the analysis, the each obtained APEs were properly diluted in methanol (1:40). For all of the experiments (including enzymatic activities), the absorbances were measured using an Epoch 256695 spectrophotometer microplate reader (BioTek, Winooski, VT, USA).
2.4.1. TPC
The phenolic content of each APEs was measured by the Folin–Ciocalteu reagent method, as reported previously with some modifications [10]. Briefly, an aliquot (30 µL) of diluted methanolic extract was placed in a 96-well microplate, and 150 µL of Folin–Ciocalteu diluted reagent (1:10) was added, followed by 120 µL of sodium carbonate (7.5%). The reaction was performed for 30 min in the dark. The absorbance was measured at 765 nm. Using a gallic acid standard curve, the results were expressed as grams of gallic acid equivalents per g of dry matter (mg GAE/g DM).
2.4.2. TFC
The ability of AlCl3 to form a stable complex with the keto and hydroxy groups of both flavonols and flavanols was measured according to a previous report [10]. Briefly, 30 µL of the diluted extract was placed on a microplate, then 10 µL of AlCl3 (10%) and the same volume of 1 M sodium acetate were added, followed by 250 µL of distilled water. The microplate was shaken and kept in the dark at room temperature for 30 min. The blank contained all of the reagents, except the extract, for which solvent was substituted. Absorbance was measured at 415 nm. Using a quercetin standard curve, TFC was calculated as mg quercetin equivalents/per gram of dry matter (QuE/g DM).
2.4.3. DPPH Antioxidant Capacity
The ability of APE to scavenge DPPH radicals was assessed according to another study with modifications [8]. Methanolic DPPH (280 µL) with constant absorbance (1.1 nm) was reacted with 20 µL of appropriately diluted extract in a microplate and immediately kept in the dark for 20 min. Absorbance was measured at 517 nm using the solvent as blank. The percentage of inhibition of the DPPH radical was calculated as follows:
where Abs sample is the absorbance of the sample containing the extract, and Abs control is the absorbance of the DPPH reagent with solvent. Antioxidant activity is expressed as Trolox equivalents per gram of dry matter (TE/g DM).
2.5. HPLC–UV/vis Individual Phenolics Determination
HPLC analysis was performed to identify the primary phenolic compounds in the avocado peel. The APEs were filtered (0.2 µm) and analyzed. The chromatograms were determined simultaneously at 280 and 520 nm using a YL9111 binary pump (Schachambeck, Germany) and a YL9120 UV–vis detector comprised of a Kromasil 100-5C18 column (20 × 4.6 mm) using Clarity software. The separation of phenolic components was performed according to procedures described in another study [14]. Briefly, mobile phase A containing water/acid acetic (3%) and a B phase of 100% acetonitrile were employed in a gradient profile: at zero time, the ratio was 95% A and 5% B; after 45 min, the ratio was adjusted to 85% A and 15% B; to 65% A and 35% B at 60 min; to 50% A and 50% B at 65 min; finally, to 100% B at 70 min. Between injections, the column was equilibrated for 30 min under the initial conditions. Calibration curves based on standards were employed for the identification and quantification of the phenolic content in APEs, expressed as micrograms per gram of dry matter (µg/g DM).
2.6. Enzymatic Activity Measurement
The enzymatic activity of PAL and CHS was measured using the same alkaline buffer, although sodium phosphate buffer was employed for PPO activity. Briefly, 1 g of sample was sonicated for 5 min with 20 mL of buffer containing 0.1 M of sodium tetraborate and 0.1 M of boric acid, pH 8.8. The extracts were filtered and centrifuged for 30 min, 10,000× g at 4 °C, and kept on ice.
Protein content was measured using a bicinchoninic acid (BCA) protein assay kit (Thermos Fisher Scientific, Waltham, USA).
Sample blanks were considered for all of the experiments, and these contained all of the reagents except the extract, which was substituted by buffer. Absorbances were measured and converted into enzymatic activity expressed as mol per min per µg of protein (M/min × µg of protein), applying the already known molar coefficients (M−1 cm−1); ε = 17,400 [15], ε = 29,400 [16], and ε = 1260 [17] for PAL, CHS, and PPO, respectively.
2.6.1. PAL Activity
The assessment of the PAL enzyme was conducted based on other work [15]; 0.1 mL of enzymatic extract was added to 0.9 mL L-phenylalanine (1 mg/mL) and vortexed. The mixture was immediately incubated at 40 °C for 30 min, and the reaction was then stopped with 0.25 mL of 5 N hydrochloric acid and incubation in a cold bath. Finally, 5 mL of distilled water was added. The absorbance was measured at 290 nm.
2.6.2. CHS Activity
The activity of CHS enzyme was measured according to another study [18], with modifications. Briefly, 0.1 mL of the supernatant was mixed gently with 0.1 mL of 10 mM potassium cyanide, and 1.9 mL of Tris-HCl buffer (pH 7.8) was added. Consequently, 0.01 g of chalcone was diluted in a mixture of ethanol/glycerol (1:1), and 0.01 mL was mixed with the enzyme extract. The reaction was performed for 1 min at 30 °C. The absorbance was measured at 360 nm.
2.6.3. PPO Activity
The activity of PPO was analyzed by employing the supernatant obtained from the sonication of 1 g of sample with 20 mL of sodium phosphate buffer (pH 7), following the methodology reported elsewhere [19]. Briefly, 50 µL of the extract was mixed with 1 mL of catechol (150 mM) for 5 min. Absorbances were measured at 412 nm.
2.7. Statistical Analysis
Means and standard deviations were calculated. Statistical analysis was performed using STATGRAPHICS Centurion XVI.I. First, all of the dependent variables were tested for normality by a Shapiro–Wilk test. Then, one-way ANOVA and Tukey’s (p < 0.05) posthoc test were applied to address the differences. Finally, the correlation coefficients between the variables were determined using Pearson’s analysis at two significance levels (p < 0.05 and p < 0.001).
3. Results and Discussion
3.1. Size and Ripeness State Evaluation of Avocado Fruits
Firmness and color are two maturity indexes that are commonly associated with the ripeness of avocado fruits. Firmness (N) and color (L*, a *, and b *) values among the S, M, and L avocado groups did not show statistically significant differences (Table 1). Thus, the ripeness state was considered equal in all of the assessed samples. The results are comparable to values reported previously [10,11,12], which showed that firmness of around 6–13 N corresponds to ready-to-eat avocado fruits. It is likely that the L* values of ready-to-eat “Hass” avocado are in the range of 22–27.

Table 1.
Avocado ripeness state by size groupings.
For this study, peels from ready-to-eat avocados were assessed because the content of polyphenols is higher at this stage due to the breakdown of chlorophyll, which allows the release of secondary pigments such as anthocyanins, flavonoids, and tannins [20,21].
3.2. Phytochemicals and Antioxidant Characteristics of the APEs
Table 2 shows the results of the phenolic assessment of the APE of different size groups. No differences (p > 0.05) were found in TPC between S and M APEs, although TPC was lower in the L group (p < 0.05). The TFC in the APE of S was significantly higher than that of M and L, which were nonsignificantly different. Similar to TPC, the APEs of S and M displayed higher DPPH scavenging capacity (p > 0.05), and that of L was lower (p < 0.05).

Table 2.
Phytochemical characteristics of the avocado peel extract from small (S), medium (M), and large (L) fruit sizes.
Morais et al. [8] indicated that stressors alter the metabolism of the avocado plant; thus, the vegetative tissue phenolic content is not always similar to that of the fruit. The results presented here are in agreement with those reported elsewhere [22]. In the latter study of antioxidant development in the mesocarp of avocado groups from different bloom periods, the authors concluded that phenolic content was higher in small fruit due to the concentrating effect per unit of flesh; however, this is more likely to be associated with the impact of stressors. Evidence supporting phenolic increments due to water stress in grape peel was reported by Villangó et al. [23] and Roby et al. [24]. The authors of the first study claimed that peel from Vitis vinifera L. grown with water stress presented a higher concentration of anthocyanins and phenolic constituents, and a low fruit mass was also observed. Similar results were observed for rice (Oryza sativa L) [25], numerous wild plant species from gypsum habitats (salt marshes) [26], and wheat leaves (Triticum aestivum L) [27]. Concerning other stressors, phenolic variability was also caused by temperature [7] and nutrients [28].
3.3. HPLC–UV/vis of Individual Phenolic Content in S, M, and L APEs
Figure 1 represents the phenolic composition variability of the APEs from S, M, and L groups. The concentration (µg/g of DM) of each identified phenolic compound is in the supplementary material (Table S1). Regarding the HPLC–UV/VIS analysis, the main phenolic presented in APEs was the flavanol (−)-epicatechin (Figure 1, peak 3), whose concentration was highest (p < 0.05) in S (24.1 ± 2.2), followed by M and L (18.5 ± 0.1 and 4.8 ± 0.5, respectively). (+)-Catechin hydrate (Figure 1, peak 1) was equally (p > 0.05) concentrated in S and M (5.3 ± 1.3 and 5.1 ± 0.1), but lower (p < 0.05) in L (1.6 ± 0.3). Kaempferol (Figure 1, peak 11) was statistically similar in all of the APEs (5.1 ± 0.2, 5.8 ± 1.0, and 5.6 ± 0.2). The flavonols rutin and quercetin were identified in all of the samples (Figure 1, peaks 8 and 9). The levels of the first compound were similar (p > 0.05) in S, M, and L APEs (0.6 ± 0.2, 0.2 ± 0.3, and 1.1 ± 0.0, respectively), whereas the second compound was greater in L and S (p > 0.05) than in M APE (0.2 ± 0.3). Regarding the flavanones naringin, (±)-naringenin, and hesperidin (Figure 1, peaks 6, 7, and 10, respectively), the first compound was observed in S and M at the same level (p > 0.05; 3.6 ± 0.7 and 2.9 ± 0.6, respectively); it was significantly lower in the L APE (1.2 ± 0.0). Similarly, narangin presented higher contents in S and M APEs (4.1 ± 0.2 and 5.6 ± 1.3, respectively; p > 0.05), which were significantly higher than in the L APE (1.5 ± 0.1). Hesperidin was the second principal phenolic in all of the APEs, with similar concentrations in all cases (p > 0.05; 11.9, 6.9 ± 0.6, and 9.3 ± 0.9, for S, M, and L, respectively). Phenolic acids appeared in the order of concentration caffeic > coumaric > ferulic (Figure 1, peaks 2, 4, and 5, respectively). All of the acids were equally concentrated in S and M (p > 0.05; caffeic: 2.4 ± 0.2 and 2.3 ± 0.2; coumaric: 1.9 ± 0.2 and 1.6 ± 0.2; ferulic: 0.7 ± 0.2 and 0.4 ± 0.1, respectively), although in L APE, the content of all of the identified acids was lower (p < 0.05; 0.9 ± 0.0 and 0.4 ± 0.1 for caffeic and coumaric, respectively), and ferulic was not detected.
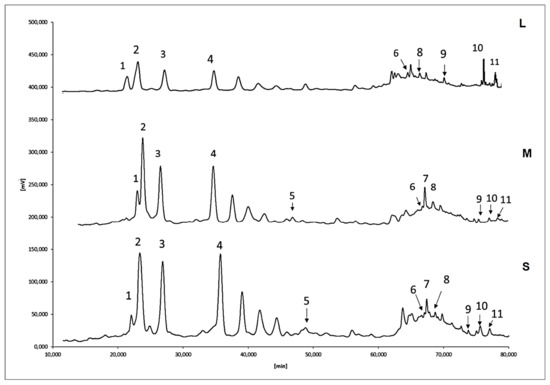
Figure 1.
Individual phenolics identified avocado peel extracts (APEs) from small (S), medium (M), and large (L) fruit. The number on each peak represents 1—(+)-catechin hydrate; 2—caffeic acid; 3—(−)-epicatechin; 4—coumaric acid; 5—ferulic acid; 6—(±)-naringenin; 7—naringin; 8—rutin hydrate; 9—quercetin; 10—hesperidin; 11—kaempferol.
The exocarp (peel) role in avocado fruit is the protection of the fruit; thus, phenolics are highly concentrated as a defense mechanism [24]. (−)-Epicatechin has been reported [29] as the principal flavonoid in avocado that protects the fruit against microbes. Similarly, phenolic acids, quercetin, naringin, and kaempferol have been associated with the antioxidant response of fruit tissues to biotic and abiotic stressors [4,18,23,24].
The significant role played by each phenolic compound in the protective metabolism of plants can be extrapolated to humans. The opportunities of polyphenol-rich extracts, particularly from industry byproducts, were discussed above. Similarly, the purified compounds have a large number of applications in food, pharmaceuticals, and bioindustries [30,31,32]. Recently, other authors have reported [21,33] the high phenolic content in avocado byproducts, and their findings support APEs being particularly rich in catechin derivatives. Molecules in monomeric epicatechin/catechin and polymerized forms (proanthocyanidins (PACs)) are of potential interest due to their high antioxidants and microbial toxicity [34,35]. For example, it was found that the combination of catechins with antibiotics increased the inhibition of resistant strains of Staphylococcus aureus [36,37]. In addition, Helicobacter pylori was shown to be sensitive to PACs from APE [38]. Furthermore, the PACs in APE were demonstrated to positively modulate internal microbiota and protect the colonic mucosa [39,40]. Therefore, therapeutic drugs and antimicrobial agents can be developed based on APE, including for application in the COVID-19 pandemic. In this sense, it is known that polyphenols prevent and ameliorate influenza symptoms [41]. It was reported that phenolic compounds interfere with the replication cycle of the coronavirus in vitro [42]. Consequently, it has been suggested that catechins, quercetin, and hesperidin, among other flavonoids, have the potential to be used in the design of coadjutant therapies against viral infection [43].
Phenolic acids, such as caffeic, coumaric, and ferulic acids, have been described as anticarcinogenic, antimutagenic, anti-inflammatory, antioxidant, and antimicrobial as well [44]. Furthermore, the incorporation of ferulic acid in food thermic processing results in a reduction in carcinogens derived from Maillard reactions [45]. Other potential applications of the polyphenol content in APE could be bioremediation [46], cosmetics [47], and feed supplements for the reduction of methane production [48].
3.4. PAL, CHS, and PPO Activities in the Peel of Avocado Sorted by Size
Similar to previous results, variability was observed among enzymatic activities of PAL, CHS, and PPO among avocado fruit sizes (Figure 2). The resulting enzymatic measurements indicated that S had higher (p < 0.05) activities of PAL (154.5 ± 13.8) compared to M and L (99.3 ± 6.6 and 6.8 ± 2.4, respectively). The same tendency was observed in CHS for S, M, and L (p < 0.05; 193.3 ± 5.2, 182.2 ± 7.5, 155.9 ± 3.1, respectively). No differences (p > 0.05) were observed regarding PPO. In all of the measurements, the lower activity was presented by the L type. These results contrast with those in previously cited work [22]. These authors reported high enzymatic activities of catalase, ascorbate peroxidase, and glutathione reductase per gram of mesocarp for the “normal” avocado phenotype, whereas the small phenotype presented higher superoxide dismutase and PPO activities due to the “concentrating effect” of the small phenotype.
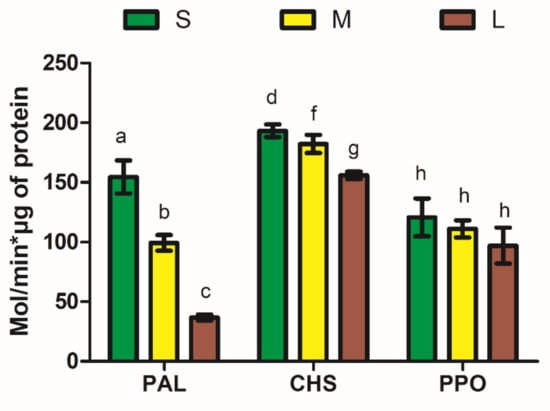
Figure 2.
Enzymatic activities in avocado peel extracts (APEs) of small (S), medium (M), and large (L) avocados. Abbreviations: PAL—phenylalanine ammonia-lyase; CHS—chalcone synthase; PPO—polyphenol oxidase. Different letters over each column indicate significant differences according to Tukey’s test (p < 0.05).
Phenolic metabolism is driven by the activity of PAL catalyzing the transformation of L-phenylalanine into cinnamic acid; then, the activity of CHS drives the synthesis of flavonoids produced by the union of the malonyl coenzyme with cinnamic acid. PPO is a copper-containing enzyme used for phenolic oxidation into o-quinones as a defense mechanism against some biotic stressors [19]. Studies have revealed that CHS is located in the epidermal and cortex cells or the elongation zone [7]. Therefore, enzyme and flavonoid synthesis coexists in the same intracellular space. Stressors such as water, temperature, and UV-C and -B radiation lead to PAL and CHS overexpression for the synthesis of phenolics [49,50]. By contrast, PPO appears not to be profoundly affected by different water regimes, as indicated elsewhere [51].
It is notable that peel from the S avocado group is a valuable source of enzymes involved in phenolic metabolism; in particular, PPO has attracted attention in this research. Avocado PPO has been found to possess a high level of enzymatic activity, which represents a significant problem in avocado processing [52]. Several sources, such as potato peel, were found to provide raw material as a source of PPO for bioremediation of phenol-contaminated water, organic compounds, and biosensors, in addition to products useful for other analytical applications [19]. Therefore, avocado peel is a potential source of PPO that has not been investigated to date.
3.5. Statistical Correlation between the Phytochemical Parameters and Avocado Physiology
Significative positive correlations were found among the response variables in S, M, and L avocado fruits (Table 3). Strong correlations (p < 0.001) were observed in TPC with DPPH inhibition and with CHS. Similarly, PAL and CHS were highly correlated (p < 0.001) with weight and volume. In addition, TPC, TFC, and DPPH were correlated (p < 0.05) with PAL. TFC showed the same correlation with CHS (p < 0.001). Regarding PPO, it was only correlated (p < 0.05) with PAL and inversely with weight. Strong negative correlations (p < 0.001) were presented between TFC with weight and volume. Similarly, PAL and CHS demonstrated the same tendency. TPC was inversely correlated (p < 0.05) with weight, volume, and DPPH scavenging capacity.

Table 3.
Pearson’s correlations among enzymatic activities and fruit weight and volume.
Similar correlations have been reported in previous studies, i.e., between DPPH scavenging inhibition and TPC and flavonoids [18] and between phenolic composition and enzymatic activity due to abiotic and biotic stressors affecting the plant [53] and hormones [54]. No correlation between phenols and PPO was observed in the other water-stressed fruits in an earlier report [51]. Nevertheless, comparative studies correlating fruit phenotypes, phenolic composition, and key enzymes that act in the phenolic metabolism have not been performed. Therefore, this work is the first to elucidate the relationship between fruit byproducts, fruit size, phenolic content, and the factors that induce variability and their impact on biorefinery.
In terms of the circular economy, numerous accounts of successful exploitation of byproducts in biorefining have been reported. The utilization of food byproducts and eco-friendly technologies (such as ultrasound, microwaves, pressurized liquids, and others) for processing are now popular research topics for companies aiming to obtain biomolecules, fibers, polysaccharides, pectin, and proteins, among numerous other elements [55,56,57]. These changes have increased the potential of the bioeconomy and the biorefinery industry [58]. For example, the orange industry generates significant byproduct waste, including low-quality fruit. It has been noted that these byproducts are rich in phytochemicals and other valuable compounds, such as pectin, ethanol, and essential oils. Following the implementation of the biorefinery concept, it was concluded that these changes are economical and practical, although new opportunities such as the implementation of green technologies and the development of novel engineering processes remain to be addressed [59].
Similarly, a biorefinery approach has been proposed for potato peel and reviewed for other tropical fruits [60]. Avocado has been assessed for the integral exploitation of the whole fruit into oil, encapsulated phytochemicals, xylitol, ethanol, and energy [61]. These studies indicated that the high cost of the raw material was the principal factor affecting production. Nevertheless, it was concluded that the biorefinery concept is economically and environmentally viable if the energy requirements are reduced. In this sense, formerly, we studied the impact of easy, scalable green extraction methods (ultrasound and microwave) on the obtainment of extracts that are high in phenolic compounds from avocado peel [10]. The results demonstrated that the implementation of these methods, separately and in combination, significantly reduced energy and raw material consumption by up to 75% and 54.8%, respectively, compared to conventional extraction. Therefore, the integration of green processing and the phytochemical characteristics of the small, underpriced avocado size fruits studied herein will be pivoting for the bioeconomy of the avocado industry.
4. Conclusions
As a live organism, avocado orchards produce different sized fruits; those with a larger size are better valorized than the smaller fruits that are usually discarded or utilized for processing. In this study, our hypothesis, stated at the beginning of this work, was probed. Greater TPC, TFC, and inhibition of DPPH radicals, higher content of individual flavonoids and phenolic acids, in addition to the higher activities of PAL, CHS, and PPO in the APEs of small-sized “Hass” avocados compared to the large-sized type were demonstrated. Peels from small avocado fruits are an excellent source of phenolic compounds and enzymes, with multiple industrial applications on food, pharmaceuticals, biochemistry, and analytics. Moreover, it is recommended that eco-friendly extraction technologies to enhance the bioeconomy of the avocado industry and promote sustainability in a biorefinery framework be implemented. Consequently, the utilization of avocado byproducts is pivoting to provide healthy, wholesome, and high-quality foods and to reduce environmental pollution and economic losses.
Supplementary Materials
The following are available online at https://www.mdpi.com/2311-7524/6/4/91/s1. Supplementary Material 1; Table S1: Phenolic content in S, M, and L avocado peel extract (APE).
Author Contributions
G.B.-M.—HPLC–UV/vis analysis; I.T.-M.—analytic performance, writing, and discussion; C.C.-A. and J.A.-E.—result analysis, discussion, and revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agencia Nacional de Investigación y Desarrollo—ANID (formerly CONICYT), grant number 21171483, CONICYT FONDECYT grant numbers 1130463 and 11611557, and the internal grant from Dirección de Investigación DIUBB (083009-2R, 122509 and132209 GI/C).
Acknowledgments
Igor Trujillo is grateful to ANID and the Bio-Bio University.
Conflicts of Interest
The authors declare no conflict of interest. The data for this study are available from the corresponding author upon reasonable request.
References
- Schaffer, B.; Wolstenholme, N.; Whiley, A. The Avocado, Botany, Production and Uses, 2nd ed.; Schaffer, B., Wolstenholme, B.N., Whiley, A., Eds.; CABI: Oxfordshire, UK, 2013; ISBN 9788578110796. [Google Scholar]
- Silber, A.; Naor, A.; Cohen, H.; Bar-Noy, Y.; Yechieli, N.; Levi, M.; Noy, M.; Peres, M.; Duari, D.; Narkis, K.; et al. Irrigation of ‘Hass’ avocado: Effects of constant vs. temporary water stress. Irrig. Sci. 2019, 37, 451–460. [Google Scholar] [CrossRef]
- Cowan, A.K.; Cripps, R.F.; Richings, E.W.; Taylor, N.J. Fruit size: Towards an understanding of the metabolic control of fruit growth using avocado as a model system. Physiol. Plant. 2001, 111, 127–136. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Celedón, J.M.; Gil, P.M.; Ferreyra, R.; Maldonado, P.; Barrera, C. Sensitivity and Variability of Two Plant Water Stress Indicators: Exploring Criteria for Choosing a plant Monitoring Method for Avocado Irrigation Management. Chil. J. Agric. Res. 2012, 72, 379–387. [Google Scholar] [CrossRef]
- Colombo, R.; Papetti, A. Avocado (Persea americana Mill.) by-products and their impact: From bioactive compounds to biomass energy and sorbent material for removing contaminants. A review. Int. J. Food Sci. Technol. 2019, 54, 943–951. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Participation of phenylalanine ammonia-lyase (PAL) in increased phenolic compounds in fresh cold stressed walnut (Juglans regia L.) kernels. Postharvest Biol. Technol. 2015, 104, 17–25. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Schmidt, E.M.; Bonafe, E.G.; Eberlin, M.N.; Sawaya, A.C.H.F.; Visentainer, J.V. Antioxidant activity, phenolics and UPLC-ESI(-)-MS of extracts from different tropical fruits parts and processed peels. Food Res. Int. 2015, 77, 392–399. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A.; Puranik, N.M.; Jambhulkar, S.J.; Kulkarni, B.D. Valorization of potato peel: A biorefinery approach. Crit. Rev. Biotechnol. 2018, 38, 218–230. [Google Scholar] [CrossRef]
- Trujillo-Mayol, I.; Alarcón-Enos, J.; Céspedes-Acuña, C.; Silva, F.L. Improvement of the polyphenol extraction from avocado peel by assisted ultrasound and microwaves. Food Process Eng. 2019, 42, 1–11. [Google Scholar] [CrossRef]
- Villa-Rodríguez, J.A.; Molina-Corral, F.J.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Effect of maturity stage on the content of fatty acids and antioxidant activity of “Hass” avocado. Food Res. Int. 2011, 44, 1231–1237. [Google Scholar] [CrossRef]
- Herrera-González, J.A.; Salazar-García, S.; Martínez-Flores, H.E.; Ruiz-García, J.E. Indicadores preliminares de madurez fisiológica y comportamiento postcosecha del fruto de aguacate méndez. Rev. Fitotec. Mex. 2017, 40, 55–63. [Google Scholar] [CrossRef]
- Márquez, C.J.; Yepes, D.P.; Sanchez, L. Changes physical-chemical of avocado (Persea americana Mill. cv. “Hass”) in postharvest for two municipalities of antioquia. Temas Agrar. 2014, 19, 32–47. [Google Scholar] [CrossRef]
- Sahpazidou, D.; Geromichalos, G.D.; Stagos, D.; Apostolou, A.; Haroutounian, S.A.; Tsatsakis, A.M.; Tzanakakis, G.N.; Hayes, A.W.; Kouretas, D. Anticarcinogenic activity of polyphenolic extracts from grape stems against breast, colon, renal and thyroid cancer cells. Toxicol. Lett. 2014, 230, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, T.; Balawejder, M. Impact of ozonation process on the level of selected oxidative stress markers in raspberries stored at room temperature. Food Chem. 2019, 298, 125093. [Google Scholar] [CrossRef]
- Ardila, H.D.; Martínez, S.T.; Higuera, B.L. Levels of constitutive flavonoid biosynthetic enzymes in carnation (Dianthus caryophyllus L.) cultivars with differential response to Fusarium oxysporum f. sp. dianthi. Acta Physiol. Plant. 2013, 35, 1233–1245. [Google Scholar] [CrossRef]
- Kumar, V.B.A.; Mohan, T.C.K.; Murugan, K. Purification and kinetic characterization of polyphenol oxidase from Barbados cherry (Malpighia glabra L.). Food Chem. 2008, 110, 328–333. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Nasiri, A.; Jaafar, H.Z.E.; Baghdadi, A.; Ahmad, I. Changes in phytochemical synthesis, chalcone synthase activity and pharmaceutical qualities of sabah snake grass (Clinacanthus nutans L.) in relation to plant age. Molecules 2014, 19, 17632–17648. [Google Scholar] [CrossRef]
- Niphadkar, S.S.; Vetal, M.D.; Rathod, V.K. Purification and Characterization of Polyphenol Oxidase from Waste Potato Peel by Aqueous Two-Phase Extraction. Prep. Biochem. Biotechnol. 2015, 45, 632–649. [Google Scholar] [CrossRef]
- Cox, K.A.; McGhie, T.K.; White, A.; Woolf, A.B. Skin colour and pigment changes during ripening of “Hass” avocado fruit. Postharvest Biol. Technol. 2004, 31, 287–294. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Pasini, F.; Caboni, M.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as valuable tools for the determination of phenolic and other polar compounds in the edible part and by-products of avocado. Food Sci. Technol. 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Y.; Khuong, T.; Lovatt, C.J. Developmental differences in antioxidant compounds and systems in normal and small-phenotype fruit of “Hass” avocado (Persea americana Mill.). Sci. Hortic. 2016, 206, 15–23. [Google Scholar] [CrossRef]
- Villangó, S.; Szekeres, A.; Bencsik, O.; Láposi, R.; Pálfi, Z.; Zsófi, Z. The effect of postveraison water deficit on the phenolic composition and concentration of the Kékfrankos (Vitis vinifera L.) berry. Sci. Hortic. 2016, 209, 113–116. [Google Scholar] [CrossRef]
- Roby, G.; Harbertson, J.F.; Adams, D.A.; Matthews, M.A. Berry size and vine water deficits as factors in Winegrape composition: Anthocyanins and tannins. Aust. J. Grape Wine Res. 2004, 10, 100–107. [Google Scholar] [CrossRef]
- Quan, N.; Anh, L.; Khang, D.; Tuyen, P.; Toan, N.; Minh, T.; Minh, L.; Bach, D.; Ha, P.; Elzaawely, A.; et al. Involvement of Secondary Metabolites in Response to Drought Stress of Rice (Oryza sativa L.). Agriculture 2016, 6, 23. [Google Scholar] [CrossRef]
- Bautista, I.; Boscaiu, M.; Lidón, A.; Llinares, J.V.; Lull, C.; Donat, M.P.; Mayoral, O.; Vicente, O. Environmentally induced changes in antioxidant phenolic compounds levels in wild plants. Acta Physiol. Plant. 2016, 38, 1–15. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef]
- Keller, M.; Rogiers, S.Y.; Schultz, R. Nitrogen and ultraviolet radiation modify grapewines′ susceptibility to powdery midew. Vitis 2003, 42, 87–94. [Google Scholar]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I.C.R.F. Bioactive characterization of Persea americana Mill. by-products: A rich source of inherent antioxidants. Ind. Crops Prod. 2018, 111, 212–218. [Google Scholar] [CrossRef]
- Neilson, A.P.; O’Keefe, S.F.; Bolling, B.W. High-Molecular-Weight Proanthocyanidins in Foods: Overcoming Analytical Challenges in Pursuit of Novel Dietary Bioactive Components. Annu. Rev. Food Sci. Technol. 2016, 7, 43–64. [Google Scholar] [CrossRef]
- Mayer, R.; Stecher, G.; Wuerzner, R.; Silva, R.C.; Sultana, T.; Trojer, L.; Feuerstein, I.; Krieg, C.; Abel, G.; Popp, M.; et al. Proanthocyanidins: Target compounds as antibacterial agents. J. Agric. Food Chem. 2008, 56, 6959–6966. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Xiao, K.; Li, B.; Jiang, W.; Peng, W.; Zheng, J.; Zhou, H. The combination of catechin and epicatechin gallate from Fructus crataegi potentiates β-lactam antibiotics against Methicillin-Resistant Staphylococcus aureus (MRSA) in vitro and in vivo. Int. J. Mol. Sci. 2013, 14, 1802–1821. [Google Scholar] [CrossRef] [PubMed]
- Miklasińska, M.; Kȩpa, M.; Wojtyczka, R.D.; Idzik, D.; Dziedzic, A.; Wąsik, T.J. Catechin hydrate augments the antibacterial action of selected antibiotics against Staphylococcus aureus clinical strains. Molecules 2016, 21, 244. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Garcia, A.; Aranda, M.; Saéz, V.; Zúñiga, F.; Alarcón, J.; Avello, M.; Pastene, E. One-step purification of two semi-synthetic epicatechin adducts prepared from avocado peels procyanidins by centrifugal partition chromatography and evaluation of their anti-inflammatory effects on adenocarcinoma gastric cells infected with Helicobacter pylori. J. Chil. Chem. Soc. 2018, 4, 4222–4228. [Google Scholar] [CrossRef]
- Wong, X.; Carrasco-Pozo, C.; Escobar, E.; Navarrete, P.; Blachier, F.; Andriamihaja, M.; Lan, A.; Tomé, D.; Cires, M.J.; Pastene, E.; et al. Deleterious Effect of p-Cresol on Human Colonic Epithelial Cells Prevented by Proanthocyanidin-Containing Polyphenol Extracts from Fruits and Proanthocyanidin Bacterial Metabolites. J. Agric. Food Chem. 2016, 64, 3574–3583. [Google Scholar] [CrossRef]
- Cires, M.J.; Navarrete, P.; Pastene, E.; Carrasco-Pozo, C.; Valenzuela, R.; Medina, D.A.; Andriamihaja, M.; Beaumont, M.; Blachier, F.; Gotteland, M. Protective Effect of an Avocado Peel Polyphenolic Extract Rich in Proanthocyanidins on the Alterations of Colonic Homeostasis Induced by a High-Protein Diet. J. Agric. Food Chem. 2019, 67, 11616–11626. [Google Scholar] [CrossRef]
- Sawai-Kuroda, R.; Kikuchi, S.; Shimizu, Y.K.; Sasaki, Y.; Kuroda, K.; Tanaka, T.; Yamamoto, T.; Sakurai, K.; Shimizu, K. A polyphenol-rich extract from Chaenomeles sinensis (Chinese quince) inhibits influenza A virus infection by preventing primary transcription in vitro. J. Ethnopharmacol. 2013, 146, 866–872. [Google Scholar] [CrossRef]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of flavonoids against coronavirus infection. Chem. Biol. Interact. 2020, 328, 109211. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Rivas-García, L.; Varela-López, A.; Llopis, J.; Battino, M.; Sánchez-González, C. Do nutrients and other bioactive molecules from foods have anything to say in the treatment against COVID-19? Environ. Res. 2020, 191, 110053. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Silván, J.M.; Assar, S.H.; Srey, C.; Dolores, M.; Ames, J.M. Control of the Maillard reaction by ferulic acid. Food Chem. 2011, 128, 208–213. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Naidu, R. Bioremediation potential of natural polyphenol rich green wastes: A review of current research and recommendations for future directions. Environ. Technol. Innov. 2015, 4, 17–28. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Pintado, M.M.E.; Aguilar, C.N. Avocado by-products: Nutritional and functional properties. Trends Food Sci. Technol. 2018, 80, 51–60. [Google Scholar] [CrossRef]
- Geerkens, C.H.; Schweiggert, R.M.; Steingass, H.; Boguhn, J.; Rodehutscord, M.; Carle, R. Influence of apple and citrus pectins, processed mango peels, a phenolic mango peel extract, and gallic acid as potential feed supplements on in vitro total gas production and rumen methanogenesis. J. Agric. Food Chem. 2013, 61, 5727–5737. [Google Scholar] [CrossRef]
- Rubin, I.; Oliveira, D.; Rodrigues, G.; Severo, J.; Renard, C.M.G.C.; Clasen, F.; Valmor, C. Plant Physiology and Biochemistry Preharvest UV-C radiation influences physiological, biochemical, and transcriptional changes in strawberry cv. Camarosa. Plant Physiol. Biochem. 2016, 108, 391–399. [Google Scholar] [CrossRef]
- Sheng, K.; Zheng, H.; Shui, S.S.; Yan, L.; Liu, C.; Zheng, L. Comparison of postharvest UV-B and UV-C treatments on table grape: Changes in phenolic compounds and their transcription of biosynthetic genes during storage. Postharvest Biol. Technol. 2018, 138, 74–81. [Google Scholar] [CrossRef]
- Cirilli, M.; Caruso, G.; Gennai, C.; Urbani, S.; Frioni, E.; Ruzzi, M.; Servili, M.; Gucci, R.; Poerio, E.; Muleo, R. The role of polyphenoloxidase, peroxidase, and β-glucosidase in phenolics accumulation in Olea europaea L. Fruits under different water regimes. Front. Plant Sci. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Toledo, L.; Aguirre, C. Enzymatic browning in avocado (Persea americana) revisited: History, advances, and future perspectives. Crit. Rev. Food Sci. Nutr. 2017, 57, 3860–3872. [Google Scholar] [CrossRef] [PubMed]
- Schovánková, J.; Opatová, H. Changes in phenols composition and activity of phenylalanine-ammonia lyase in apples after fungal infections. Hortic. Sci. 2011, 38, 1–10. [Google Scholar] [CrossRef]
- Koca, N.; Karaman, Ş. The effects of plant growth regulators and L-phenylalanine on phenolic compounds of sweet basil. Food Chem. 2015, 166, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Matharu, A.S.; de Melo, E.M.; Houghton, J.A. Opportunity for high value-added chemicals from food supply chain wastes. Bioresour. Technol. 2016, 215, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A. Side Streams of Plant Food Processing as a Source of Valuable Compounds: Selected Examples. Annu. Rev. Food Sci. Technol. 2017, 8, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Ciriminna, R.; Carnaroglio, D.; Delisi, R.; Arvati, S.; Tamburino, A.; Pagliaro, M. Industrial Feasibility of Natural Products Extraction with Microwave Technology. ChemistrySelect 2016, 1, 549–555. [Google Scholar] [CrossRef]
- López, J.A.S.; Li, Q.; Thompson, I.P. Biorefinery of waste orange peel. Crit. Rev. Biotechnol. 2010, 30, 63–69. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Mohd Adzahan, N.; Abdul Rahman, R.; Zainal Abedin, N.H.; Hussain, N.; Sulaiman, R.; Chong, G.H. Current trends of tropical fruit waste utilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 335–361. [Google Scholar] [CrossRef]
- Dávila, J.A.; Rosenberg, M.; Castro, E.; Cardona, C.A. A model biorefinery for avocado (Persea americana Mill.) processing. Bioresour. Technol. 2017, 243, 17–29. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).