Effect of High CO2 Treatment and MA Packaging on Sensory Quality and Physiological-Biochemical Characteristics of Green Asparagus (Asparagus officinalis L.) during Postharvest Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Packaging Materials, Storage Conditions, and Replication
2.3. Microbiological Analysis
2.4. Sensory Quality
2.5. Atmosphere Analysis
2.6. Physicochemical and Biochemical Traits
2.7. Statistical Analysis
3. Results and Discussion
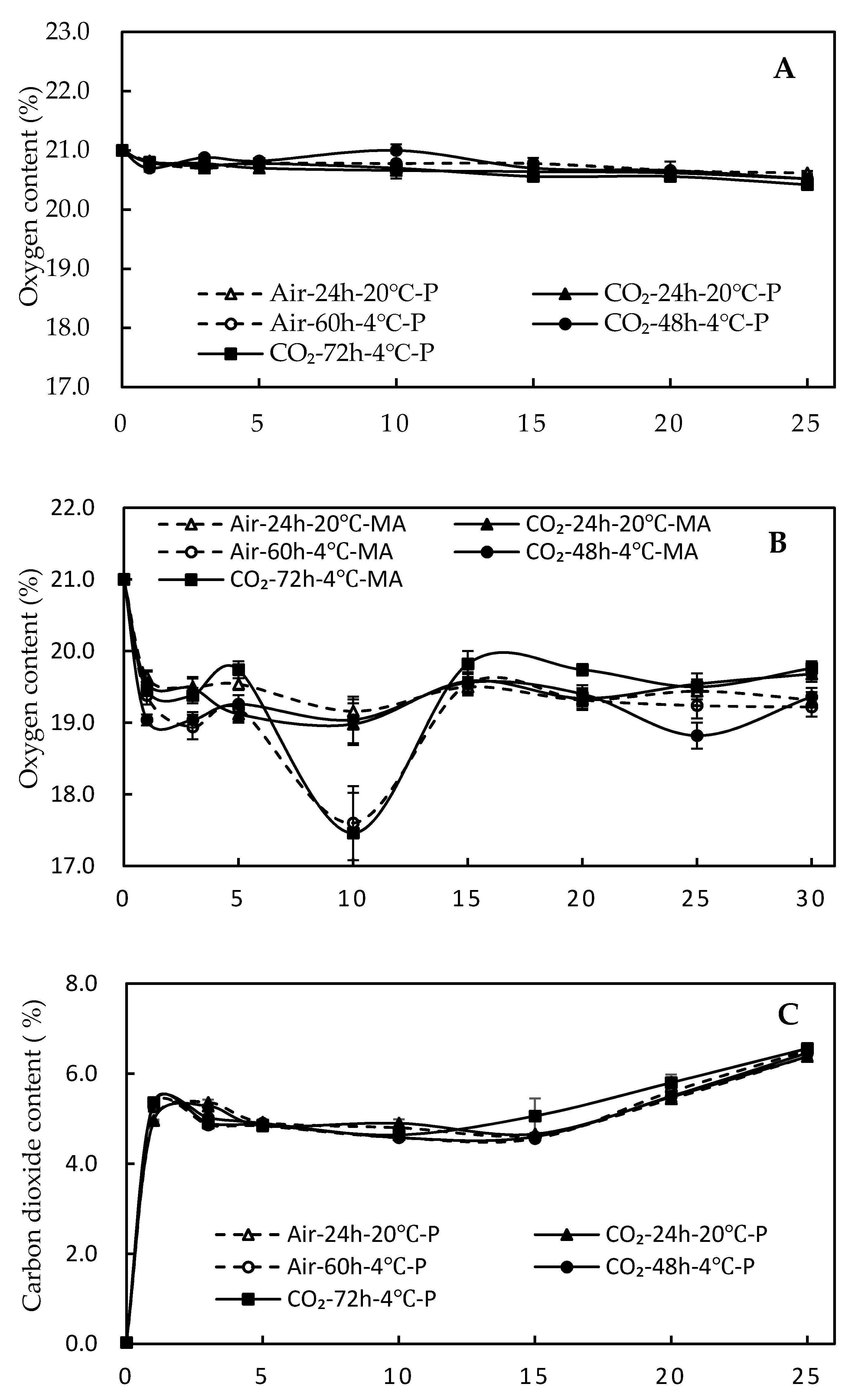
3.1. Atmosphere Analysis in All Packages
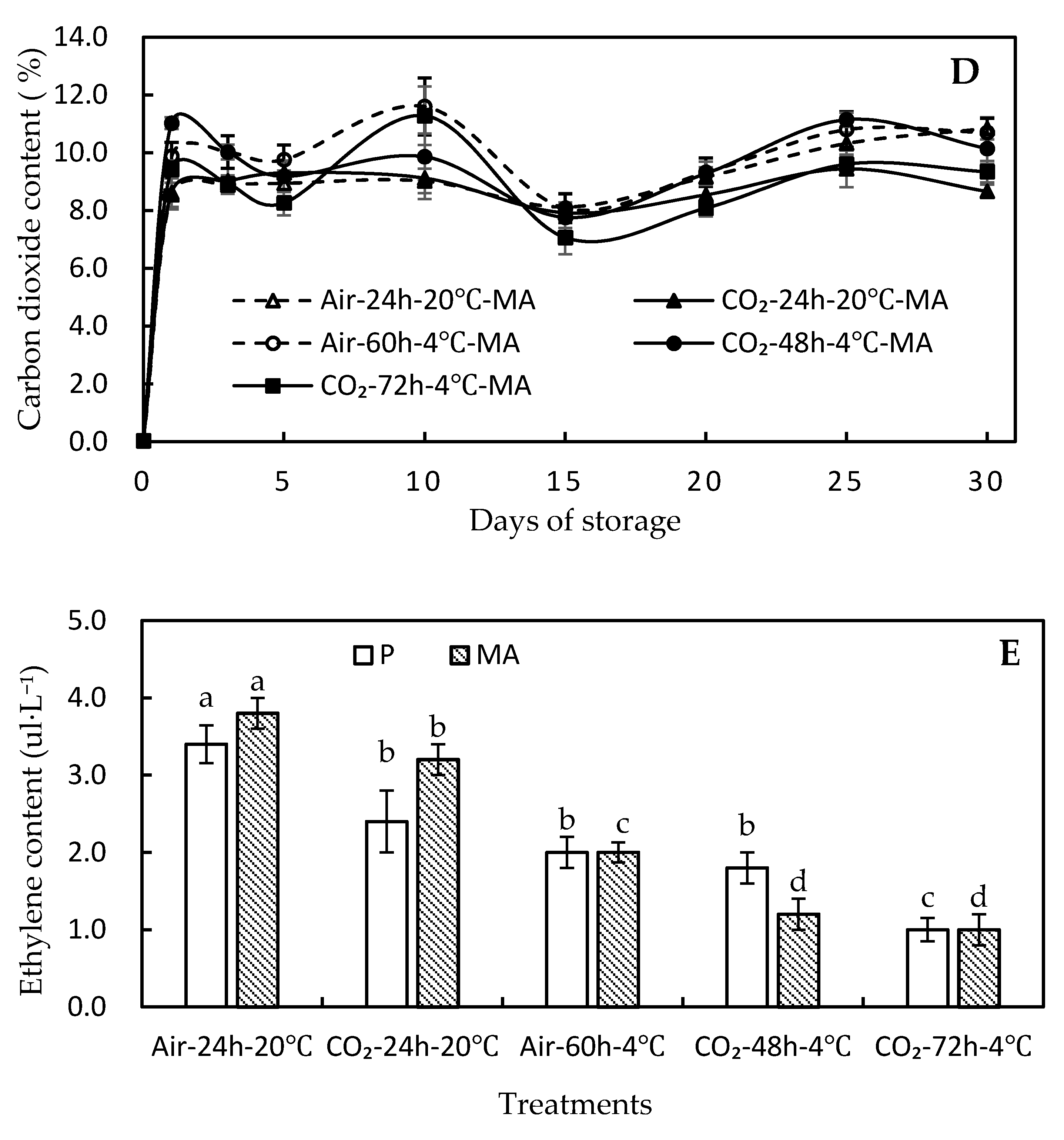
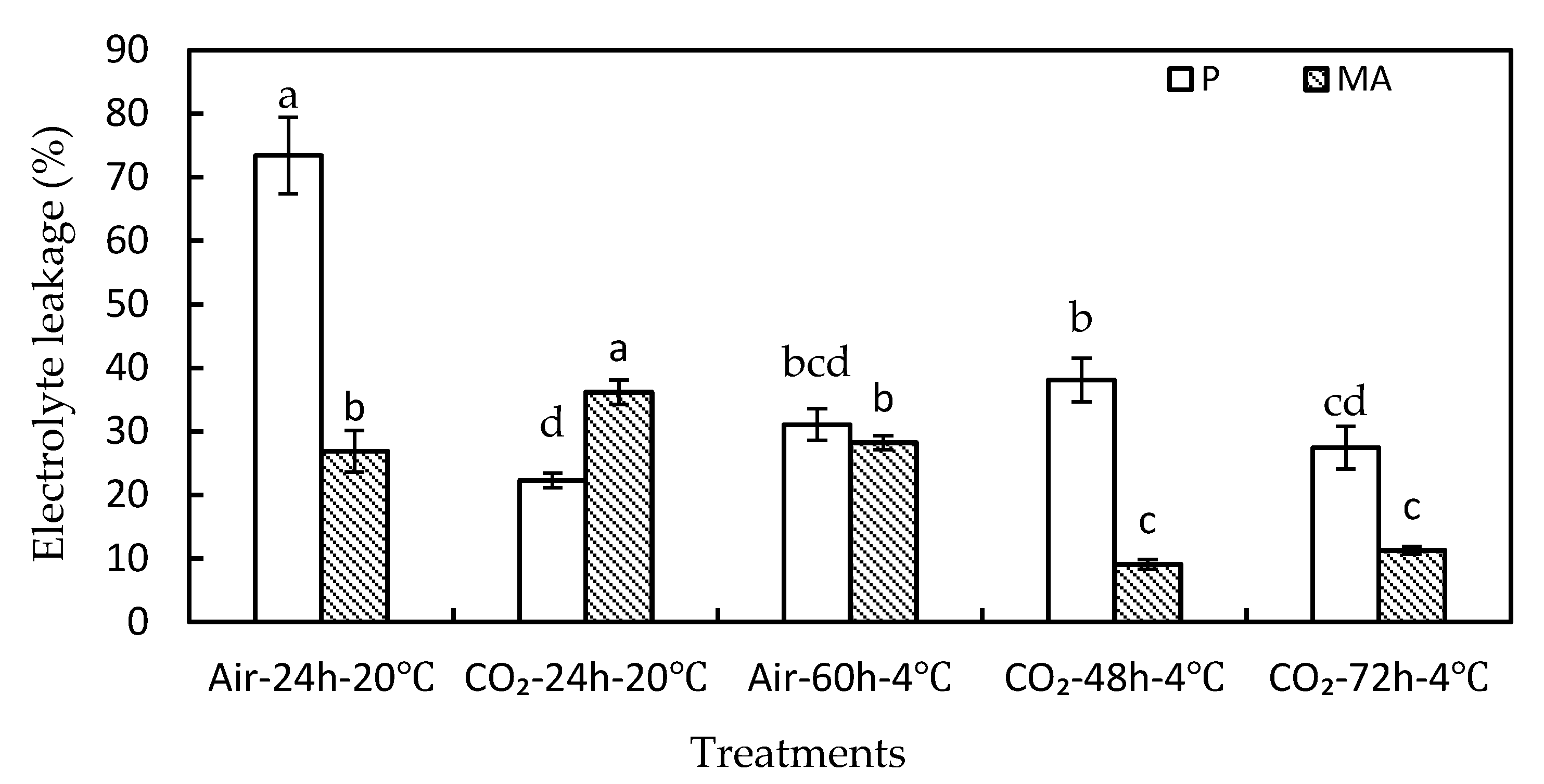
3.2. Effect of High-CO2 Treatments on Physiochemical Traits
3.3. Effect of High-CO2 Treatments on Biochemical Traits
3.4. Microbiological Analysis
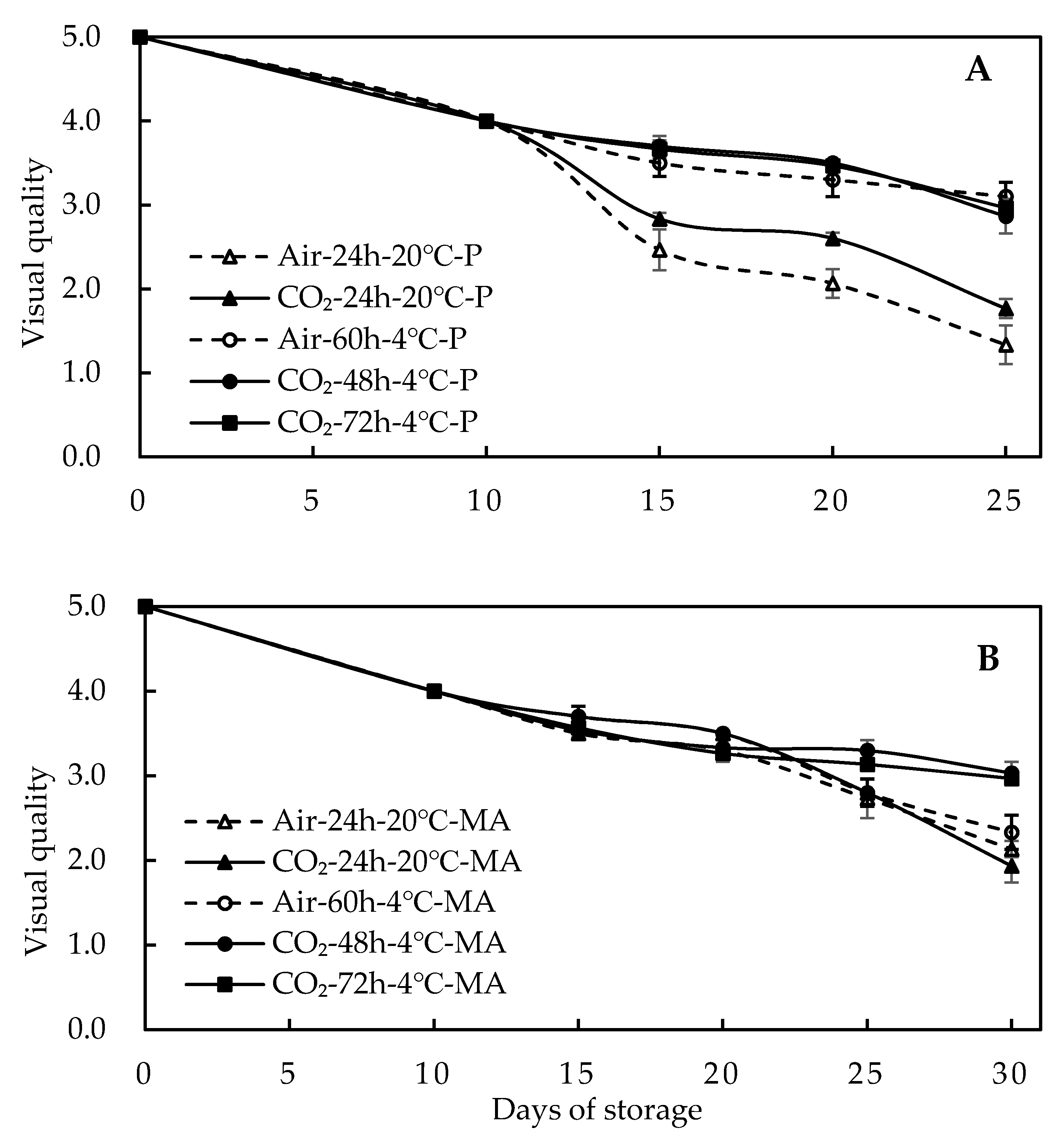
3.5. Effect of High-CO2 Treatments on Sensory Quality
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nikaido, K.; Jishi, T.; Maeda, T.; Suzuki, T.; Araki, H. Quality change of asparagus spears stored with snow cooling. JSHS 2014, 83, 327–334. [Google Scholar] [CrossRef]
- Song, L.; Zeng, W.; Wu, A.; Picard, K.; Lampugnani, E.R.; Cheetamun, R.; Beahan, C.; Cassin, A.; Lonsdale, A.; Doblin, M.S.; et al. Asparagus spears as a model to study heteroxylan biosynthesis during secondary wall development. PLoS ONE 2015, 10, e0123878. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, M.; Wang, S.J.; Wu, Z.H. Effect of 1-methylcyclopropene treatment on green asparagus quality during cold storage. Int. Agrophys. 2012, 26, 407–411. [Google Scholar] [CrossRef]
- Inoue, T.; Murai, T.; Natsuaki, T. An effective system for detecting Iris yellow spot virus transmission by Thrips tabaci. Plant Pathol. 2010, 59, 422–428. [Google Scholar] [CrossRef]
- Qian, L.; He, S.; Liu, X.; Huang, Z.; Chen, F.; Gui, F. Effect of elevated CO2 on the interaction between invasive thrips, Frankliniella occidentalis, and its host kidney bean, Phaseolus vulgaris. Pest Manag. Sci. 2018, 74, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Noctor, G. High CO2 primes plant biotic stress defences through redox-linked pathways. Plant Physiol. 2016, 172, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Y.; Wang, K.D.; Wang, L.; Li, D.; Yan, J.W.; Ban, Z.J.; Luo, Z.S.; Li, L.; Yang, D.M. Effect of superatmospheric oxygen exposure on strawberry (Fragaria× ananassa Fuch.) volatiles, sensory and chemical attributes. Postharvest Biol. Technol. 2018, 142, 60–71. [Google Scholar] [CrossRef]
- Kim, S.K. Insecticidal effect of carbon dioxide treatment on onion thrips (Thrips tabaci: Thripidae: Thysanoptera). J. Agric. Life Environ. Sci. 2017, 29, 87–93. [Google Scholar] [CrossRef]
- Seki, M.; Murai, T. Insecticidal effect of high carbon dioxide atmospheres on thrips eggs oviposited in plant tissue. Appl. Èntomol. Zoöl. 2012, 47, 433–436. [Google Scholar] [CrossRef]
- Watkins, C.B.; Manzano-Mendez, J.E.; Nock, J.F.; Zhang, J.; Maloney, K.E. Cultivar variation in response of strawberry fruit to high carbon dioxide treatments. J. Sci. Food Agric. 1999, 79, 886–890. [Google Scholar] [CrossRef]
- Rosales, R.; Fernandez-Caballero, C.; Romero, I.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Molecular analysis of the improvement in rachis quality by high CO2 levels in table grapes stored at low temperature. Postharvest Biol. Technol. 2013, 77, 50–58. [Google Scholar] [CrossRef]
- Teles, C.S.; Benedetti, B.C.; Gubler, W.D.; Crisosto, C.H. Prestorage application of high carbon dioxide combined with controlled atmosphere storage as a dual approach to control Botrytis cinerea in organic ‘Flame Seedless’ and ‘Crimson Seedless’ table grapes. Postharvest Biol. Technol. 2014, 89, 32–39. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Li, Y.; Yang, M.; Shi, C.; Zheng, C. Studies of postharvest berry abscission of ‘Kyoho’ table grapes during cold storage and high oxygen atmospheres. Postharvest Biol. Technol. 2007, 43, 95–101. [Google Scholar] [CrossRef]
- Blanch, M.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. The relationship between bound water and carbohydrate reserves in association with cellular integrity in Fragaria vesca stored under different conditions. Food Bioprocess Technol. 2015, 8, 875–884. [Google Scholar] [CrossRef]
- Lwin, W.W.; Srilaong, V.; Boonyaritthongchai, P.; Wongs-Aree, C.; Pongprasert, N. Electrostatic atomised water particles reduces postharvest lignification and maintain asparagus quality. Sci. Hortic. 2020, 271, 109487. [Google Scholar] [CrossRef]
- Barry, C.S. The stay-green revolution: Recent progress in deciphering the mechanisms of chlorophyll degradation in higher plants. Plant Sci. 2009, 176, 325–333. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.S.; Li, J.H.; Yang, M.Y.; Yan, J.W.; Lu, H.Y.; Li, D.; Chen, C.K.; Aghdam, M.S.; Wu, B.; et al. Morphological and quality characterization of grape berry and rachis in response to postharvest 1-methylcyclopropene and elevated oxygen and carbon dioxide atmospheres. Postharvest Biol. Technol. 2019, 153, 107–117. [Google Scholar] [CrossRef]
- Chandra, D.; Lee, J.S.; Hong, Y.P.; Park, M.H.; Choi, A.J.; Kim, J.G. Short-term application of CO2 gas: Effects on physicochemical, microbial, and sensory qualities of “Charlotte” strawberry during storage. J. Food Saf. 2019, 39, e12597. [Google Scholar] [CrossRef]
- Lu, Y.T.; Li, D.; Li, L.; Belwal, T.; Xu, Y.Q.; Lin, X.Y.; Duan, Z.H.; Luo, Z.S. Effects of elevated CO2 on pigment metabolism of postharvest mandarin fruit for degreening. Food Chem. 2020, 318, 126462. [Google Scholar] [CrossRef]
- Sanchez-Ballesta, M.T.; Romero, I.; Jiménez, J.B.; Orea, J.M.; González-Ureña, Á.; Escribano, M.I.; Merodio, C. Involvement of the phenylpropanoid pathway in the response of table grapes to low temperature and high CO2 levels. Postharvest Biol. Technol. 2007, 46, 29–35. [Google Scholar] [CrossRef]
- Wang, L.X.; Choi, I.L.; Lee, J.H.; Kang, H.M. The effect of high CO2 treatment and MA packaging on asparagus quality and shelf life during cold storage. J. Agric. Life Environ. Sci. 2019, 31, 41–49. [Google Scholar] [CrossRef]
- Yoon, H.S.; Choi, I.L.; Heo, J.Y.; Kim, J.Y.; Han, S.J.; Kang, H.M. Influence of hot water immersion and map pre-treatments on sterilization and asparagus spear qualities during cold storage. Hortic. Sci. Technol. 2018, 36, 756–765. [Google Scholar] [CrossRef]
- Mosquera, M.I.M.; Rojas, B.G.; Guerrero, L.G. Measurement of chlorophyllase activity in olive fruit (Oleaeuropaea). J. Biol. Chem. 1994, 116, 6. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ. Introductory Chapter: Postharvest Physiology and Technology of Horticultural Crops. Postharvest Handl. 2017, 1–5. [Google Scholar]
- Tudela, J.A.; Hernandeza, N.; Perez-Vicente, A.; Gil, M.I. Growing season climates affect quality of fresh-cut lettuce. Postharvest Biol. Technol. 2017, 123, 60–68. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest Technology of Horticultural Crops (Vol. 3311), 3rd ed.; University of California, Agriculture and Natural Resources: Davis, CA, USA, 2002. [Google Scholar]
- Siomos, A.S.; Sfakiotakis, E.M.; Dogras, C.C. Modied atmosphere packaging of white asparagus spears: Composition, color and textural quality responses to temperature and light. Sci. Hortic. 2000, 84, 1–13. [Google Scholar] [CrossRef]
- Beever, D.J.; Yearsley, C.W.; Hogg, M.G. Effect of post-harvest fumigation on quality of asparagus spears. N. Z. J. Agric. Res. 2011, 28, 537–543. [Google Scholar] [CrossRef]
- Kou, J.J.; Wei, C.Q.; Zhao, Z.H.; Guan, J.F.; Wang, W.J. Effects of ethylene and 1-methylcyclopropene treatments on physiological changes and ripening-related gene expression of ‘Mopan’ persimmon fruit during storage. Postharvest Biol. Technol. 2020, 166, 111185. [Google Scholar] [CrossRef]
- Liu, E.C.; Niu, L.F.; Yi, Y.; Wang, L.M.; Ai, Y.W.; Zhao, Y.; Wang, H.X.; Min, T. Expression analysis of erfs during storage under modified atmosphere packaging (High-concentration of CO2) of fresh-cut lotus root. HortScience 2020, 55, 216–223. [Google Scholar] [CrossRef]
- Lipton, W.J. Postharvest Biology of Fresh Asparagus. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 69–155. [Google Scholar] [CrossRef]
- Kato, M.; Hayakawa, Y.; Hyodo, H.; Ikoma, Y.; Yano, M. Wound-induced ethylene synthesis and expression and formation of 1-aminocyclopropane-1-carboxylate (ACC) synthase, ACC oxidase, phenylalanine ammonia-lyase, and peroxidase in wounded mesocarp tissue of Cucurbita maxima. Plant Cell Physiol. 2000, 41, 440–447. [Google Scholar] [CrossRef]
- Park, M.H. Sucrose delays senescence and preserves functional compounds in Asparagus officinalis L. Biochem. Biophys. Res. Commun. 2016, 480, 241–247. [Google Scholar] [CrossRef] [PubMed]
- An, J.S.; Zhang, M.; Wang, S.J.; Tang, J.M. Physical, chemical and microbiological changes in stored green asparagus spears as affected by coating of silver nanoparticles-PVP. LWT-Food Sci. Technol. 2008, 41, 1100–1107. [Google Scholar] [CrossRef]
- Weichmann, J. The effect of controlled atmosphere storage on the sensory and nutritional quality of fruits and vegetables. Hort. Rev. 1986, 8, 1–127. [Google Scholar]
- Holcroft, D.M.; Kader, A.A. Carbon dioxide–induced changes in color and anthocyanin synthesis of stored strawberry fruit. HortScience 1999, 34, 1244–1248. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Park, J.J.; Lee, J.J.; Lee, W.Y. Combined effect of chitosan coating and modified atmosphere packaging on fresh-cut cucumber. Food Sci. Nutr. 2019, 7, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.M.; Zhang, X.L.; Jiang, X.Y.; Huang, S.H.; Pang, X.Q.; Qu, H.X.; Zhang, Z.Q. Quality retention and selective gene expression of Chinese flowering cabbage as affected by atmosphere gas composition. J. Food Process. Preserv. 2020, 9. [Google Scholar] [CrossRef]
- Ramin, A.A.; Modares, B. Improving postharvest quality and storage life of green olives using CO2. Hortic. Environ. Biotechnol. 2008, 49, 403–408. [Google Scholar]
- Taye, A.M.; Tilahun, S.; Park, D.S.; Seo, M.H.; Jeong, C.S. Effects of continuous application of CO2 on fruit quality attributes and shelf life during cold storage in cherry tomato. Hortic. Sci. Technol. 2017, 35, 300–313. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ferrer, M.; Harper, C.; Pérez-Muntoz, F.; Chaparro, M. Modified atmosphere packaging of minimally processed mango and pineapple fruits. J. Food Sci. Technol. 2002, 67, 3365–3371. [Google Scholar] [CrossRef]
- Sanchez-Ballesta, M.T.; Jimenez, J.B.; Romero, I.; Orea, J.M.; Maldonado, R.; Urena, A.G.; Escribano, M.I.; Merodio, C. Effect of high CO2 pretreatment on quality, fungal decay and molecular regulation of stilbene phytoalexin biosynthesis in stored table grapes. Postharvest Biol. Technol. 2006, 42, 209–216. [Google Scholar] [CrossRef]
- Poubol, J.; Izumi, H. Shelf life and microbial quality of fresh-cut mango cubes stored in high CO2 atmospheres. J. Food Sci. 2005, 70, M69–M74. [Google Scholar] [CrossRef]
- Meredith, H.; Valdramidis, V.; Rotabakk, B.T.; Sivertsvik, M.; McDowell, D.; Bolton, D.J. Effect of different modified atmospheric packaging (MAP) gaseous combinations on Campylobacter and the shelf-life of chilled poultry fillets. Food Microbiol. 2014, 44, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.Å.; Moen, B.; Rødbotten, M.; Berget, I.; Pettersen, M.K. Effect of vacuum or modified atmosphere packaging (MAP) in combination with a CO2 emitter on quality parameters of cod loins ( Gadus morhua ). Food Packag Shelf Life 2016, 9, 29–37. [Google Scholar] [CrossRef]
- Kamihira, M.; Taniguchi, M.; Kobayashi, T. Sterilization of microorganisms with supercritical carbon dioxide. Agric. Biol. Chem. 2014, 51, 407–412. [Google Scholar] [CrossRef]
- Kafkaletou, M.; Christopoulos, M.V.; Tsantili, E. Short-term treatments with high CO2 and low O2 concentrations on quality of fresh goji berries (Lycium barbarum L.) during cold storage. J. Sci. Food Agric. 2017, 97, 5194–5201. [Google Scholar] [CrossRef] [PubMed]
- Tudela, J.A.; Marín, A.; Garrido, Y.; Cantwell, M.; Medina-Martínez, M.S.; Gil, M.I. Off-odour development in modified atmosphere packaged baby spinach is an unresolved problem. Postharvest Biol. Technol. 2013, 75, 75–85. [Google Scholar] [CrossRef]
- Mattos, L.M.; Moretti, C.L.; Ferreira, M.D. Modified Atmosphere Packaging for perishable plant products. Polypropylene 2012, 95, 1–12. [Google Scholar]
- Li, D.; Li, L.; Xiao, G.; Limwachiranon, J.; Xu, Y.; Lu, H.; Yang, D.; Luo, Z. Effects of elevated CO2 on energy metabolism and gamma-aminobutyric acid shunt pathway in postharvest strawberry fruit. Food Chem. 2018, 265, 281–289. [Google Scholar] [CrossRef]
- Romero, I.; Casillas-Gonzalez, A.C.; Carrazana-Villalba, S.J.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Impact of high CO2 levels on heat shock proteins during postharvest storage of table grapes at low temperature. Functional in vitro characterization of VVIHSP18.1. Postharvest Biol. Technol. 2018, 145, 108–116. [Google Scholar] [CrossRef]
- Vazquez-Hernandez, M.; Romero, I.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Deciphering the role of cbf/dreb transcription factors and dehydrins in maintaining the quality of table grapes cv. autumn royal treated with high CO2 levels and stored at 0 degrees C. Front. Plant Sci. 2017, 8, 1591. [Google Scholar] [CrossRef] [PubMed]
- Blanch, M.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. Water distribution and ionic balance in response to high CO2 treatments in strawberries (Fragaria vesca L. cv. Mara de Bois). Postharvest Biol. Technol. 2012, 73, 63–71. [Google Scholar] [CrossRef]



| Treatments | 24 h-20 °C | 48 h-4 °C | 72 h-4 °C | |
|---|---|---|---|---|
| Air | P z | Air-24 h-20 °C-P | Air-60 h-4 °C-P | |
| MA | Air-24 h-20 °C-MA | Air-60 h-4 °C-MA | ||
| CO2 | P | CO2-24 h-20 °C-P | CO2-48 h-4 °C-P | CO2-72 h-4 °C-P |
| MA | CO2-24 h-20 °C-MA | CO2-48 h-4 °C-MA | CO2-72 h-4 °C-MA | |
| Treatments | Firmness (N) | SSC (°Brix) | ||||
|---|---|---|---|---|---|---|
| Tip | Stem | |||||
| P | MA | P | MA | P | MA | |
| Initial day | 7.52 b z | 7.96 b | 7.52 b | 7.96 a | 4.75 a | 4.75 a |
| Air-24 h-20 °C | 11.02 a | 12.08 a | 7.76 ab | 8.50 a | 4.11 b | 3.40 c |
| CO2-24 h-20 °C | 10.30 a | 10.80 a | 8.82 a | 7.26 a | 4.17 b | 3.13 d |
| Air-60 h-4 °C | 10.18 a | 11.70 a | 7.32 b | 8.96 a | 4.30 b | 3.68 bc |
| CO2-48 h-4 °C | 10.56 a | 12.22 a | 8.90 a | 8.02 a | 4.30 b | 3.78 b |
| CO2-72 h-4 °C | 10.04 a | 11.92 a | 8.28 ab | 8.02 a | 4.09 b | 3.56 c |
| Treatments | Hue Angle (◦) | Chlorophyll (mg·mL−1) | Chlorophyllase Activity (U·g−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tip | Stem | Tip | Stem | Tip | Stem | |||||||
| P | MA | P | MA | P | MA | P | MA | P | MA | P | MA | |
| Initial day | 125 a z | 125 a | 122 a | 122 a | 10.0 a | 10.0 a | 8.3 a | 8.3 a | 4.4 c | 4.4 e | 3.4 c | 3.4 c |
| Air-24 h-20 °C | 120 c | 122 b | 119 cd | 121 b | 4.5 c | 4.7 c | 4.7 c | 4.2 d | 20.7 a | 14.4 bc | 8.2 bc | 9.4 bc |
| CO2-24 h-20 °C | 120 c | 112 c | 118 d | 118 c | 3.9 e | 4.0 e | 4.2 d | 4.2 d | 14.3 b | 12.5 cd | 17.6 a | 11.7 b |
| Air-60 h-4 °C | 121 bc | 122 b | 121 b | 119 b | 5.0 b | 5.6 b | 4.8 c | 5.3 c | 20.1 a | 21.8 a | 13.6 ab | 11.4 b |
| CO2-48 h-4 °C | 122 b | 122 b | 121 b | 121 b | 5.2 b | 5.7 b | 5.4 b | 5.7 b | 13.1 b | 10.3 d | 15.1 a | 13.6 ab |
| CO2-72 h-4 °C | 120 c | 122 b | 119 c | 119 b | 4.1 d | 4.5 d | 4.2 d | 4.3 d | 14.2 b | 16.2 b | 15.8 a | 18.8 a |
| Treatments | Number of Microorganisms (log CFU·g−1) | |||||
|---|---|---|---|---|---|---|
| Total Aerobic Bacteria | Yeast and Mold | |||||
| Initial | P | MA | Initial | P | MA | |
| Air-24 h-20 °C | 4.42 a z | 5.06 b | 5.57 a | 0.00 a | 0.00 a | 0.00 a |
| CO2-24 h-20 °C | 3.90 b | 4.43 c | 4.94 b | 0.00 a | 0.00 a | 0.00 a |
| Air-60 h-4 °C | 4.41 a | 5.35 a | 4.50 c | 0.00 a | 0.00 a | 0.00 a |
| CO2-48 h-4 °C | 3.48 c | 4.11 d | 4.31 c | 0.00 a | 0.00 a | 0.00 a |
| CO2-72 h-4 °C | 3.95 b | 4.29 c | 5.11 b | 0.00 a | 0.00 a | 0.00 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-X.; Choi, I.-L.; Kang, H.-M. Effect of High CO2 Treatment and MA Packaging on Sensory Quality and Physiological-Biochemical Characteristics of Green Asparagus (Asparagus officinalis L.) during Postharvest Storage. Horticulturae 2020, 6, 84. https://doi.org/10.3390/horticulturae6040084
Wang L-X, Choi I-L, Kang H-M. Effect of High CO2 Treatment and MA Packaging on Sensory Quality and Physiological-Biochemical Characteristics of Green Asparagus (Asparagus officinalis L.) during Postharvest Storage. Horticulturae. 2020; 6(4):84. https://doi.org/10.3390/horticulturae6040084
Chicago/Turabian StyleWang, Li-Xia, In-Lee Choi, and Ho-Min Kang. 2020. "Effect of High CO2 Treatment and MA Packaging on Sensory Quality and Physiological-Biochemical Characteristics of Green Asparagus (Asparagus officinalis L.) during Postharvest Storage" Horticulturae 6, no. 4: 84. https://doi.org/10.3390/horticulturae6040084
APA StyleWang, L.-X., Choi, I.-L., & Kang, H.-M. (2020). Effect of High CO2 Treatment and MA Packaging on Sensory Quality and Physiological-Biochemical Characteristics of Green Asparagus (Asparagus officinalis L.) during Postharvest Storage. Horticulturae, 6(4), 84. https://doi.org/10.3390/horticulturae6040084






