A Horticultural Medium Established from the Rapid Removal of Phytotoxins from Winery Grape Marc
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polyphenolic Compound Depletion
2.3. Depleted Marc Properties
2.4. Depleted Marc Compost Blend Properties
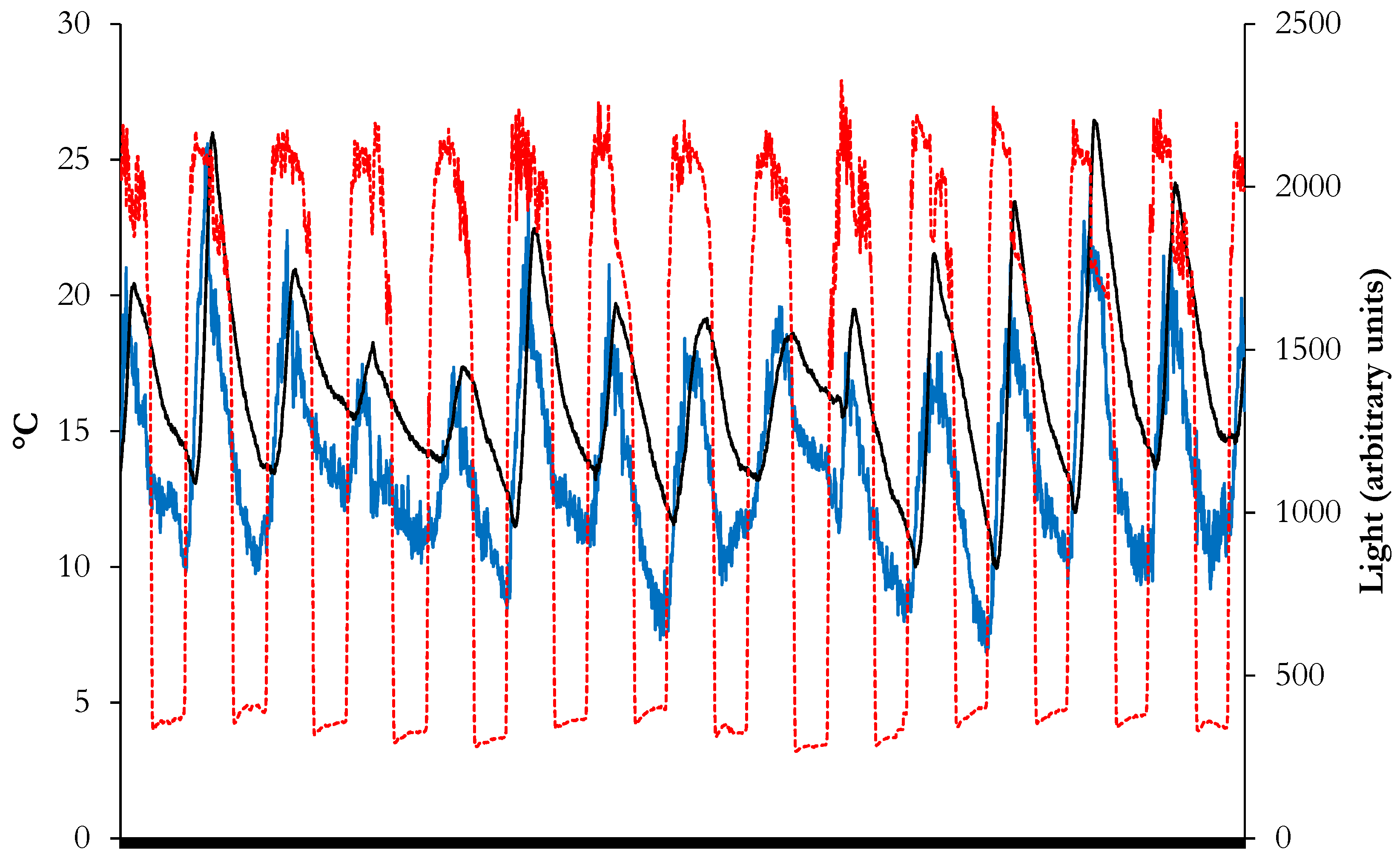
2.5. Seedling Emergence Tests
2.6. Statistical Methods
3. Results and Discussion
3.1. Depleted Marc Characteristics
3.2. Depleted Marc Blends
3.3. Seedling Emergence
3.4. Nutrient Profiles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Varzakas, T.H. Fruit/Fruit juice waste management: Treatment methods and potential uses of treated waste. In Waste Management for the Food Industries; Academic Press: Amsterdam, The Netherlands, 2008; pp. 569–628. [Google Scholar]
- Carmona, E.; Moreno, M.T.; Avilés, M.; Ordovás, J. Use of grape marc compost as substrate for vegetable seedlings. Sci. Hortic. 2012, 137, 69–74. [Google Scholar] [CrossRef]
- Greenwood, S.L.; Edwards, G.R.; Harrison, R. Supplementing grape marc to cows fed a pasture-based diet as a method to alter nitrogen partitioning and excretion. J. Dairy Sci. 2012, 95, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Moate, P.J.; Williams, S.R.O.; Torok, V.A.; Hannah, M.C.; Ribaux, B.E.; Tavendale, M.H.; Eckard, R.J.; Auldist, M.J.; Wales, W.J. Grape marc reduces methane emissions when fed to dairy cows. J. Dairy Sci. 2014, 97, 5073–5087. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Shukla, S. Fermentation of food wastes for generation of nutraceuticals and supplements. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peña, E., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 707–734. [Google Scholar]
- Santos, N.W.; Santos, G.T.D.; Silva-Kazama, D.C.; Grande, P.A.; Pintro, P.M.; de Marchi, F.E.; Jobim, C.C.; Petit, H.V. Production, composition and antioxidants in milk of dairy cows fed diets containing soybean oil and grape residue silage. Livest. Sci. 2014, 159, 37–45. [Google Scholar] [CrossRef]
- Zalikarenab, L.; Pirmohammadi, R.; Teimuriyansari, A. Chemical composition and digestibility of dried white and red grape pomace for ruminants. J. Anim. Vet. Adv. 2007, 6, 1079–1082. [Google Scholar]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical characterisation of the solid by-products and residues from the winery and distillery industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Gómez-Brandóón, M.; Lores, M.; Insam, H.; Domínguez, J. Strategies for recycling and valorization of grape marc. Crit. Rev. Biotechnol. 2019, 39, 437–450. [Google Scholar] [CrossRef]
- Kritsotakis, I.K.; Kabourakis, E.M. Grape vine waste and giant reed biomass composts as peat and mineral fertilizer substitutes for producing organic tomato transplants. J. Crop. Improv. 2011, 25, 664–679. [Google Scholar] [CrossRef]
- Burg, P.; Vítěz, T.; Turan, J.; Burgová, J. Evaluation of grape pomace composting process. ACTA Univ. Agric. Fac Silvicult. 2014, 62, 875–881. [Google Scholar] [CrossRef]
- Devesa-Ray, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology; John Wiley & Sons Ltd.: Chichester, UK, 2001. [Google Scholar]
- Garcia-Jares, C.; Vazquez, A.; Lamas, J.P.; Pajaro, M.; Alvarez-Casas, M.; Lores, M. Antioxidant white grape seed phenolics: Pressurized liquid extracts from different varieties. Antioxidants 2015, 4, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, A.M. HPLC-DAD detection of changes in phenol content of red berry skins during ripening. Eur. Food Res. Technol. 2013, 237, 555–564. [Google Scholar] [CrossRef]
- Niu, S.; Hao, F.; Mo, H.; Jiang, J.; Wang, H.; Liu, C.; Fan, X.; Zhang, Y. Phenol profiles and antioxidant properties of white skinned grapes and their coloured genotypes during growth. Biotechnol. Biotec. Eq. 2017, 31, 58–67. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef]
- Jelley, R.E.; Herbst-Johnstone, M.; Klaere, S.; Pilkington, L.I.; Grose, C.; Martin, D.; Barker, D.; Fedrizzi, B. Optimization of ecofriendly extraction of bioactive monomeric phenolics and useful flavor precursors from grape waste. ACS Sustain. Chem. Eng. 2016, 4, 5060–5067. [Google Scholar] [CrossRef]
- Mojzer, E.B.; Hrnčič, M.K.; Škerget, M.; Željko, K.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- McDOnnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Man, A.; Gâz, A.Ș.; Mare, A.D.; Berța, L. Effects of low-molecular weight alcohols on bacterial viability. Rev. Română Med. Lab. 2017, 25, 335–343. [Google Scholar] [CrossRef]
- Curzons, A.D.; Constable, D.C.; Cunningham, V.L. Solvent selection guide: A guide to the integration of environmental, health and safety criteria into the selection of solvents. Clean. Prod. Proc. 1999, 1, 82–90. [Google Scholar] [CrossRef]
- Olejar, K.; Ray, S.; Ricci, A.; Kilmartin, P. Superior antioxidant polymer films created through the incorporation of grape tannins in ethyl cellulose. Cellulose 2014, 21, 4545–4556. [Google Scholar] [CrossRef]
- Olejar, K.J.; Ray, S.; Kilmartin, P.A. Enhanced antioxidant activity of polyolefin films integrated with grape tannins. J. Sci. Food Agric. 2016, 96, 2825–2831. [Google Scholar] [CrossRef] [PubMed]
- Tapas, A.R.; Sakarkar, D.M.; Kakde, R.B. Flavonoids as nutraceuticals: A review. Trop. J. Pharm. Res. 2008, 7, 1089–1099. [Google Scholar] [CrossRef]
- Marlborough Research Centre. Blenhiem Weather Station. Available online: https://www.mrc.org.nz/blenheim-weather-station (accessed on 3 August 2009).
- Olejar, K.J.; Ricci, A.; Swift, S.; Zujovic, Z.; Gordon, K.C.; Fedrizzi, B.; Vesari, A.; Kilmartin, P.A. Characterization of an antioxidant and antimicrobial extract from cool climate, white grape marc. Antioxidants 2019, 8, 232. [Google Scholar] [CrossRef]
- Paradelo, R.; Moldes, A.B.; Gonzalez, D.; Barral, M.T. Plant tests for determining the suitability of grape marc composts as components of plant growth media. Waste Manag. Res. 2012, 30, 1059–1065. [Google Scholar] [CrossRef]
- Naeth, M.A.; Bailey, A.W.; Chanasyk, D.S.; Pluth, D.J. Water holding capacity of litter and soil organic matter in mixed prairie and fescue grassland ecosystems of Alberta. J. Range Manag. 1991, 44, 13–17. [Google Scholar] [CrossRef]
- Topp, G.C.; Parkin, G.W.; Ferre, T.P.A. Chapter 70: Soil Water Content. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; p. 940. [Google Scholar]
- Leelamanie, D.A.L.; Karube, J.; Yoshida, A. Characterizing water repellency indices: Contact angle and water drop penetration time of hydrophobized sand. Soil Sci. Plant. Nutr. 2008, 54, 179–187. [Google Scholar] [CrossRef]
- Hamza, M.A.; Anderson, W.K. Soil compaction in cropping systems: A review of the nature, causes and possible solutions. Soil Till Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- Håkansson, I.; Lipiec, J. A review of the usefulness of relative bulk density values in studies of soil structure and compaction. Soil Till Res. 2000, 53, 71–81. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. OCSPP 850.4230: Early seedling growth toxicity test. In Ecological Effects Test Guidelines; Office of Chemical Safety and Pollution Prevention; US Environmental Protection Agency: Washington, DC, USA, 2012. [Google Scholar]
- Ros, M.; Garcia, C.; Hernandez, T. The use of urban organic wastes in the control of erosion in a semiarid Mediterranean soil. Soil Use Manag. 2001, 17, 292–293. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Jakobek, L.; Šeruga, M. Influence of solvent and temperature on extraction of phenolic compounds from grape seed, antioxidant activity and colour of extract. Int. J. Food Sci. Technol. 2009, 44, 2394–2401. [Google Scholar] [CrossRef]
- Gentili, R.; Ambrosini, R.; Montagnani, C.; Caronni, S.; Citterio, S. Effect of soil pH on the growth, reproductive investment and pollen allergenicity of Ambrosia artemisiifolia L. Front. Plant Sci. 2018, 9, 1335. [Google Scholar] [CrossRef] [PubMed]
- Mohammadshirazi, F.; McLaughlin, R.A.; Heitman, J.L.; Brown, V.K. A multi-year study of tillage and amendment effects on compacted soils. J. Environ. Manag. 2017, 203 Pt 1, 533–541. [Google Scholar] [CrossRef]
- Ahmad, D. Rate of water absorption by soil clods under confined and unconfined conditions. Malays. J. Soil Sci. 1997, 1, 65–76. [Google Scholar]
- An, N.; Tang, C.-S.; Xu, S.-K.; Gong, X.-P.; Shi, B.; Inyang, H.I. Effects of soil characteristics on moisture evaporation. Eng. Geol. 2018, 239, 126–135. [Google Scholar] [CrossRef]
- Alessi, J.; Power, J.F. Corn emergence in relation to soil temperature and seedling depth. Agron. J. 1971, 63, 717–719. [Google Scholar] [CrossRef]
- Australian Standards. Potting Mixes; AS3743-1989; Standards Australia: Sydney, Australia, 1989. [Google Scholar]
- Standards New Zealand. Composts, Soil Conditioners and Mulches; NZS4454:2005; New Zealand Standards: Wellington, New Zealand, 2005. [Google Scholar]
- Achmon, Y.; Harrold, D.R.; Claypool, J.T.; Stapleton, J.J.; Vander Gheynst, J.S.; Simmons, C.W. Assessment of tomato and wine processing solid wastes as soil amendments for biosolarization. Waste Manag. 2016, 48, 156–164. [Google Scholar] [CrossRef]
- Emino, E.R.; Warman, P.R. Biological assay for compost quality. Compost. Sci. Util. 2004, 12, 342–348. [Google Scholar] [CrossRef]
- Ozdener, Y.; Kutbay, H.G. Toxicity of copper, cadmium, nickel, lead and zinc on seed germination and seedling growth in Eruca sativa. Fresen. Environ. Bull. 2009, 18, 26–31. [Google Scholar]
- Paradelo, R.; Cendon, Y.; Moldes, A.B.; Barral, M.T. Relationship between heavy metals and phytotoxicity in compost. Ciencia Technol. Alim. 2008, 6, 143–151. [Google Scholar] [CrossRef][Green Version]
- El Sebaaly, Z.; Kfoury, L.; Nabhan, G.; Shaban, N.; Sassine, Y.N. Use of local composted winery waste for lettuce production in Labanon. AGROFOR Int. J. 2017, 2, 99–107. [Google Scholar]
- Liebman, D. Integration of soil, crop and weed management in low-external-input farming systems. Weed Res. 2000, 40, 27–47. [Google Scholar] [CrossRef]
- Tamet, V.; Boiffin, J.; Dürr, C.; Souty, N. Emergence and early growth of an epigeal seedling (Daucus carota L.): Influence of soil temperature, sowing depth, soil crusting and seed weight. Soil Till Res. 1996, 40, 25–38. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytologist. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Paul, D. Osmotic stress adaptions in rhizobacteria. J. Basic Microb. 2013, 53, 101–110. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Nagaz, K.; Masmoudi, M.M.; Mechlia, N.B. Impacts of irrigation regimes with saline water on carrot productivity and soil salinity. J. Saudi Soc. Agric. Sci. 2012, 11, 19–27. [Google Scholar] [CrossRef]
- Schmidhalter, U.; Oertli, J.J. Germination and seedling growth of carrots under salinity and moisture stress. Plant Soil 1991, 132, 243–251. [Google Scholar] [CrossRef]
- Qian, J.H.; Doran, J.W.; Walters, D.T. Maize plant contributions to root zone available carbon and microbial transformations of nitrogen. Soil Biol. Biochem. 1997, 29, 1451–1462. [Google Scholar] [CrossRef]
- RJ Hill Laboratories Ltd. 2016. Available online: https://www.hill-laboratories.com (accessed on 10 June 2019).
- Food and Agriculture Organization of the United Nations. Plant Nutrition for Food Security: A Guide for Integrated Nutrient Management; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]

| % Marc Concentration | pH | Bulk Density (g/V) | Moisture Content (%) | Water Penetration (sec) |
|---|---|---|---|---|
| 0 | 7.02 ± 0.06 a z | 0.40 ± 0.16 nd | 91.3 ± 0.0 a | 3.8 ± 2.5 a |
| 25 | 6.85 ± 0.03 b | 0.42 ± 0.02 | 89.9 ± 0.0 b | 2.5 ± 1.3 a |
| 50 | 6.76 ± 0.01 c | 0.50 ± 0.04 | 85.0 ± 0.0 d | 1.8 ± 1.0 a |
| 75 | 6.42 ± 0.01 d | 0.51 ± 0.01 | 87.6 ± 0.0 c | 4.0 ± 2.6 a |
| 100 | 5.58 ± 0.01 e | 0.26 ± 0.11 | 79.7 ± 0.0 e | 48 ± 37 b |
| % Marc Concentration | Carrot | |||||
| % Emergence | % Viability | Root (cm) | Stalk (cm) | Root z (mg/plant) | Stalk (mg/plant) | |
| 0 | 72.5 ± 17.7 nd y | 90.0 ± 2.5 nd | 58 ± 17 b | 21 ± 5 nd | 0.91 ± 0.01 ab | 1.67 ± 0.25 nd |
| 25 | 65.0 ± 14.1 | 96.7 ± 4.7 | 72 ± 14 a | 20 ± 5 | 0.98 ± 0.07 ab | 1.56 ± 0.01 |
| 50 | 90.0 ± 0.0 | 88.9 ± 0.0 | 72 ± 15 a | 21 ± 3 | 1.19 ± 0.02 a | 1.32 ± 0.03 |
| 75 | 87.5 ± 17.7 | 91.7 ± 2.4 | 68 ± 13 ab | 22 ± 6 | 1.25 ± 0.11 a | 1.51 ± 0.28 |
| 100 | 77.5 ± 3.5 | 90.2 ± 5.0 | 64 ± 18 ab | 22 ± 3 | 0.53 ± 0.24 b | 1.38 ± 0.16 |
| Corn | ||||||
| % Marc Concentration | % Emergence | % Viability | Root (cm) | Stalk (cm) | Root z (mg/plant) | Stalk z (mg/plant) |
| 0 | 75.0 ± 25.2 nd | 100 ± 0 nd | 30 ± 6 a | 21 ± 6 a | 155 ± 23 ab | 87.9 ± 49.9 nd |
| 25 | 80.0 ± 28.3 | 100 ± 0 | 17 ± 3 b | 16 ± 3 b | 121 ± 26 bc | 71.0 ± 24.6 |
| 50 | 75.0 ± 10.0 | 100 ± 0 | 16 ± 4 b | 17 ± 3 ab | 180 ± 4 a | 82.8 ± 31.6 |
| 75 | 70.0 ± 25.8 | 100 ± 0 | 15 ± 3 bc | 17 ± 3 ab | 143 ± 7 abc | 85.7 ± 14.7 |
| 100 | 90.0 ± 11.5 | 100 ± 0 | 11 ± 3 c | 14 ± 5 b | 110 ± 17 c | 45.5 ± 25.2 |
| Analysis | Raw Marc | Depleted Marc | Blend 50:50 | Compost | Accepted Range [21] |
|---|---|---|---|---|---|
| Electrical Conductivity (dS/m) | 4.9 z | 0.6 | 1.8 | 3.7 | 0.5–1.8 |
| Nitrate (mg/L) | 41 | 2 | 2 | 2 | 20–80 |
| Ammonium (mg/L) | <1 | <1 | 2 | <1 | 1–20 |
| Phosphorus (mg/L) | 214 | 55 | 13 | 2 | 5–20 |
| Potassium (mg/L) | 2170 | 218 | 375 | 538 | 20–80 |
| Sulfur (mg/L) | 31 | 9 | 137 | 439 | |
| Calcium (mg/L) | 42 | 11 | 65 | 290 | 30–70 |
| Magnesium (mg/L) | 48 | 2 | 12 | 48 | 7–25 |
| Sodium (mg/L) | 19 | 3 | 51 | 123 | 5–40 |
| Iron (mg/L) | 1.0 | 3.4 | 9.5 | 9.9 | 20.0–50.0 |
| Zinc (mg/L) | 1.2 | 1.0 | 5.7 | 7.8 | 0.3–10.0 |
| Copper (mg/L) | 1.0 | 0.3 | 0.3 | 0.3 | 0.4–10 |
| Boron (mg/L) | 3.2 | 0.6 | 0.2 | 0.2 | 0.1–0.7 |
| Total Carbon (%) | 48 | 53 | 40 | 33 | |
| Total Nitrogen (%) | 1.7 | 2.5 | 1.8 | 1.5 | |
| Dry Matter (%) | 28.6 | 23.2 | 31.5 | 38.1 | |
| C/N Ratio | 28 | 22 | 22 | 23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olejar, K.J.; Vandermeer, C.; Fedrizzi, B.; Kilmartin, P.A. A Horticultural Medium Established from the Rapid Removal of Phytotoxins from Winery Grape Marc. Horticulturae 2019, 5, 69. https://doi.org/10.3390/horticulturae5040069
Olejar KJ, Vandermeer C, Fedrizzi B, Kilmartin PA. A Horticultural Medium Established from the Rapid Removal of Phytotoxins from Winery Grape Marc. Horticulturae. 2019; 5(4):69. https://doi.org/10.3390/horticulturae5040069
Chicago/Turabian StyleOlejar, Kenneth J., Charlotte Vandermeer, Bruno Fedrizzi, and Paul A. Kilmartin. 2019. "A Horticultural Medium Established from the Rapid Removal of Phytotoxins from Winery Grape Marc" Horticulturae 5, no. 4: 69. https://doi.org/10.3390/horticulturae5040069
APA StyleOlejar, K. J., Vandermeer, C., Fedrizzi, B., & Kilmartin, P. A. (2019). A Horticultural Medium Established from the Rapid Removal of Phytotoxins from Winery Grape Marc. Horticulturae, 5(4), 69. https://doi.org/10.3390/horticulturae5040069






