Toxicity of Piperine Amide Analogs toward the Tomato Pinworm Tuta absoluta (Lepidoptera: Gelechiidae) and Risk Assessment for Two Predators
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Insecticidal Bioassays
2.2.1. Amide’s Susceptibility Bioassay
2.2.2. Dose-Response Bioassay
2.2.3. Time-Response Bioassay
2.2.4. Sublethal Effects on Weight Gain and Leaf Consumption
2.2.5. Risk Assessment for T. absoluta Predators
3. Results
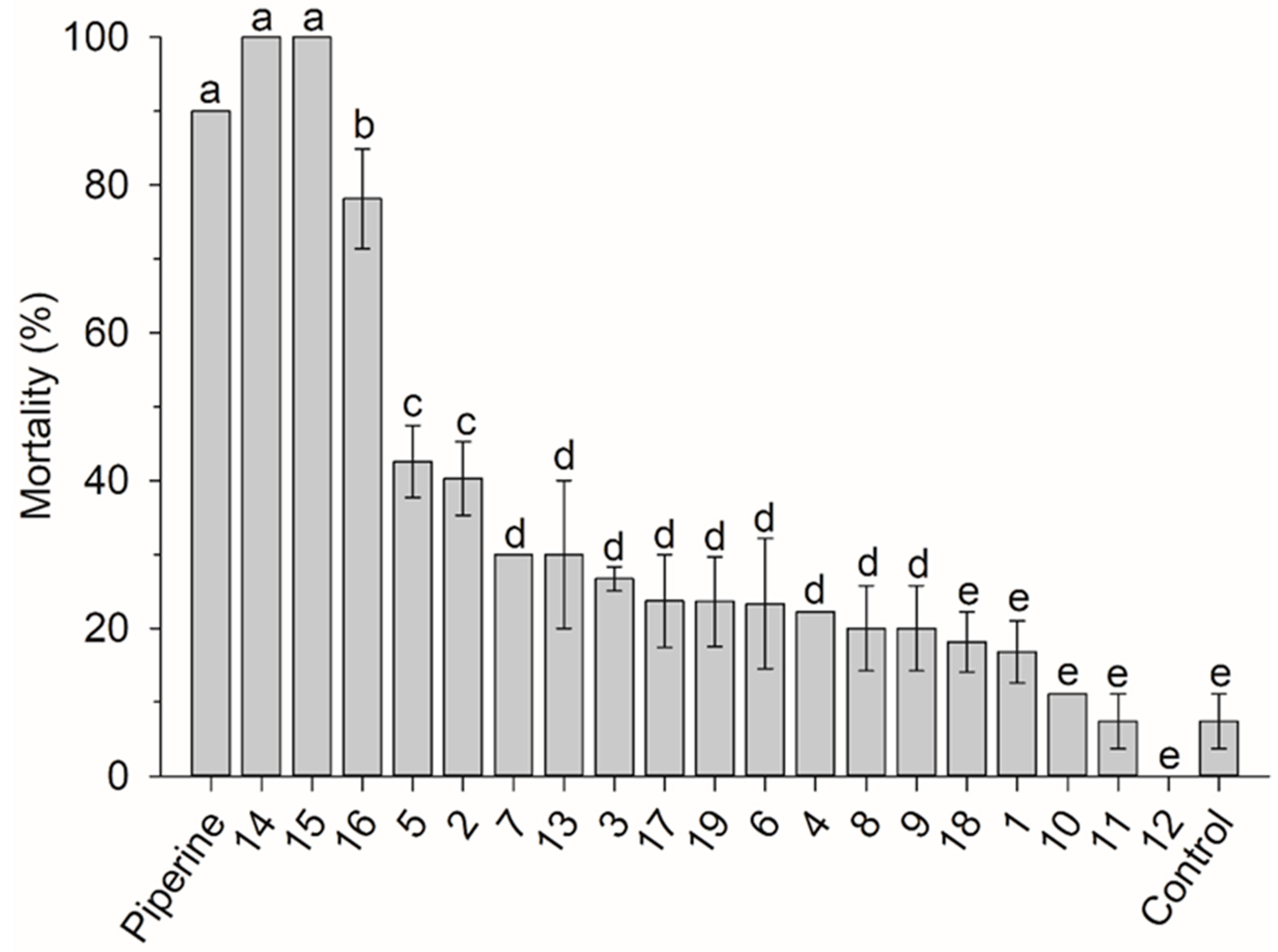
3.1. Amide Susceptibility Bioassay
3.2. Dose-Response Bioassay
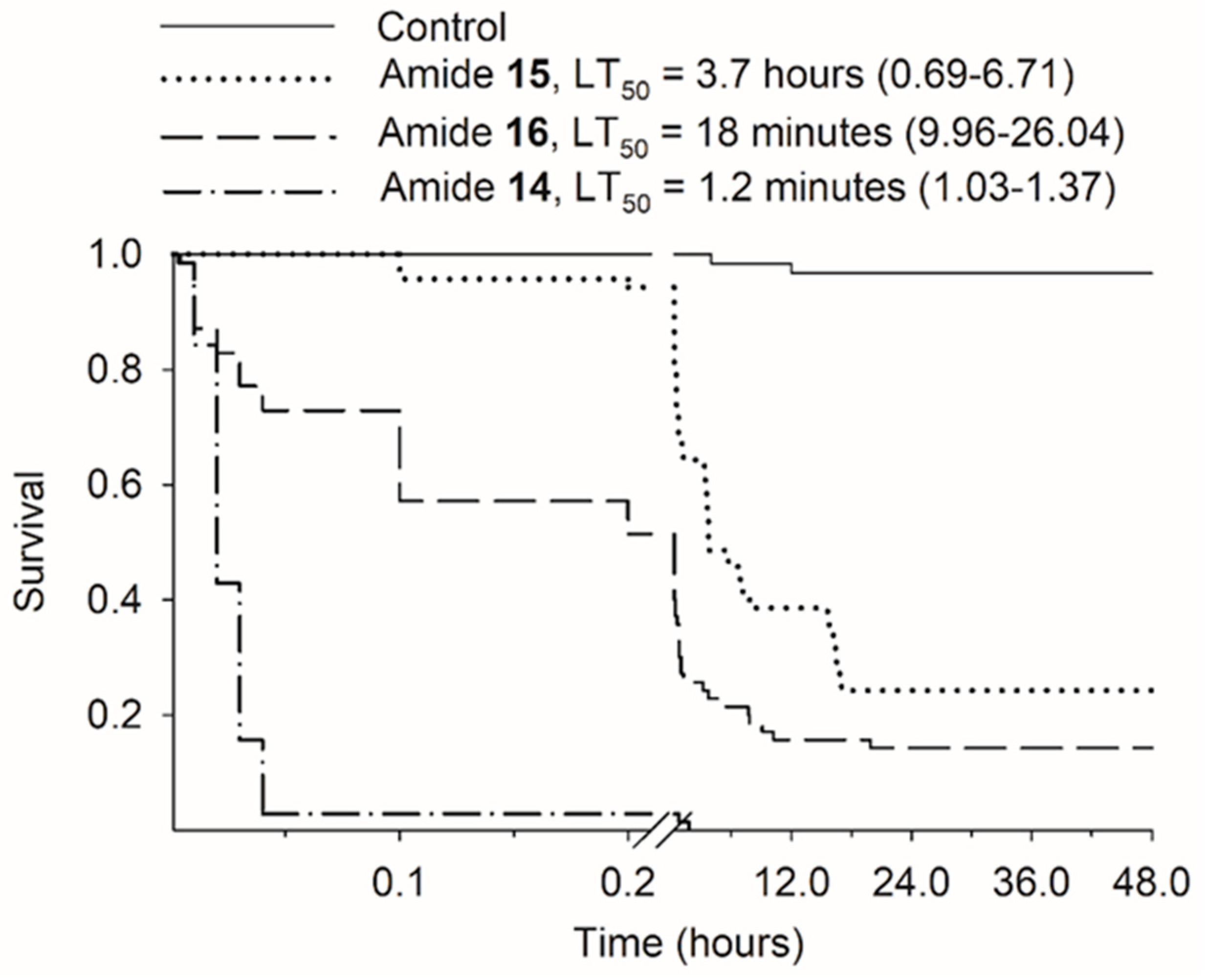
3.3. Time-Response Bioassay
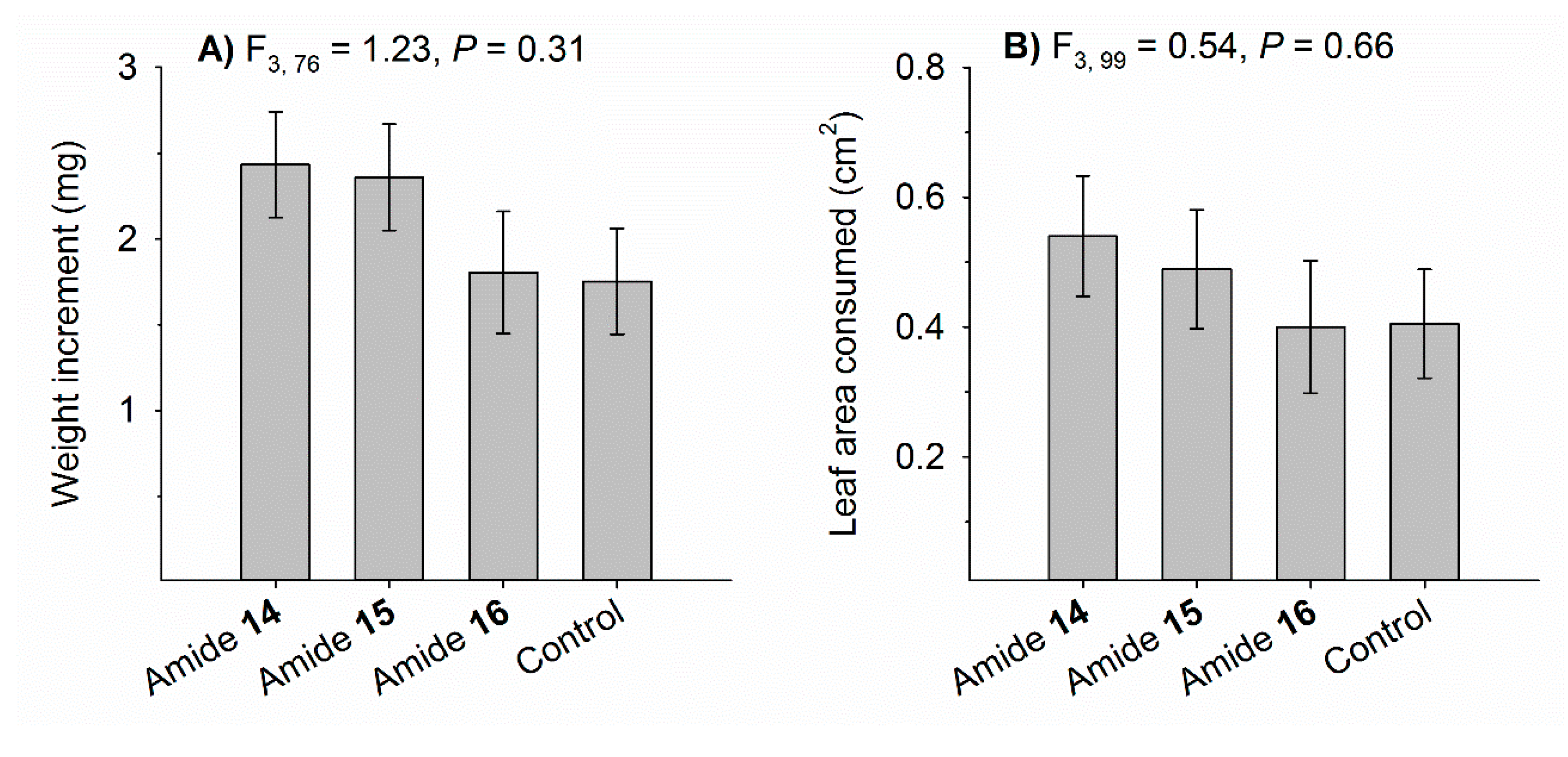
3.4. Sublethal Effects on Weight Gain and Leaf Consumption
3.5. Risk Assessment for T. absoluta Predators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miranda, M.M.M.; Picanço, M.; Zanuncio, J.C.; Guedes, R.N.C. Ecological life table of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Biocontrol Sci. Technol. 1998, 8, 597–606. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Picanço, M.C. The tomato borer Tuta absoluta in South America: Pest status, management and insecticide resistance. EPPO Bull. 2012, 42, 211–216. [Google Scholar] [CrossRef]
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: The new threat to tomato world production. J. Pest Sci. 2011, 84, 403–408. [Google Scholar] [CrossRef]
- Campos, M.R.; Biondi, A.; Adiga, A.; Guedes, R.N.C.; Desneux, N. From the Western Palaearctic region to beyond: Tuta absoluta 10 years after invading Europe. J. Pest Sci. 2017, 90, 787–796. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.-H.; Desneux, N. Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef]
- Pfeiffer, D.G.; Muniappan, R.; Sall, D.; Diatta, P.; Diongue, A.; Dieng, E.O. First record of Tuta absoluta (Lepidoptera: Gelechiidae) in Senegal. Florida Entomol. 2013, 96, 661–662. [Google Scholar] [CrossRef]
- Brévault, T.; Sylla, S.; Diatte, M.; Bernadas, G.; Diarra, K. Tuta absoluta Meyrick (Lepidoptera: Gelechiidae): A new threat to tomato production in sub-Saharan Africa. Afr. Entomol. 2014, 22, 441–444. [Google Scholar] [CrossRef]
- Tropea Garzia, G.; Siscaro, G.; Biondi, A.; Zappalà, L. Tuta absoluta, a South American pest of tomato now in the EPPO region: Biology, distribution and damage. EPPO Bull. 2012, 42, 205–210. [Google Scholar] [CrossRef]
- Picanço, M.; Leite, G.L.D.; Guedes, R.N.C.; Silva, E.A. Yield loss in trellised tomato affected by insecticidal sprays and plant spacing. Crop Prot. 1998, 17, 447–452. [Google Scholar] [CrossRef]
- Campos, M.R.; Silva, T.B.M.; Silva, W.M.; Silva, J.E.; Siqueira, H.A.A. Spinosyn resistance in the tomato borer Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Pest Sci. 2015, 88, 405–412. [Google Scholar] [CrossRef]
- Siqueira, H.A.A.; Guedes, R.N.C.; Fragoso, D.B.; Magalhaes, L.C. Abamectin resistance and synergism in Brazilian populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Int. J. Pest Manag. 2001, 47, 247–251. [Google Scholar] [CrossRef]
- Roditakis, E.; Vasakis, E.; García-Vidal, L.; Martínez-Aguirre, M.R.; Rison, J.L.; Haxaire-Lutun, M.O.; Nauen, R.; Tsagkarakou, A.; Bielza, P. A four-year survey on insecticide resistance and likelihood of chemical control failure for tomato leaf miner Tuta absoluta in the European/Asian region. J. Pest Sci. 2018, 91, 421–435. [Google Scholar] [CrossRef]
- Silva, G.A.; Picanço, M.C.; Bacci, L.; Crespo, A.L.B.; Rosado, J.F.; Guedes, R.N.C. Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Manag. Sci. 2011, 67, 913–920. [Google Scholar] [CrossRef]
- Campos, M.R.; Silva, T.B.; Silva, W.M.; Silva, J.E.; Siqueira, H.A. Susceptibility of Tuta absoluta (Lepidoptera: Gelechiidae) Brazilian populations to ryanodine receptor modulators. Pest Manag. Sci. 2015, 71, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, P.C.; Picanço, M.C.; Pereira, E.J.G.; Martins, J.C.; Chediak, M.; Guedes, R.N.C. Spatial and temporal variation in the control failure likelihood of the tomato leaf miner, Tuta absoluta. Ann. Appl. Biol. 2013, 162, 50–59. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, M.; Kreutzweiser, D.; Mitchell, E.A.D.; Morrissey, C.A.; Noome, D.A.; Van Der Sluijs, J.P. Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ. Sci. Pollut. Res. 2015, 22, 119–134. [Google Scholar] [CrossRef]
- Cônsoli, F.L.; Parra, J.R.P.; Hassan, S.A. Side-effects of insecticides used in tomato fields on the egg parasitoid Trichogramma pretiosum Riley (Hym., Trichogrammatidae), a natural enemy of Tuta absoluta (Meyrick) (Lep., Gelechiidae). J. Appl. Entomol. 1998, 122, 43–47. [Google Scholar] [CrossRef]
- Resende, G.C.; Alvarenga, E.S.; Araújo, T.A.; Campos, J.N.; Picanço, M.C. Toxicity to Diaphania hyalinata, selectivity to non-target species and phytotoxicity of furanones and phthalide analogues. Pest Manag. Sci. 2016, 72, 1772–1777. [Google Scholar] [CrossRef][Green Version]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef]
- Isman, M.B.; Akhtar, Y. Plant natural products as a source for developing environmentally acceptable insecticides. In Insecticides Design Using Advanced Technologies; Springer: Berlin/Heidelberg, Germany, 2007; pp. 235–248. [Google Scholar]
- Tavares, W.S.; Cruz, I.; Petacci, F.; Freitas, S.S.; Serrão, J.E.; Zanuncio, J.C. Insecticide activity of piperine: Toxicity to eggs of Spodoptera frugiperda (Lepidoptera: Noctuidae) and Diatraea saccharalis (Lepidoptera: Pyralidae) and phytotoxicity on several vegetables. J. Med. Plants Res. 2011, 5, 5301–5306. [Google Scholar]
- Brito, E.F.; Baldin, E.L.L.; Silva, R.C.M.; Ribeiro, L.P.; Vendramim, J.D. Bioactivity of Piper extracts on Tuta absoluta (Lepidoptera: Gelechiidae) in tomato. Pesqui. Agropecuária Bras. 2015, 50, 196–202. [Google Scholar] [CrossRef]
- Paula, V.F.; Barbosa, L.C.A.; Demuner, A.J.; Piló-Veloso, D.; Picanço, M.C. Synthesis and insecticidal activity of new amide derivatives of piperine. Pest Manag. Sci. 2000, 56, 168–174. [Google Scholar] [CrossRef]
- Qu, H.; Yu, X.; Zhi, X.; Lv, M.; Xu, H. Natural-product-based insecticidal agents 14. Semisynthesis and insecticidal activity of new piperine-based hydrazone derivatives against Mythimna separata Walker in vivo. Bioorg. Med. Chem. Lett. 2013, 23, 5552–5557. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.S.; Lima, L.F.; Previato, J.O.; Previato, L.M.; Heise, N.; Lima, M.E.F. Toxic effects of natural piperine and its derivatives on epimastigotes and amastigotes of Trypanosoma cruzi. Bioorg. Med. Chem. Lett. 2004, 14, 3555–3558. [Google Scholar] [CrossRef]
- Aguiar, A.R.; Alvarenga, E.S.; Lopes, M.C.; Santos, I.B.; Galdino, T.V.; Picanço, M.C. Active insecticides for Diaphania hyalinata selective for the natural enemy Solenopsis saevissima. J. Environ. Sci. Health Part B 2016, 51, 579–588. [Google Scholar] [CrossRef]
- Farias, E.S.; Silva, E.M.P.; Teixeira, M.G.; Ferreira, J.S.; Alvarenga, E.S.; Picanço, M.C. Phthalides as promising insecticides against Tuta absoluta (Lepidoptera: Gelechiidae). J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2018, 53, 49–56. [Google Scholar] [CrossRef]
- Alvarenga, E.S.; Carneiro, V.M.T.; Resende, G.C.; Picanço, M.C.; Farias, E.D.S.; Lopes, M.C. Synthesis and insecticidal activity of an oxabicyclolactone and novel pyrethroids. Molecules 2012, 17, 13989–14001. [Google Scholar] [CrossRef]
- Moreira, M.D.; Picanço, M.C.; Barbosa, L.C.A.; Guedes, R.N.C.; Barros, E.C.; Campos, M.R. Compounds from Ageratum conyzoides: Isolation, structural elucidation and insecticidal activity. Pest Manag. Sci. 2007, 63, 615–621. [Google Scholar] [CrossRef]
- Jelihovschi, E.; Faria, J.C.; Allaman, I.B. ScottKnott: A package for performing the Scott–Knott clustering algorithm in R. TEMA (São Carlos) 2014, 15, 3–17. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- BRASIL. Manual de Protocolo Para Testes de Eficácia de Produtos Desinfestantes; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2004.
- Scott, I.M.; Jensen, H.; Scott, J.G.; Isman, M.B.; Arnason, J.T.; Philogène, B.J.R. Botanical insecticides for controlling agricultural pests: Piperamides and the Colorado potato beetle Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Arch. Insect Biochem. Physiol. 2003, 54, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Cantrell, C.L.; Meepagala, K.M.; Wedge, D.E.; Tabanca, N.; Schrader, K.K. Natural toxins for use in pest management. Toxins 2010, 2, 1943–1962. [Google Scholar] [CrossRef] [PubMed]
- Blazka, M. Acute toxicity and eye irritancy. In Principles and Methods of Toxicology, 5th ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 1131–1177. [Google Scholar]
- Rathburn, C.B. Insecticide formulations—Types and uses: A review. J. Am. Mosq. Control Assoc. 1985, 1, 80–84. [Google Scholar]
- Elliott, M.; Farnham, A.W.; Janes, N.F.; Johnson, D.M.; Pulman, D.A.; Sawicki, R.M. Insecticidal amides with selective potency against a resistant (Super-kdr) strain of houseflies (Musca domestica L.). Agric. Biol. Chem. 1986, 50, 1347–1349. [Google Scholar] [CrossRef]
- Okwute, S.K.; Egharevba, H.O. Piperine-type amides: Review of the chemical and biological characteristics. Int. J. Chem. 2013, 5, 99–122. [Google Scholar] [CrossRef]
- Whitehead, S.R.; Bowers, M.D. Chemical ecology of fruit defence: Synergistic and antagonistic interactions among amides from Piper. Funct. Ecol. 2014, 28, 1094–1106. [Google Scholar] [CrossRef]
- Scott, I.M.; Jensen, H.R.; Philogène, B.J.R.; Arnason, J.T. A review of Piper spp. (Piperaceae) phytochemistry, insecticidal activity and mode of action. Phytochem. Rev. 2008, 7, 65–75. [Google Scholar] [CrossRef]
- van der Werf, H.M.G. Assessing the impact of pesticides on the environment. Agric. Ecosyst. Environ. 1996, 60, 81–96. [Google Scholar] [CrossRef]
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating pesticide degradation in the environment: Blind spots and emerging opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef]
- Moriarty, F. The sublethal effects of synthetic insecticides on insects. Biol. Rev. 1969, 44, 321–356. [Google Scholar] [CrossRef]
- Wang, D.; Gong, P.; Li, M.; Qiu, X.; Wang, K. Sublethal effects of spinosad on survival, growth and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae). Pest Manag. Sci. 2009, 65, 223–227. [Google Scholar] [CrossRef]
- Janmaat, A.F.; Bergmann, L.; Ericsson, J. Effect of low levels of Bacillus thuringiensis exposure on the growth, food consumption and digestion efficiencies of Trichoplusia ni resistant and susceptible to Bt. J. Invertebr. Pathol. 2014, 119, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Sterk, G.; Hassan, S.A.; Baillod, M.; Bakker, F.; Bigler, F.; Blümel, S.; Bogenschütz, H.; Boller, E.; Bromand, B.; Brun, J.; et al. Results of the seventh joint pesticide testing programme carried out by the IOBC/WPRS-Working Group ‘Pesticides and Beneficial Organisms’. BioControl 1999, 44, 99–117. [Google Scholar] [CrossRef]
- Tomé, H.V.V.; Ramos, G.S.; Araújo, M.F.; Santana, W.C.; Santos, G.R.; Guedes, R.N.C.; Maciel, C.D.; Newland, P.L.; Oliveira, E.E. Agrochemical synergism imposes higher risk to Neotropical bees than to honeybess. R. Soc. Open Sci. 2017, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Valles, S.M.; Perera, O.P.; Strong, C.A. Gene structure and expression of the glutathione S-transferase, SiGSTS1, from the red imported fire ant, Solenopsis invicta. Arch. Insect Biochem. Physiol. 2006, 61, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Rashid, T.; Feng, G. Esterase in imported fire ants, Solenopsis invicta and S. Richteri (Hymenoptera: Formicidae): Activity, kinetics and variation. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, L.; Cui, R.; Zeng, X.; Gao, X. Cloning and expression of multiple cytochrome P450 genes: Induction by fipronil n workers of the red imported fire ant (Solenopsis invicta Buren). PLoS ONE 2016, 11, e0150915. [Google Scholar] [CrossRef]
- Araújo, T.A.; Picanço, M.C.; Ferreira, D.O.; Campos, J.N.; Arcanjo, L.P.; Silva, G.A. Toxicity and residual effects of insecticides on Ascia monuste and predator Solenopsis saevissima. Pest Manag. Sci. 2017, 73, 2259–2266. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Snyder, W.E. Biodiversity and Insect Pests: Key Issues for Sustainable Management; Gurr, G.M., Wratten, S.D., Snyder, W.E., Read, D.M.Y., Eds.; Wiley-Blackwell: Chichester, UK, 2012. [Google Scholar]
- Hull, L.A.; Beers, E.H. Ecological selectivity: Modifying chemical control practices to preserve natural enemies. In Biology Control in Agriculture IPM System; Elsevier: Amsterdam, The Netherlands, 1985; pp. 103–122. [Google Scholar]
- Picanço, M.C.; Oliveira, I.R.; Rosado, J.F.; Silva, F.M.; Gontijo, P.C.; Silva, R.S. Natural biological control of Ascia monuste by the social wasp Polybia ignobilis (Hymenoptera: Vespidae). Sociobiology 2010, 56, 67–76. [Google Scholar]


| Compound | N a | Slope b | LD50 (μg/mg) b | Chi-squared | P-Valued |
|---|---|---|---|---|---|
| Amide 14 | 240 | 3.14 (2.29–3.99) | 3.68 (2.83–4.47) | 0.22 | 0.90 |
| Amide 15 | 300 | 5.29 (3.82–6.76) | 6.46 (5.85–7.20) | 1.82 | 0.61 |
| Amide 16 | 420 | 2.47 (1.96–2.98) | 13.52 (11.06–15.95) | 3.34 | 0.65 |
| Piperine c | 300 | 1.79 (1.38–2.20) | 3.80 (2.92–4.81) | 0.11 | 0.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, E.; Farias, E.; Ribeiro, A.; Alvarenga, E.; Aguiar, A.; Ferreira, J.; Picanço, M. Toxicity of Piperine Amide Analogs toward the Tomato Pinworm Tuta absoluta (Lepidoptera: Gelechiidae) and Risk Assessment for Two Predators. Horticulturae 2019, 5, 70. https://doi.org/10.3390/horticulturae5040070
Pereira E, Farias E, Ribeiro A, Alvarenga E, Aguiar A, Ferreira J, Picanço M. Toxicity of Piperine Amide Analogs toward the Tomato Pinworm Tuta absoluta (Lepidoptera: Gelechiidae) and Risk Assessment for Two Predators. Horticulturae. 2019; 5(4):70. https://doi.org/10.3390/horticulturae5040070
Chicago/Turabian StylePereira, Elba, Elizeu Farias, Arthur Ribeiro, Elson Alvarenga, Alex Aguiar, Jhulyana Ferreira, and Marcelo Picanço. 2019. "Toxicity of Piperine Amide Analogs toward the Tomato Pinworm Tuta absoluta (Lepidoptera: Gelechiidae) and Risk Assessment for Two Predators" Horticulturae 5, no. 4: 70. https://doi.org/10.3390/horticulturae5040070
APA StylePereira, E., Farias, E., Ribeiro, A., Alvarenga, E., Aguiar, A., Ferreira, J., & Picanço, M. (2019). Toxicity of Piperine Amide Analogs toward the Tomato Pinworm Tuta absoluta (Lepidoptera: Gelechiidae) and Risk Assessment for Two Predators. Horticulturae, 5(4), 70. https://doi.org/10.3390/horticulturae5040070






