Punicalagin Content and Antifungal Activity of Different Pomegranate (Punica ganatum L.) Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants Used for Extraction and Fungus
2.2. Preparation of Powders and Extracts
2.3. Mycelial Growth Inhibition Assay
2.4. Determination of Total Phenolic Content
2.5. Acidity and pH Analysis
2.6. HPLC–UV Analysis of Extracts
2.7. Statistical Analysis
3. Results Discussion
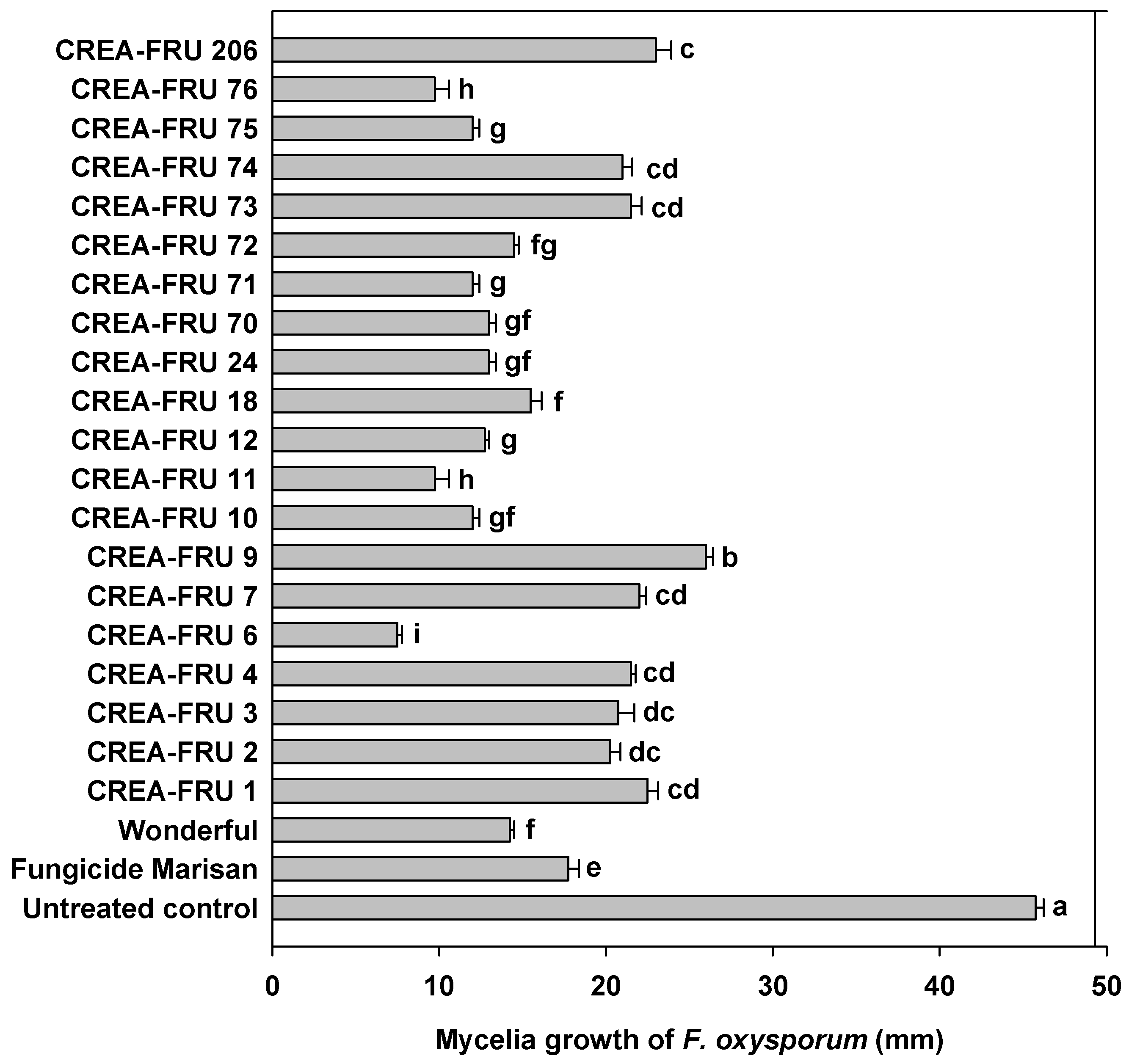
3.1. Mycelial Growth Inhibition Assays
3.2. Solvent Extraction Efficiency
3.3. Total Phenolic, Punicalagin Content, Ellagic Acid Content, Acidity, pH and Correlation Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ulrike, A.F.; Reinhold, C.; Dietmar, R.K. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MS(n). Food Chem. 2011, 127, 807–821. [Google Scholar]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073–2085. [Google Scholar] [CrossRef] [PubMed]
- Gill, N.S.; Dhawan, S.; Jain, A.; Arora, R.; Bali, M. Antioxidant and anti-ulcerogenic activity of wild Punica granatum ethanolic seed extract. Res. J. Med. Plants 2012, 6, 47–55. [Google Scholar] [CrossRef]
- Malik, A.; Mukhtar, H. Prostate cancer prevention through pomegranate fruit. Cell Cycle 2006, 5, 371–373. [Google Scholar] [PubMed]
- Sturgeon, S.R.; Ronnenberg, A.G. Pomegranate and breast cancer: Possible mechanisms of prevention. Nutr. Rev. 2010, 68, 122–128. [Google Scholar] [CrossRef]
- Kaneria, M.J.; Chanda, S.V. The effect of sequential fractionation technique on the various efficacies of pomegranate (Punica granatum L.). Food Anal. Method. 2013, 6, 164–175. [Google Scholar] [CrossRef]
- Quattrucci, A.; Ovidi, E.; Tiezzi, A.; Vinciguerra, V.; Balestra, G.M. Biological control of tomato bacterial speck using Punica granatum fruit peel extract. Crop Prot. 2013, 46, 18–22. [Google Scholar] [CrossRef]
- Romanazzi, G.; Smilanick, J.L.; Feliziani, E.; Droby, S. Integrated management of postharvest gray mold on fruits crops. Postharvest Biol. Technol. 2016, 113, 69–76. [Google Scholar] [CrossRef]
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal activity of pomegranate peel extract against fusarium wilt of tomato. Eur. J. Plant Pathol. 2017, 147, 229–238. [Google Scholar] [CrossRef]
- Rongai, D.; Sabatini, N.; Pulcini, P.; Di Marco, C.; Storchi, L.; Marrone, A. Effect of pomegranate peel extract on shelf life of 1 fresh strawberries: Phytochemical analysis, antifungal activity and possible mechanisms involved. J. Food Sci. Technol. 2018, 55, 2702–2711. [Google Scholar] [CrossRef]
- Pareek, S.; Valero, D.; Serrano, M. Postharvest biology and technology of pomegranate. J. Sci. Food Agric. 2015, 95, 2360–2379. [Google Scholar] [CrossRef] [PubMed]
- Paschalidis, K.A.; Moschou, P.N.; Toumi, I.; Roubelakis–Angelakis, K.A. Polyamine anabolic/catabolic regulation along the woody grapevine plant axis. J. Plant Physiol. 2009, 166, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Paschalidis, K.; Feng, J.C.; Song, J.; Liu, J.H. Polyamine catabolism in plants: A universal process with diverse functions. Front. Plant Sci. 2019, 10, 561. [Google Scholar] [CrossRef]
- Landi, L.; Feliziani, E.; Romanazzi, G. Expression of defense genes in strawberry fruits treated with different resistance inducers. J. Agric. Food Chem. 2014, 62, 3047–3056. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Reyes, J.G.; Spadaro, D.; Gullinoa, M.L.; Garibaldi, A. Efficacy of plant essential oils on postharvest control of rot caused by fungi on four cultivars of apples in vivo. Flavour Fragr. J. 2010, 25, 171–177. [Google Scholar] [CrossRef]
- Nabigol, A.; Morshedi, H. Evaluation of the antifungal activity of the Iranian thyme essential oils on the postharvest pathogens of Strawberry fruits. Afr. J. Biotechnol. 2011, 10, 9864–9869. [Google Scholar]
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal activity of some botanical extracts on Fusarium oxysporum. Open Life Sci. 2015, 10, 409–416. [Google Scholar]
- Foss, S.R.; Nakamura, C.V.; Ueda-Nakamura, T.; Cortez, D.A.G.; Endo, E.H.; Dias Filho, B.P. Antifungal activity of pomegranate peel extract and isolated compound punicalagin against dermatophytes. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 32–38. [Google Scholar] [CrossRef]
- Ferrara, G.; Giancaspro, A.; Mazzeo, A.; Giove, S.-L.; Matarrese, A.M.S.; Pacucci, C.; Punzi, R.; Trani, A.; Gambacorta, G.; Blanco, A.; et al. Characterization of pomegranate (Punica granatum L.) genotypes collected in Puglia region, Southeastern Italy. Sci. Hortic. 2014, 178, 70–78. [Google Scholar] [CrossRef]
- Hmid, I.; Elothmania, D.; Hanine, H.; Oukablic, A.; Mehinagic, E. Comparative study of phenolic compounds and their antioxidant attributes of eighteen pomegranate (Punica granatum L.) cultivars grown in Morocco. Arab. J. Chem. 2017, 10, 2675–2684. [Google Scholar] [CrossRef]
- Radunić, M.; Jukić Špika, M.; Goreta Ban, S.; Gadže, J.; Díaz-Pérez, J.; MacLean, D. Physical and chemical properties of pomegranate fruit accessions from Croatia. Food Chem. 2015, 177, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, R.; Fawole, O.A.; Stander, M.A.; Opara, U.L. Preharvest and postharvest factors influencing bioactive compounds in pomegranate (Punica granatum L.). Sci. Hortic. 2014, 178, 114–123. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analyses: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Wang, Z.; Pan, Z.; Ma, H.; Atungulu, G.G. Extract of phenolics from pomegranate peels. Open Food Sci. J. 2011, 5, 17–25. [Google Scholar] [CrossRef]
- Venkataramanamma, D.; Aruna, P.; Singh, R.P. Standardization of the conditions for extraction of polyphenols from pomegranate peel. J. Food Sci. Technol. 2016, 53, 2497–2503. [Google Scholar] [CrossRef] [PubMed]
- Anis, Z.; Sulaiman, O.; Hashim, R.; Mehdi, S.H.; Ghalib, R.M. Radical scavenging activity, total phenol content and antifungal activity of Cinnamomum Iners. Wood. Iran. J. Energy Environ. 2012, 3, 74–78. [Google Scholar] [CrossRef]
- Ifesan, B.O.T.; Fashakin, J.F.; Ebosele, F.; Oyerinde, A.S. Antioxidant and antimicrobial properties of selected plant leaves. Eur. J. Med. Plants 2013, 3, 465–473. [Google Scholar] [CrossRef]
- El-Khateeb, A.Y.; Elsherbiny, E.A.; Tadros, L.K.; Ali, S.M.; Hamed, H.B. Phytochemical analysis and antifungal activity of fruit leaves extracts on the mycelial growth of fungal plant pathogens. J. Plant Pathol. Microbiol. 2013, 4, 199. [Google Scholar] [CrossRef]
- Orak, H.H.; Demïrcï, A.Ş.; Gümüş, T. Antibacterial and antifungal activity of pomegranate (Punica granatum L. cv.) peel. Electron. J. Environ. Agric. Food Chem 2011, 10, 1958–1969. [Google Scholar]
- Oliveira, C.E.V.; Stamford, T.L.M.; Neto, N.J.G.; Souza, E.L. Inhibition of Staphylococcus aureus in broth and meat broth using synergies of phenolics and organic acids. Int. J. Food Microbiol. 2010, 137, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, L.; Fan, L.; Ding, Y.; Zhao, S.; Liu, Y.; Ma, C. Optimization of extraction and enrichment of phenolics from pomegranate (Punica granatum L.) leaves. Ind. Crops Prod. 2013, 42, 587–594. [Google Scholar] [CrossRef]
| Genotype Code | Geographic Origins | Pomegranate Type | Pomegranate Juice |
|---|---|---|---|
| Wonderful | Middle Eastern | Commercial | Sour/Sweet |
| CREA-FRU 1 | Mediterranean | Wild | Sweet |
| CREA-FRU 2 | Middle Eastern | Wild | Sour |
| CREA-FRU 3 | Middle Eastern | Wild | Sour |
| CREA-FRU 4 | Middle Eastern | Wild | Sour |
| CREA-FRU 6 | Middle Eastern | Wild | Sour |
| CREA-FRU 7 | Middle Eastern | Wild | Sour |
| CREA-FRU 9 | Middle Eastern | Wild | Sour |
| CREA-FRU 10 | Mediterranean | Wild | Sour |
| CREA-FRU 11 | Middle Eastern | Wild | Sour |
| CREA-FRU 12 | Mediterranean | Wild | Sweet |
| CREA-FRU 18 | Middle Eastern | Wild | Sour |
| CREA-FRU 24 | Middle Eastern | Wild | Sour |
| CREA-FRU 70 | Mediterranean | Commercial | Sweet |
| CREA-FRU 71 | Mediterranean | Commercial | Sweet |
| CREA-FRU 72 | Middle Eastern | Wild | Sweet |
| CREA-FRU 73 | Mediterranean | Commercial | Sweet |
| CREA-FRU 74 | Mediterranean | Wild | Sour |
| CREA-FRU 75 | Mediterranean | Wild | Sour |
| CREA-FRU 76 | Mediterranean | Wild | Sour |
| CREA-FRU 206 | Middle Eastern | Wild | Sour/Sweet |
| Treatment | Dose | Mycelial Growth of F. oxysporum | Extraction Efficiency | ||
|---|---|---|---|---|---|
| mm | % | ||||
| Untreated control | 45.7 | ±0.41 a | |||
| Synthetic fungicide | 0.15% | 17.5 | ±0.64 b | ||
| WE | 1% | 16.5 | ±0.64 bc | 18.1 | ±0.55 a |
| ME | 1% | 16.5 | ±0.63 bc | 17.4 | ±0.31 a |
| EE | 1% | 15.5 | ±0.30 c | 13.8 | ±0.32 d |
| PAEE80-20 | 1% | 15.5 | ±0.64 c | 17.4 | ±0.37 a |
| PAEE40-60 | 1% | 16.0 | ±0.40 c | 16.8 | ±0.23 b |
| PAEE20-80 | 1% | 15.7 | ±0.48 c | 16.2 | ±0.14 c |
| F = 451.12 P < 0.001 | F = 24.154 P < 0.001 | ||||
| Genotype Code | pH | Acidity | TPC | EA | PC | ||
|---|---|---|---|---|---|---|---|
| PC (α + β) | PαC | PβC | |||||
| meq NaOH g−1DW | mg GAE g−1DW | mg g−1DW | mg g−1DW | mg g−1DW | mg g−1DW | ||
| Wonderful | 4.07 a | 1.38 e | 432.7 c | 14.6 d | 216.8 c | 95.1 c | 121.3 c |
| CREA-FRU 1 | 4.24 a | 1.11 f | 241.6 e | 6.1 g | 176.9 d | 38.1 f | 138.8 c |
| CREA-FRU 2 | 4.12 a | 1.60 de | 261.0 e | 9.6 f | 87.8 f | 19.6 g | 68.2 f |
| CREA-FRU 3 | 4.18 a | 1.30 e | 444.3 c | 9.0 f | 144.3 de | 35.3 f | 109.0 d |
| CREA-FRU 4 | 4.19 a | 1.75 cd | 311.0 d | 7.4 g | 97.8 f | 24.4 g | 73.4 e |
| CREA-FRU 6 | 3.92 ab | 2.90 a | 612.7 a | 7.5 g | 476.7 a | 120.6 b | 356.1 a |
| CREA-FRU 7 | 4.15 a | 1.80 c | 402.8 c | 10.0 f | 169.4 d | 34.4 f | 135.0 c |
| CREA-FRU 9 | 3.90 ab | 1.65 d | 146.0 f | 0.6 i | 1.6 l | 1.3 i | 0.3 i |
| CREA-FRU 10 | 3.69 b | 3.15 a | 529.3 b | 27.3 b | 318.9 b | 128.7 b | 190.15 b |
| CREA-FRU 11 | 4.02 a | 2.65 b | 514.9 b | 6.0 g | 369.2 b | 183.8 a | 185.4 b |
| CREA-FRU 12 | 3.93 ab | 1.25 e | 448.0 c | 40.1 a | 145.6 de | 78.9 d | 66.7 f |
| CREA-FRU 18 | 3.75 b | 2.35 bc | 448.7 c | 8.5 fg | 118.4 e | 50.0 e | 68.5 f |
| CREA-FRU 24 | 3.92 ab | 1.45 e | 514.4 b | 15.4 d | 130.1 e | 75.2 d | 46.4 g |
| CREA-FRU 70 | 3.92 ab | 1.75 cd | 347.2d | 18.0 c | 69.3 g | 29.7 fg | 39.6 g |
| CREA-FRU 71 | 4.04 a | 1.85 c | 572.3 a | 12.3 e | 254.9 c | 91.8 c | 163.1 bc |
| CREA-FRU 72 | 4.01 a | 1.7 d | 510.5 b | 10.8 f | 128.6 e | 60.5 e | 68.1 f |
| CREA-FRU 73 | 3.90 ab | 1.65 d | 237.7 e | 4.6 h | 4.9 i | 3.8 h | 0.5 i |
| CREA-FRU 74 | 3.88 ab | 1.60 de | 295.5 de | 7.0 g | 92.0 f | 57.7 e | 37.3 g |
| CREA-FRU 75 | 3.92 ab | 2.00 c | 585.8 a | 31.3 b | 319.9 b | 129.3 b | 190.4 b |
| CREA-FRU 76 | 4.02 a | 2.65 b | 514.9 b | 37.8 a | 453.6 a | 128.5 b | 325.1 a |
| CREA-FRU 206 | 4.03 a | 1.35 e | 249.6 e | 8.2 fg | 25.9 h | 21.6 g | 4.3 h |
| MG | PC | EA | TPC | TA | pH | |
|---|---|---|---|---|---|---|
| pH | 0.13 | 0.01 | 0.24 | 0.50 | 0.26 | |
| TA | 0.38 | 0.56 | 0.07 | 0.30 | ||
| TPC | 0.77 | 0.61 | 0.08 | |||
| EA | 0.32 | 0.09 | ||||
| PC | 0.61 | |||||
| PαC | 0.81 | |||||
| PβC | 0.60 | |||||
| MG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rongai, D.; Pulcini, P.; Di Lernia, G.; Nota, P.; Preka, P.; Milano, F. Punicalagin Content and Antifungal Activity of Different Pomegranate (Punica ganatum L.) Genotypes. Horticulturae 2019, 5, 52. https://doi.org/10.3390/horticulturae5030052
Rongai D, Pulcini P, Di Lernia G, Nota P, Preka P, Milano F. Punicalagin Content and Antifungal Activity of Different Pomegranate (Punica ganatum L.) Genotypes. Horticulturae. 2019; 5(3):52. https://doi.org/10.3390/horticulturae5030052
Chicago/Turabian StyleRongai, Domenico, Patrizio Pulcini, Giovanni Di Lernia, Paolo Nota, Pjerin Preka, and Filomena Milano. 2019. "Punicalagin Content and Antifungal Activity of Different Pomegranate (Punica ganatum L.) Genotypes" Horticulturae 5, no. 3: 52. https://doi.org/10.3390/horticulturae5030052
APA StyleRongai, D., Pulcini, P., Di Lernia, G., Nota, P., Preka, P., & Milano, F. (2019). Punicalagin Content and Antifungal Activity of Different Pomegranate (Punica ganatum L.) Genotypes. Horticulturae, 5(3), 52. https://doi.org/10.3390/horticulturae5030052




