1. Introduction
The determination of essential element concentrations in leaves is useful for improving managed plant protocols and understanding ecological relations. For the results of leaf tissue analyses to be useful, the leaf tissue sampling approaches need to be appropriate and repeatable. Therefore, knowledge of the various plant and environment traits that directly influence leaf nutrients is required to maximize the benefits of leaf tissue sampling for any given species or plant group.
As a group, cycads remain the most threatened plants worldwide [
1,
2,
3]. Much interest in cycad gardening and species conservation has created a need for practical knowledge that greatly exceeds the actual knowledge that has accumulated from applied research [
4,
5]. Therefore, cycad conservationists often lack the information that is needed to inform management decisions [
6,
7].
Reporting of leaf nutrient concentrations for various gymnosperms such as cycads has been accruing, but most cycad species remain unstudied within this research agenda [
5]. Moreover, the methods that underpin leaf sampling protocols have not been defined adequately for the plant group. We have recently shown that plant height and soil nutrient status influenced
Cycas micronesica leaf nutrients [
8]. Many other sources of variation that may influence plant leaf nutrients, such as position of tissue within large compound leaves, leaf canopy position, and incident light, have not been studied for cycads to date.
Our objective was to determine the influence of location along the rachis of mature
C. micronesica tree leaves on macro- and micro-nutrient concentrations. Leaf age was included to determine if the influence of rachis position on tissue concentrations was stable regardless of age. Using this species as the first case study of cycads, the results will improve leaf sampling protocols for cycad tissue analysis studies by revealing if leaflet position influences leaf elements. New information on location-specific nutrient allocations will improve our knowledge of how element transfers within a cycad plant fit into biogeochemical cycling. Since age [
9,
10] and location along the pine needle [
11] have been shown to influence gymnosperm tissue element concentrations, we predicted that both age and location along the rachis would exert an influence on
C. micronesica tissue element relations.
2. Materials and Methods
We studied in situ
C. micronesica populations in Yap, where soil series was formed in residuum derived from green, chlorite, and talc schist (clayey-skeletal, mixed, isohyperthermic lithic tropudalfs) [
12]. Yap is located ~820 km SW of Guam and ~420 km NE of Palau, and is therefore in the middle of the native range of the species. A soil sample from 0–15 cm depth was collected from beneath the leaf span of each sampled tree and combined into a composite sample. The pH was 6.0 and the elemental content was 5.3 mg·g
−1 N, 12.8 µg·g
−1 P, 97.1 µg·g
−1 K, 2.1 mg·g
−1 Ca, 1.5 mg·g
−1 Mg, 14.0 µg·g
−1 Mn, 310.2 µg·g
−1 Fe, 3.7 µg·g
−1 Cu, and 8.2 µg·g
−1 Zn.
Sampling dates were 12–15 October, 2016. The trees used for this study were restricted to similar heights and similar incident light to decrease variation caused by these variables. We ensured this by directly measuring stem height and restricting sampled trees to 1.5–2.0 m height. A 0.75 m line quantum sensor (EMS-7, PP Systems, Amesbury, MA, U.S.A.) was used to quantify the incident light for each sampled plant. The percentage photosynthetically active radiation (PAR) transmission at the location of each sample was quantified by placing one sensor in the direct incident solar beam and a second sensor beneath the emergent forest canopy where each C. micronesica leaf was positioned. Incident light measurements were conducted from 11:00 to 13:00. The percent transmission of ambient light was calculated as ((sun – shade)/sun) × 100. We restricted sampled trees to fairly open canopy locations with percent PAR transmission ranging from 20% to 30%.
Cycas micronesica trees produce large, pinnately compound leaves (
Figure 1). Leaflets were sampled from the youngest and oldest leaf flushes that were comprised exclusively of green leaves. Leaf flushes containing senescing leaves were not included. Determining the sequence of existing leaf flushes on a cycad tree is an unambiguous endeavor [
8]. The trees we selected exhibited four leaf flushes of fully green leaves. We initially measured the length of each rachis, then divided by three to define four equally spaced sampling locations. The four sampling sites for each leaf included basal, 1/3 the span of the rachis, 2/3 the span of the rachis, and terminal leaflets. For regression analyses, the position of each sampled leaflet was defined as the distance from the base of the leaf petiole. For the youngest flush, the basal site ranged from 46–53 cm, the 1/3 span site ranged from 91–99 cm, the 2/3 span site ranged from 130–139 cm, and the terminal site ranged from 181–186 cm. For the oldest flush, the basal site ranged from 46–54 cm, the 1/3 span site ranged from 91–99 cm, the 2/3 span site ranged from 132–139 cm, and the terminal site ranged from 182–188 cm. For each tree, one leaf in each cardinal direction was used for leaflet harvests for each leaf age. Each of the rachis sites for these four sampled leaves for each leaf age category were treated as subsamples and were combined into one sample for each rachis position.
Leaflets were stored in paper bags in ambient conditions until they were prepared for chemical analysis. The tissue was dried at 75 °C and milled to pass through a 20-mesh screen. Total N and C were determined by dry combustion (FLASH EA1112 CHN Analyzer, Thermo Fisher, Waltham, MA, USA) [
13]. Samples were also digested by a microwave system with nitric acid and peroxide, then phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), boron (B), manganese (Mn), zinc (Zn), and copper (Cu) were quantified by inductively coupled plasma optical emission spectroscopy (Spectro Genesis; SPECTRO Analytical Instruments, Kleve, Germany) [
14].
All elemental response variables were fitted to linear and quadratic models, with position along the rachis as the independent variable. The Proc GLM procedure in SAS (SAS 9.3; SAS Institute, Cary, NC, USA) was employed for this procedure. If the regression model for old leaves differed from that for new leaves, we did not pursue further analyses. If the regression model was the same for both leaf age classes, we employed analysis of covariance to determine if leaf age influenced the slope of the model. Leaf age was designated as the covariate, and elemental response variables were treated as the dependent variable, and distance along the rachis was treated as the independent variable.
3. Results
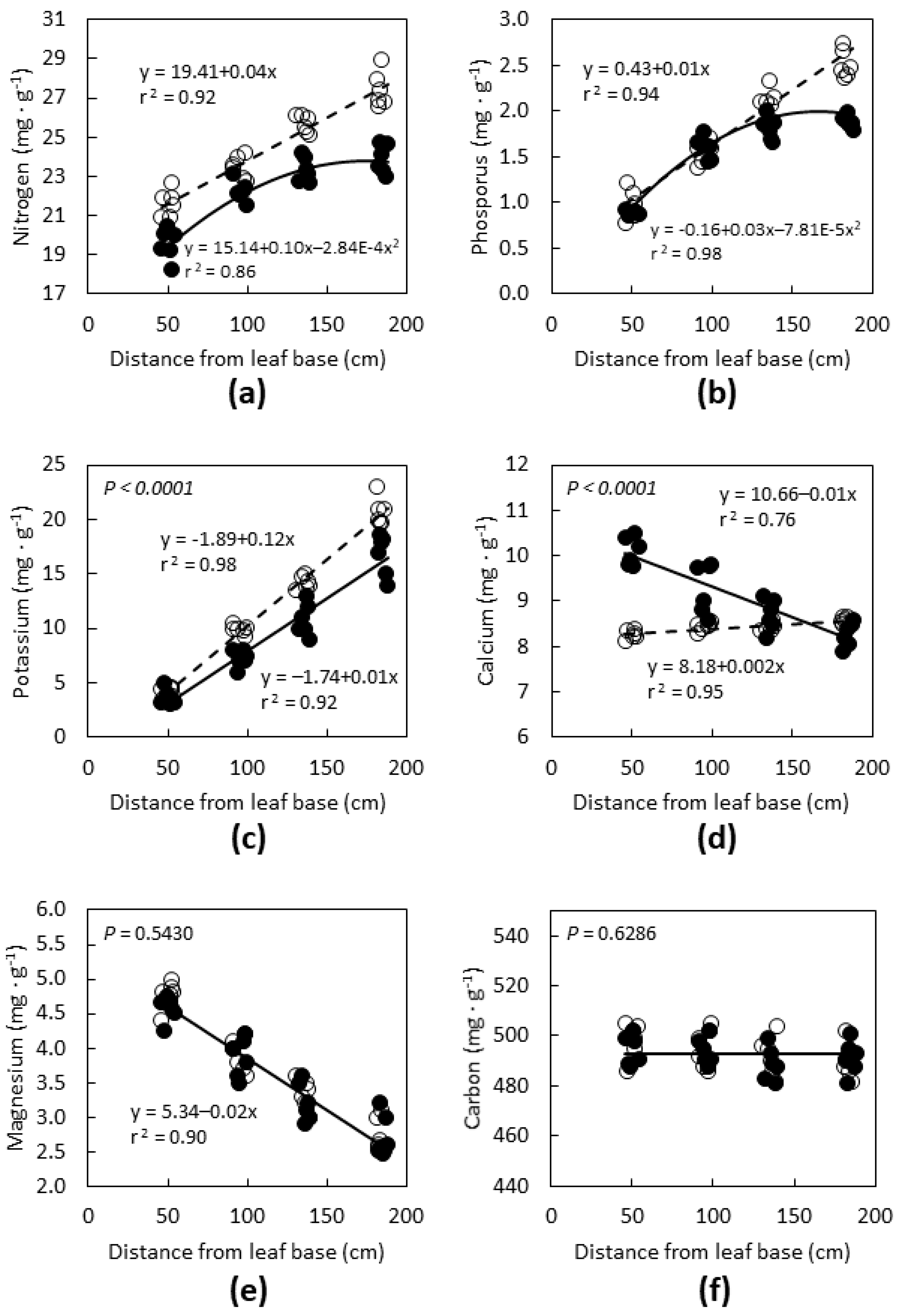
The influence of leaf age and position along the rachis differed among the macronutrients. Leaf age influenced the pattern of N concentration along the
C. micronesica leaf rachis (
Figure 2a). Young leaves exhibited a linear increase in N concentration from basal to terminal leaflets, but old leaves exhibited a quadratic pattern with regard to location along the rachis. Old leaves contained less N, regardless of position along the rachis. The patterns for P were similar to N, except the decline in P concentration with leaf age was only evident in the leaflets closest to the rachis apex (
Figure 2b). Leaf K concentration increased linearly from basal to terminal leaflets, and leaf age influenced the slope (
Figure 2c). Basal leaflets exhibited similar K concentration for the two leaf age categories. Terminal leaflets of old leaves exhibited K of about 15 mg·g
−1, but K of terminal leaflets of young leaves was about 20 mg·g
−1. Leaf Ca expressed a linear relationship with distance along the rachis, and leaf age influenced the slope (
Figure 2d). The leaflets near the rachis apex exhibited Ca concentrations that were similar for the two leaf age categories. However, older leaves exhibited progressively greater relative Ca than young leaves for leaflets near the base of the rachis. Leaf Mg concentration expressed a linear decline with distance from the leaf base, but leaf age did not influence the slope (
Figure 2e). Leaf C concentration was not influenced by position along the rachis for either leaf age (
Figure 2f).
The influence of leaf age and position along the rachis differed among the micronutrients. Leaf Fe declined linearly from basal to terminal leaflets, and leaf age influenced the slope (
Figure 3a). Old leaves contained greater Fe concentration than new leaves, regardless of position along the rachis. Leaf Mn concentration declined linearly with distance from the leaf base, but leaf age did not influence the slope (
Figure 3b). Young leaves exhibited a Zn concentration of about 20 µg·g
−1 regardless of position along the rachis (
Figure 3c). Old leaves exhibited a linear decline in Zn concentration from basal to terminal leaflets, with similar concentrations to the young leaves for the terminal leaflets. The pattern of leaf B concentration and the influence of leaf age on B were similar to those of Zn (
Figure 3d). Position along the rachis did not influence leaf Cu concentration, with a mean of 3 µg·g
−1 for both leaf age categories (
Figure 3e). The variation of Cu concentration among the replications was substantial.
4. Discussion
We have shown that many essential nutrients for plants are unevenly distributed along the rachis of a representative cycad species, and that leaves of different ages exhibited patterns that were dissimilar for some of the elements. These distribution patterns may be influenced by source–sink relations associated with soil nutrient concentrations, the relative mobility of each element, the amount of resorption in the older leaves of the mobile elements, and the amount of accumulation with leaf age of the immobile elements. We added position of sampled leaflets within leaves as a mandatory component of what was recorded and reported for future cycad leaf tissue analyses. Only two cycad leaf nutrient papers that we are aware of used methods that represented the full length of the rachis, with collection at the two extreme positions plus a midpoint position [
8,
15] or collection of all leaflets on each sampled leaf [
16]. One other cycad paper reported the position that was sampled, but the sampling was restricted to leaflets from the middle of the rachis [
17]. The majority of cycad publications including leaf tissue analysis results provide no description of location along the rachis where leaflets were sampled [
18,
19,
20,
21,
22,
23,
24,
25]. Our findings indicate that these past publications without rachis position reported are ambiguous and the methods are not repeatable.
How does general nutritional status of a plant modify the influence of leaflet position on nutrient concentrations? This question remains unanswered. Plants growing in impoverished soils may exhibit greater disparity of nutrient concentrations along a long leaf rachis. Our results indicate that the plants we sampled were not experiencing nutritional stress, as our data were within or greater than the published range for cycads in general [
16,
17,
18,
19,
20,
21,
22,
23,
24,
25] and for this species in particular [
8,
15,
23]. As a minimum, we suggest the soil nutrient status should be measured and reported accurately in future studies [
26]. Regardless, the results indicate that the position of leaflets on a cycad plant’s compound leaf may influence nutrient contents even when soils are not impoverished. Manipulative fertilization studies would best answer this question, where a range in nutritional status is experimentally created within representatives of one or more cycad species.
The individual elements exhibited dissimilar patterns with regard to rachis position and leaf age. Most elements behaved similarly for the two leaf age categories, but some did not. For example, the macronutrients N and P exhibited linear increases in young leaves from basal to terminal leaflets, but quadratic patterns in old leaves whereby greater similarity in concentrations occurred for basal leaflets than terminal leaflets. These patterns were likely created by differential resorption of the elements from the leaflets in old leaves. Leaf B, Ca, and Zn exhibited acute disparity between old and young leaf concentrations with regard to position along the rachis. Leaf Fe, K, Mg, and Mn were influenced by position along the rachis, but the two age categories behaved similarly. Finally, C and Cu were not influenced by position along the rachis for either age category. This highly contrasting behavior of the various macro- and micro-nutrients underscores the mandate to record the position along the rachis for each sampled leaf, and the relative position of sampled leaves within the canopy as an estimate of relative leaf age.
The variability in elemental concentration within an individual leaf was substantial for some elements and minimal for others. The elements exhibiting the greatest differences in concentration among the positions along the rachis were K, Mn, P, and Zn, revealing a 2.5-fold to 4.0-fold disparity for basal and terminal leaflets. These findings reveal that sampling only a portion of the length of a cycad leaf rachis may produce inaccurate results for some elements. For example, sampling at the midpoint of the rachis would provide results close to the true mean for all of the elements that exhibited a linear pattern with relation to position along the rachis, but restricting leaflet sampling to this position would overestimate N and P from older leaves, due to the quadratic model that describes the influence of position along the rachis for these two elements. Sampling leaflets from the base or apex of a cycad leaf would not provide an accurate estimate of the true leaf element concentration for any of the elements except for C and Cu.
Our oldest leaves were similar in appearance to the youngest leaves, as we avoided the oldest leaf flush that contained senescing leaves. A substantial difference in nutrient concentrations between young leaves and senesced leaves has been reported for
Cycas micronesica [
8],
Cycas nitida K.D. Hill & A. Lindstr. [
21], and
Cycas wadei Merrill [
24]. This is a common phenomenon whereby plants recycle nutrients by re-translocating them back to the stems prior to leaf senescence [
27,
28]. However, until the present study, the leaf age at which age-related senescence processes commence was unknown. We have shown that older green leaves with no visible signs of senescence exhibited reduced concentrations of K, N, and P when compared to the youngest leaves. These same older green leaves exhibited increased concentrations of B, Ca, Fe, and Zn when compared to the youngest leaves. Therefore, the modifications in leaf concentrations associated with senescence are initiated well before signs of senescence are visible in an aging
C. micronesica leaf. We suggest that leaf sampling of cycad plants in future studies should be restricted to the youngest fully expanded leaf flush unless the influence of age on response variables is an explicit objective. This would standardize the methods and improve comparisons among species and studies.
We chose to limit shade level and tree height for this initial leaflet position study in order to reduce background variability. More studies are needed to determine if plant size influences element concentrations along the length of leaves, and if incident light levels modify these relations.
The leaf behaviors of
C. micronesica may not conform to those of all other cycad species. There are 335 described cycad species currently accepted [
29]. The variation in mature leaf size is considerable among these species [
4]. Our leaves were approximately 1.9 m in length, and this relatively large leaf size may partly explain the differences in elemental concentrations within the leaf. Many other cycad species, such as
Zamia pygmaea Sims, produce diminutive compound leaves that may not exhibit the same level of heterogeneity. Alternatively, other cycad species such as
Cycas multipinnata C.J. Chen & S.Y. Yang produce leaves with a rachis more than twice as long as those of
C. micronesica, and may exhibit even greater within-leaf variability. These issues underscore that position of leaflet sampling within a cycad leaf must be recorded and reported for all future cycad leaf studies unless preliminary studies reveal no influence of leaflet position on the response variables of interest.
Our interest was in clarifying sampling protocols for cycad leaf research, but our results reveal that any angiosperm or gymnosperm with large complex leaves may exhibit heterogeneous nutrient concentrations among the microsites of the leaf. Indeed, nutrient concentrations have been shown to vary among leaflets of palmate [
30] and pinnate [
31] palm leaves. For species that produce leaves so large that sampling entire leaves is prohibitive, preliminary studies designed to understand the spatial distribution of elements within leaves are arguably mandatory for leaf nutrient research methods to be justifiable.