Postharvest UV-C Treatment, Followed by Storage in a Continuous Low-Level Ethylene Atmosphere, Maintains the Quality of ‘Kensington Pride’ Mango Fruit Stored at 20 °C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Produce
2.2. UV-C Treatment and Storage Conditions
2.3. Determination of Fruit Quality Attributes
2.3.1. Weight Loss
2.3.2. Colour
2.3.3. Firmness
2.3.4. Ethylene Production
2.3.5. Respiration Rate
2.3.6. Total Soluble Solids (TSS) and Titratable Acidity (TA)
2.4. Chemical Analysis and Antioxidant Activity Evaluation
2.4.1. Total Chlorophyll
2.4.2. Total Phenolic Content
2.4.3. Total Antioxidant Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Weight Loss
3.2. Firmness
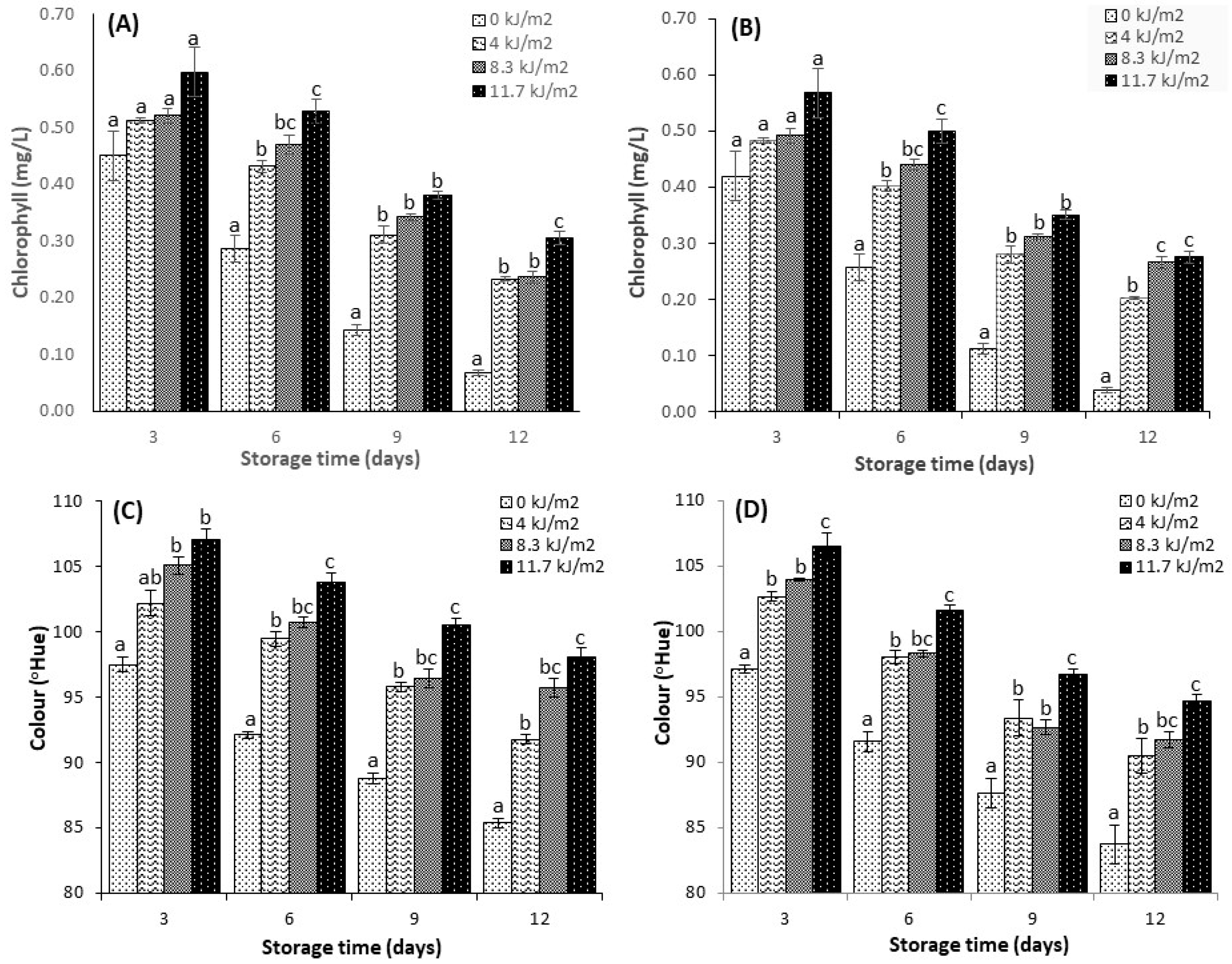
3.3. Colour
3.4. Total Chlorophyll
3.5. Ethylene Production
3.6. Respiration Rate
3.7. TSS and TA
3.8. Total Phenolic Content
3.9. Total Antioxidant Activity
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Baldwin, E.A.; Burns, J.K.; Kazokas, W.; Brecht, J.K.; Hagenmaier, R.D.; Bender, R.J.; Pesis, E. Effect of two edible coatings with different permeability characteristics on mango (Mangifera indica L.) ripening during storage. Postharvest Biol. Technol. 1999, 17, 215–226. [Google Scholar] [CrossRef]
- Narayana, C.K.; Pal, R.K.; Roy, S.K. Effect of pre-storage treatments and temperature regimes on shelf-life and respiratory behaviour of ripe baneshan mango. J. Food Sci. Technol. 1996, 33, 79–82. [Google Scholar]
- Singh, Z.; Singh, R.K.; Sane, V.A.; Nath, P. Mango-postharvest biology and biotechnology. Crit. Rev. Plant. Sci. 2013, 32, 217–236. [Google Scholar] [CrossRef]
- Ortega-Zaleta, D.; Yahia, E.M. Tolerance and quality of mango fruit exposed to controlled atmospheres at high temperatures. Postharvest Biol. Technol. 2000, 20, 195–201. [Google Scholar] [CrossRef]
- Thomas, P.; Joshi, M.R. Reduction of chilling injury in ripe alphonso mango fruit in cold storage by temperature conditioning. Int. J. Food Sci. Technol. 1988, 23, 447–455. [Google Scholar] [CrossRef]
- Ketsa, S.; Chidtragool, S.; Lurie, S. Prestorage heat treatment and poststorage quality of mango fruit. HortScience 2000, 35, 247–249. [Google Scholar]
- Larrigaudière, C.; Pons, J.; Torres, R.; Usall, J. Storage performance of clementines treated with hot water, sodium carbonate and sodium bicarbonate dips. J. Hortic. Sci. Biotechnol. 2002, 77, 314–319. [Google Scholar] [CrossRef]
- Charles, M.T.; Benhamou, N.; Arul, J. Physiological basis of uv-c induced resistance to Botrytis cinerea in tomato fruit—III. Ultrastructural modifications and their impact on fungal colonization. Postharvest Biol. Technol. 2008, 47, 27–40. [Google Scholar] [CrossRef]
- Guerrero-Beltrán, J.A.; Barbosa-Cánovas, G.V. Reviews: Advantages and limitations on processing foods by uv light. Food Sci. Technol. Int. 2004, 10, 137–147. [Google Scholar] [CrossRef]
- Hemmaty, S.; Moallemi, N.; Naseri, L. Effect of uv-c radiation and hot water on the calcium content and postharvest quality of apples. Span. J. Agric. Res. 2007, 5, 559–568. [Google Scholar] [CrossRef]
- Pristijono, P.; Papoutsis, K.; Scarlett, C.J.; Bowyer, M.C.; Vuong, Q.V.; Stathopoulos, C.E.; Golding, J.B. Postharvest uv-c treatment combined with 1-methylcyclopropene (1-mcp), followed by storage in continuous low-level ethylene atmosphere, improves the quality of tomatoes. J. Hortic. Sci. Biotechnol. 2017, 92, 521–529. [Google Scholar] [CrossRef]
- Maharaj, R.; Arul, J.; Nadeau, P. Effect of photochemical treatment in the preservation of fresh tomato (Lycopersicon esculentum cv. Capello) by delaying senescence. Postharvest Biol. Technol. 1999, 15, 13–23. [Google Scholar] [CrossRef]
- D’hallewin, G.; Schirra, M.; Manueddu, E.; Piga, A.; Ben-Yehoshua, S. Scoparone and scopoletin accumulation and ultraviolet-c induced resistance to postharvest decay in oranges as influenced by harvest date. J. Am. Soc. Hortic. Sci. 1999, 124, 702–707. [Google Scholar]
- Cantos, E.; Espin, J.C.; Tomas-Barberan, F.A. Postharvest stilbene-enrichment of red and white table grape varieties using uv-c irradiation pulses. J. Agric. Food Chem. 2002, 50, 6322–6329. [Google Scholar] [CrossRef] [PubMed]
- González-Aguilar, G.A.; Wang, C.Y.; Buta, J.G.; Krizek, D.T. Use of uv-c irradiation to prevent decay and maintain postharvest quality of ripe ‘tommy atkins’ mangoes. Int. J. Food Sci. Technol. 2001, 36, 767–773. [Google Scholar] [CrossRef]
- Gonzalez-Aguilar, G.; Wang, C.Y.; Buta, G.J. Uv-c irradiation reduces breakdown and chilling injury of peaches during cold storage. J. Sci. Food Agric. 2004, 84, 415–422. [Google Scholar] [CrossRef]
- Pristijono, P.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V.; Stathopoulos, C.E.; Golding, J.B. Combined postharvest uv-c and 1-methylcyclopropene (1-mcp) treatment, followed by storage continuously in low level of ethylene atmosphere improves the quality of tahitian limes. J. Food Sci. Technol. 2018, 55, 2467–2475. [Google Scholar] [CrossRef]
- Wills, R.; McGlasson, B.; Graham, D.; Joyce, D. Postharvest an Introduction to the Physiology and Handling of Fruits, Vegetables and Ornamentals, 4th ed.; University of New South Wales Press Ltd.: Sydney, Australia, 1998; p. 262. [Google Scholar]
- Wills, R.B.H.; Warton, M.A.; Ku, V.V.V. Ethylene levels associated with fruit and vegetables during marketing. Aust. J. Exp. Agric. 2000, 40, 465–470. [Google Scholar]
- Cosme Silva, G.M.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. Cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymesin isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LTW-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Liu, C.; Cai, L.; Han, X.; Ying, T. Temporary effect of postharvest uv-c irradiation on gene expression profile in tomato fruit. Gene 2011, 486, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J. Role of phytoalexins and other antimicrobial compounds from fruits and vegetables in postharvest disease resistance. In Photochemistry of Fruit and Vegetables; Thomas-Barberan, F.A., Robins, R.J., Eds.; Oxford, Science Publ.: Oxford, UK, 1997; pp. 221–241. [Google Scholar]
- Tiecher, A.; de Paula, L.A.; Chaves, F.C.; Rombaldi, C.V. Uv-c effect on ethylene, polyamines and the regulation of tomato fruit ripening. Postharvest Biol. Technol. 2013, 86, 230–239. [Google Scholar] [CrossRef]
- Liu, L.H.; Zabaras, D.; Bennett, L.E.; Aguas, P.; Woonton, B.W. Effects of uv-c, red light and sun light on the carotenoid content and physical qualities of tomatoes during post-harvest storage. Food Chem. 2009, 115, 495–500. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Escalona, V.H.; Robles, P.A.; Martínez-Hernández, G.B.; Artés, F. Effect of uv-c radiation on quality of minimally processed spinach leaves. J. Sci. Food Agric. 2009, 89, 414–421. [Google Scholar] [CrossRef]
- Costa, L.; Vicente, A.R.; Civello, P.M.; Chaves, A.R.; Martinez, G.A. Uv-c treatment delays postharvest senescence in broccoli florets. Postharvest Biol. Technol. 2006, 39, 204–210. [Google Scholar] [CrossRef]
- Pongprasert, N.; Sekozawa, Y.; Sugaya, S.; Gemma, H. A novel postharvest uv-c treatment to reduce chilling injury (membrane damage, browning and chlorophyll degradation) in banana peel. Sci. Hortic. 2011, 130, 73–77. [Google Scholar] [CrossRef]
- Imaizumi, T.; Yamauchi, M.; Sekiya, M.; Shimonishi, Y.; Tanaka, F. Responses of phytonutrients and tissue condition in persimmon and cucumber to postharvest uv-c irradiation. Postharvest Biol. Technol. 2018, 145, 33–40. [Google Scholar] [CrossRef]
- Stevens, C.; Liu, J.; Khan, V.A.; Lu, J.Y.; Wilson, C.L.; Igwegbe, E.C.K.; Kabwe, M.K.; Chalutz, E.; Droby, S. Application of hormetic uv-c for delayed ripening and reduction of rhizopus soft rot in tomatoes: The effect of tomatine on storage rot development. J. Phytopathol. 1998, 146, 211. [Google Scholar] [CrossRef]
- Wills, R.B.H.; Golding, J.B. Postharvest—An Introduction to the Physiology and Handling of Fruit and Vegetables, 6th ed.; UNSW Press: Sydney, Australia, 2016. [Google Scholar]
- Vicente, A.R.; Pineda, C.; Lemoine, L.; Civello, P.M.; Martinez, G.A.; Chaves, A.R. Uv-c treatments reduce decay, retain quality and alleviate chilling injury in pepper. Postharvest Biol. Technol. 2005, 35, 69–78. [Google Scholar] [CrossRef]
- Daiuto, E.R.; Vieites, R.L.; Remocoldi, M.A.; Carvalho, L.R.; Fumes, J.G.F. Postharvest of ‘hass’ avocados submitted to uv-c radiation. Rev. Colomb. Cienc. Hortic. 2013, 7, 149–160. [Google Scholar] [CrossRef]
- Jagadeesh, S.; Charles, M.; Gariepy, Y.; Goyette, B.; Raghavan, G.; Vigneault, C. Influence of postharvest uv-c hormesis on the bioactive components of tomato during post-treatment handling. Food Bioprocess Technol. 2011, 4, 1463–1472. [Google Scholar] [CrossRef]
- Dong, Y.H.; Mitra, D.; Kootstra, A.; Lister, C.; Lancaster, J. Postharvest stimulation of skin color in royal gala apple. J. Am. Soc. Hortic. Sci. 1995, 120, 95–100. [Google Scholar]
- Gonzalez-Aguilar, G.A.; Zavaleta-Gatica, R.; Tiznado-Hernandez, M.E. Improving postharvest quality of mango ‘haden’ by uv-c treatment. Postharvest Biol. Technol. 2007, 45, 108–116. [Google Scholar] [CrossRef]
- Zhonggao, J.; Jiechao, L.; Hui, L.; Chunling, Z.; Sixin, W.; Gongming, Y. Effect of postharvest uv-c irradiation on nutrition properties and antioxidant activity of sweet cherry during storage. J. Chin. Inst. Food Sci. Technol. 2017, 170–178. [Google Scholar]
- Cisneros-Zevallos, L. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Villegas-Ochoa, M.A.; Martínez-Téllez, M.A.; Gardea, A.A.; Ayala-Zavala, J.F. Improving antioxidant capacity of fresh-cut mangoes treated with uv-c. J. Food Sci. 2007, 72, S197–S202. [Google Scholar] [CrossRef]
- Rocha Ribeiro, S.M.; Queiroz, J.H.; Lopes Ribeiro de Queiroz, M.E.; Campos, F.M.; Pinheiro Sant’Ana, H.M. Antioxidant in mango (Mangifera indica L.) pulp. Plant Foods Hum. Nutr. 2007, 62, 13–17. [Google Scholar] [CrossRef]
- Liu, D.; Shi, J.; Colina Ibarra, A.; Kakuda, Y.; Jun Xue, S. The scavenging capacity and synergistic effects of lycopene, vitamin e, vitamin c, and β-carotene mixtures on the dpph free radical. LWT-Food Sci. Technol. 2008, 41, 1344–1349. [Google Scholar] [CrossRef]
- Zanfini, A.; Corbini, G.; La Rosa, C.; Dreassi, E. Antioxidant activity of tomato lipophilic extracts and interactions between carotenoids and alpha-tocopherol in synthetic mixtures. Lwt-Food Sci. Technol. 2010, 43, 67–72. [Google Scholar] [CrossRef]
| Storage/UV-C (kJ m−2) | Weight Loss (%) | Firmness (N) | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 d | 6 d | 9 d | 12 d | 3 d | 6 d | 9 d | 12 d | |
| <0.005 μL L−1 Ethylene | ||||||||
| 0 | 0.9 a | 1.2 a | 1.2 a | 1.4 a | 27.4 a | 24.4 a | 23.4 a | 20.7 a |
| 4 | 1.0 a | 1.2 a | 1.3 a | 1.5 a | 26.1 a | 25.7 a | 25.5 a | 22.6 a |
| 8.3 | 1.1 a | 1.3 a | 1.4 a | 1.5 a | 29.0 a | 27.0 a | 26.4 ab | 25.6 b |
| 11.7 | 1.1 a | 1.3 a | 1.4 a | 1.6 a | 32.6 b | 30.8 b | 30.3 c | 28.8 c |
| 0.1 μL L−1 Ethylene | ||||||||
| 0 | 1.1 a | 1.3 a | 1.4 a | 1.6 a | 22.9 a | 22.0 a | 21.6 a | 21.0 a |
| 4 | 0.9 a | 1.2 a | 1.4 a | 1.6 a | 24.4 a | 23.4 b | 23.1 a | 22.5 a |
| 8.3 | 1.0 a | 1.4 a | 1.5 a | 1.7 a | 25.0 a | 24.5 b | 24.3 ab | 23.9 b |
| 11.7 | 1.2 a | 1.5 a | 1.6 a | 1.7 a | 30.7 b | 28.3 b | 27.6 b | 26.0 b |
| Storage/UV-C (kJ m−2) | Respiration Rate (mL CO2 kg−1 h−1) | Ethylene Production (µL C2H4 kg−1 h−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 d | 6 d | 9 d | 12 d | 3 d | 6 d | 9 d | 12 d | |
| <0.005 μL L−1 Ethylene | ||||||||
| 0 | 47.71 a | 51.55 a | 68.47 a | 72.96 a | 0.029 a | 0.022 a | 0.023 a | 0.020 a |
| 4 | 48.48 a | 52.04 a | 66.86 a | 72.06 a | 0.024 a | 0.017 b | 0.015 b | 0.013 b |
| 8.3 | 38.65 b | 41.54 b | 55.19 b | 66.67 b | 0.025 a | 0.017 b | 0.015 b | 0.011 b |
| 11.7 | 39.73 b | 41.83 b | 53.58 b | 67.56 b | 0.026 a | 0.016 b | 0.014 b | 0.010 b |
| 0.1 μL L−1 Ethylene | ||||||||
| 0 | 54.37 a | 61.12 a | 67.74 a | 72.95 a | 0.062 a | 0.052 a | 0.052 a | 0.042 a |
| 4 | 59.22 b | 65.60 b | 67.83 a | 68.83 b | 0.047 b | 0.045 a | 0.044 a | 0.038 a |
| 8.3 | 61.14 b | 63.59 b | 64.82 b | 67.31 b | 0.040 b | 0.033 b | 0.034 b | 0.027 b |
| 11.7 | 50.97 c | 54.71 c | 57.14 c | 60.41 c | 0.029 c | 0.029 b | 0.031 b | 0.031 b |
| Storage/UV-C (kJ m−2) | TSS (°Brix) | TA (% Citric Acid) | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 d | 6 d | 9 d | 12 d | 3 d | 6 d | 9 d | 12 d | |
| <0.005 μL L−1 Ethylene | ||||||||
| 0 | 14.6 a | 15.0 a | 16.4 a | 17.1 a | 0.273 a | 0.141 a | 0.130 a | 0.109 a |
| 4 | 13.0 b | 14.4 a | 14.0 b | 14.7 b | 0.342 b | 0.194 a | 0.178 b | 0.146 b |
| 8.3 | 13.0 b | 13.8 ab | 14.0 b | 13.9 c | 0.338 b | 0.179 a | 0.159 bc | 0.119 ab |
| 11.7 | 12.5 b | 13.2 c | 13.0 c | 13.1 d | 0.501 c | 0.283 b | 0.244 c | 0.232 c |
| 0.1 μL L−1 Ethylene | ||||||||
| 0 | 14.4 a | 16.6 a | 16.6 a | 17.0 a | 0.165 a | 0.129 a | 0.117 a | 0.093 a |
| 4 | 14.1 ab | 14.1 b | 15.3 b | 16.2 a | 0.170 a | 0.142 a | 0.132 b | 0.113 b |
| 8.3 | 13.7 ab | 13.7 c | 14.3 c | 14.4 b | 0.229 a | 0.172 a | 0.153 b | 0.115 b |
| 11.7 | 13.0 b | 13.3 d | 13.4 d | 14.2 b | 0.551 b | 0.329 b | 0.255 b | 0.207 c |
| Storage/UV-C (kJ m−2) | TPC (mg Gallic Acid Equiv/g FW | Total Antioxidant Activity (% DPPH Scavenging Activity) | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 d | 6 d | 9 d | 12 d | 3 d | 6 d | 9 d | 12 d | |
| <0.005 μL L−1 Ethylene | ||||||||
| 0 | 0.533 a | 0.583 a | 0.603 a | 0.656 a | 18.23 a | 19.74 a | 20.88 a | 26.41 a |
| 4 | 0.546 a | 0.572 a | 0.681 ab | 0.735 ab | 18.09 a | 19.43 a | 19.36 a | 22.23 a |
| 8.3 | 0.608 a | 0.627 a | 0.733 b | 0.735 ab | 19.26 a | 20.60 a | 21.64 a | 28.16 ab |
| 11.7 | 0.621 a | 0.676 a | 0.826 c | 0.814 b | 20.17 a | 23.88 a | 25.46 b | 33.88 b |
| 0.1 μL L−1 Ethylene | ||||||||
| 0 | 0.515 a | 0.551 a | 0.583 a | 0.631 a | 19.16 a | 19.57 a | 20.72 a | 21.91 a |
| 4 | 0.514 a | 0.547 a | 0.642 ab | 0.698 ab | 20.09 a | 20.23 a | 19.33 a | 26.10 b |
| 8.3 | 0.568 a | 0.588 a | 0.694 b | 0.735 ab | 19.86 a | 20.23 a | 21.47 a | 27.74bc |
| 11.7 | 0.586 a | 0.642 a | 0.778 b | 0.780 b | 21.57 a | 23.55 a | 25.32 a | 32.64 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pristijono, P.; Golding, J.B.; Bowyer, M.C. Postharvest UV-C Treatment, Followed by Storage in a Continuous Low-Level Ethylene Atmosphere, Maintains the Quality of ‘Kensington Pride’ Mango Fruit Stored at 20 °C. Horticulturae 2019, 5, 1. https://doi.org/10.3390/horticulturae5010001
Pristijono P, Golding JB, Bowyer MC. Postharvest UV-C Treatment, Followed by Storage in a Continuous Low-Level Ethylene Atmosphere, Maintains the Quality of ‘Kensington Pride’ Mango Fruit Stored at 20 °C. Horticulturae. 2019; 5(1):1. https://doi.org/10.3390/horticulturae5010001
Chicago/Turabian StylePristijono, Penta, John B. Golding, and Michael C. Bowyer. 2019. "Postharvest UV-C Treatment, Followed by Storage in a Continuous Low-Level Ethylene Atmosphere, Maintains the Quality of ‘Kensington Pride’ Mango Fruit Stored at 20 °C" Horticulturae 5, no. 1: 1. https://doi.org/10.3390/horticulturae5010001
APA StylePristijono, P., Golding, J. B., & Bowyer, M. C. (2019). Postharvest UV-C Treatment, Followed by Storage in a Continuous Low-Level Ethylene Atmosphere, Maintains the Quality of ‘Kensington Pride’ Mango Fruit Stored at 20 °C. Horticulturae, 5(1), 1. https://doi.org/10.3390/horticulturae5010001






