Plant Growth Promoting Effects of Nepalese Sweet Potato Endophytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Evaluation of Plant Growth Promoting Properties
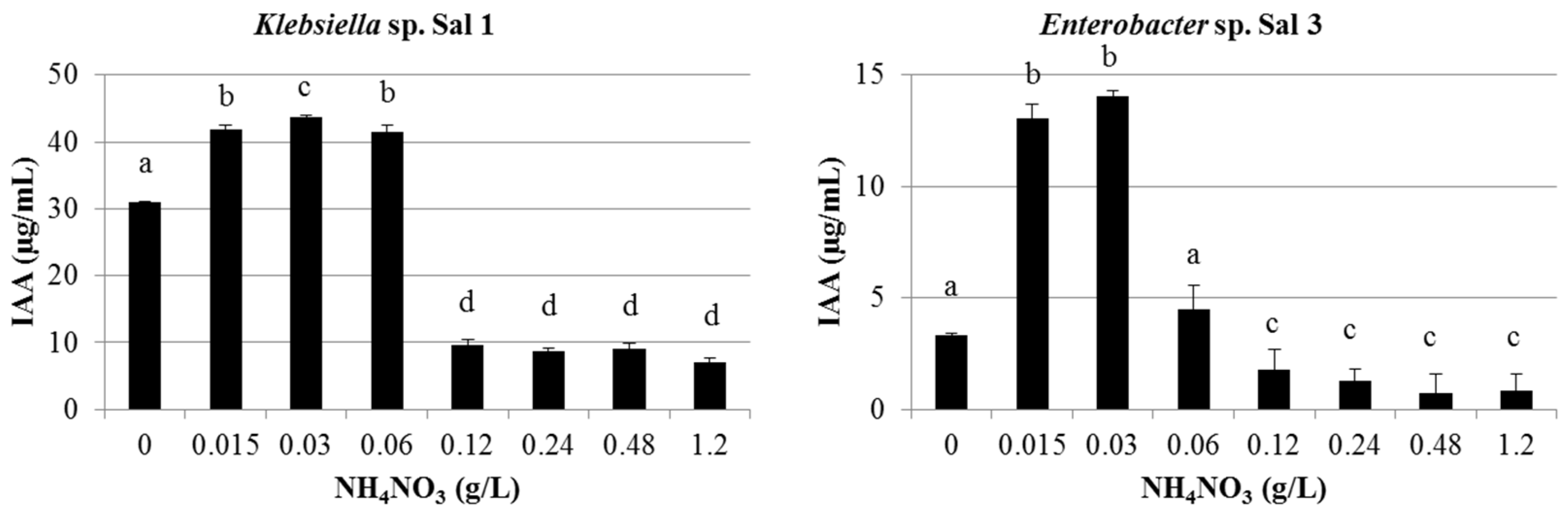
2.2.1. IAA Production
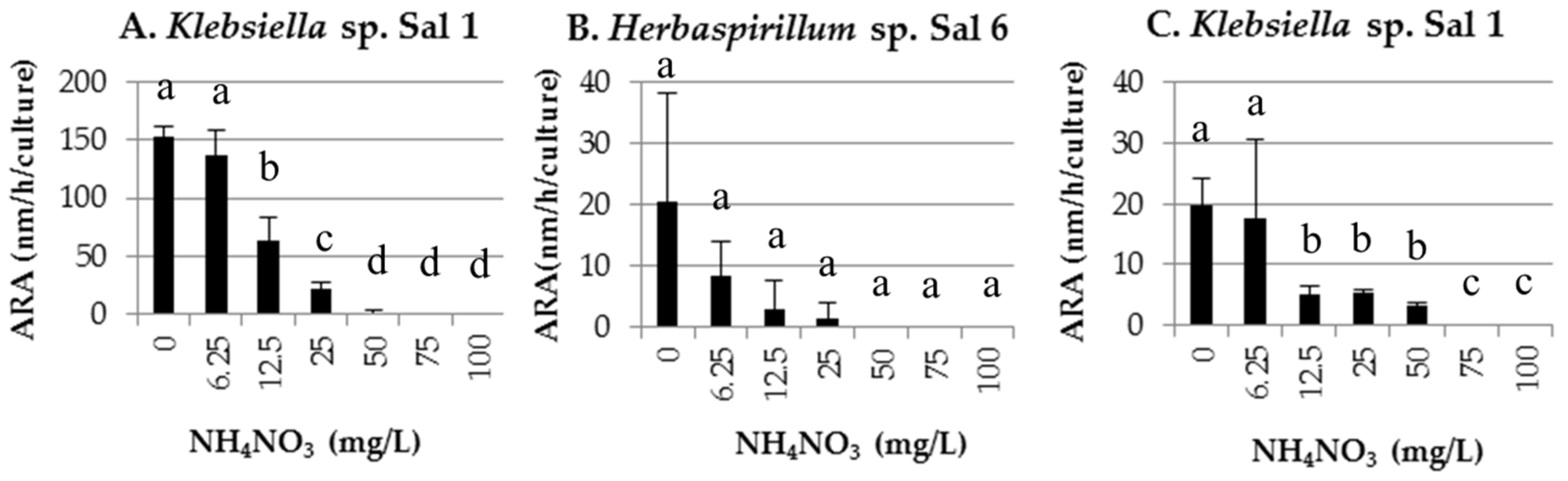
2.2.2. Nitrogen Fixation Activity
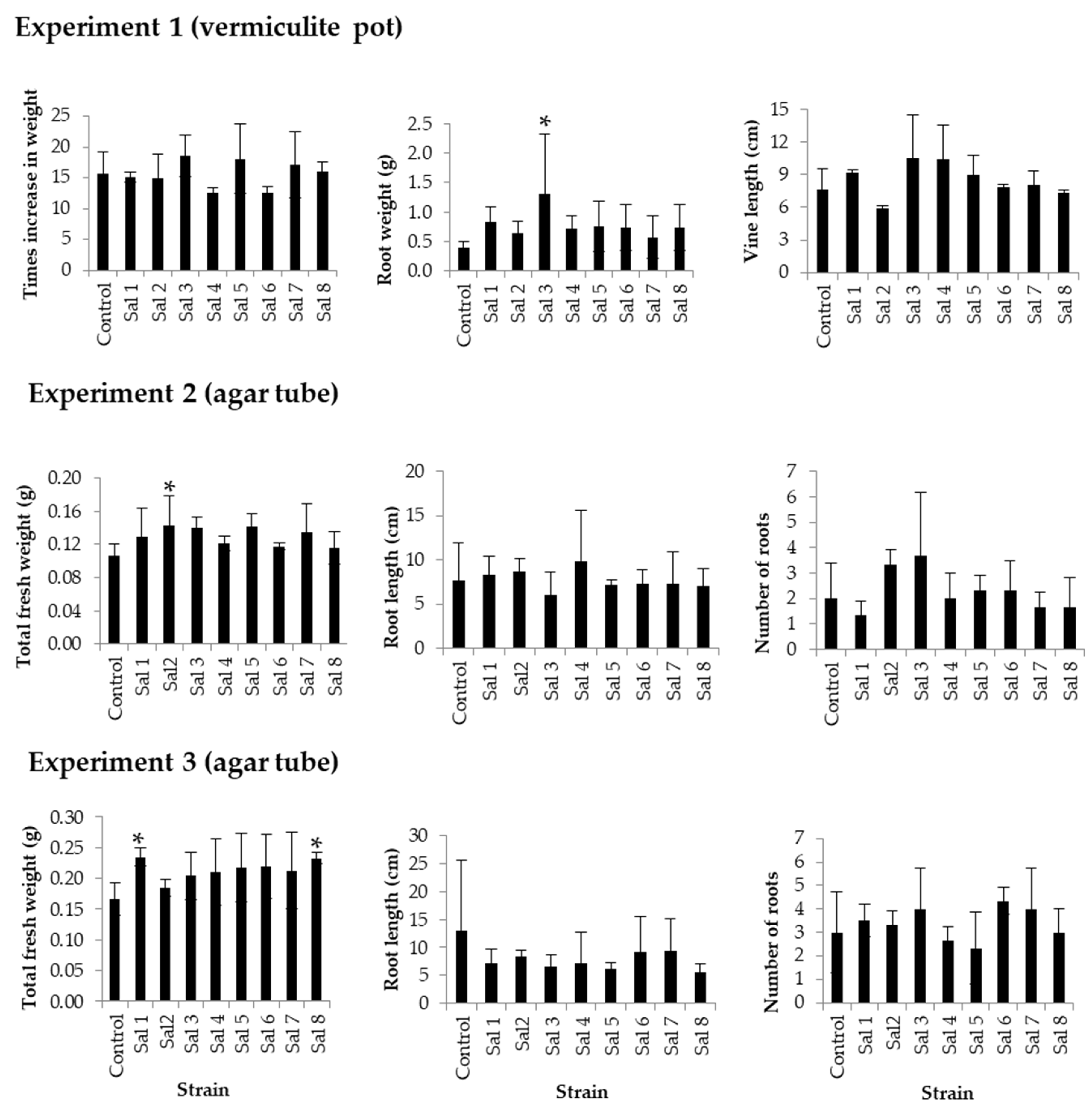
2.3. Effect of Inoculation on Sweet Potato
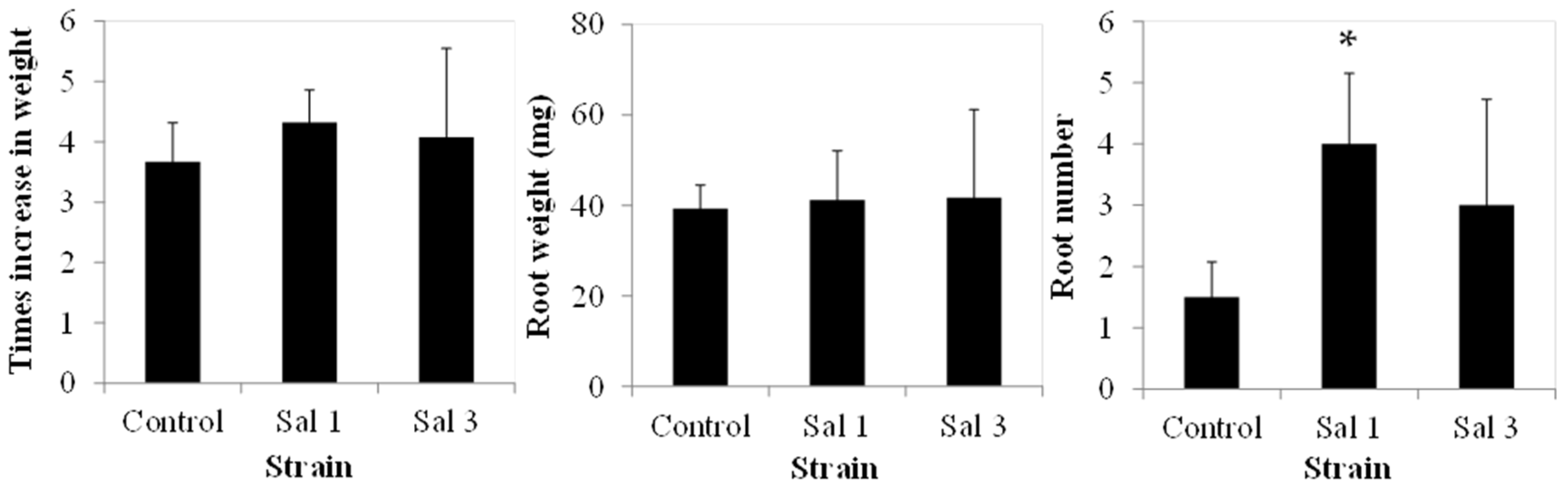
2.4. Effect of Inoculation on Tomato
2.5. Statistical Analysis
3. Results
3.1. IAA Production
3.2. Nitrogen Fixation Activity
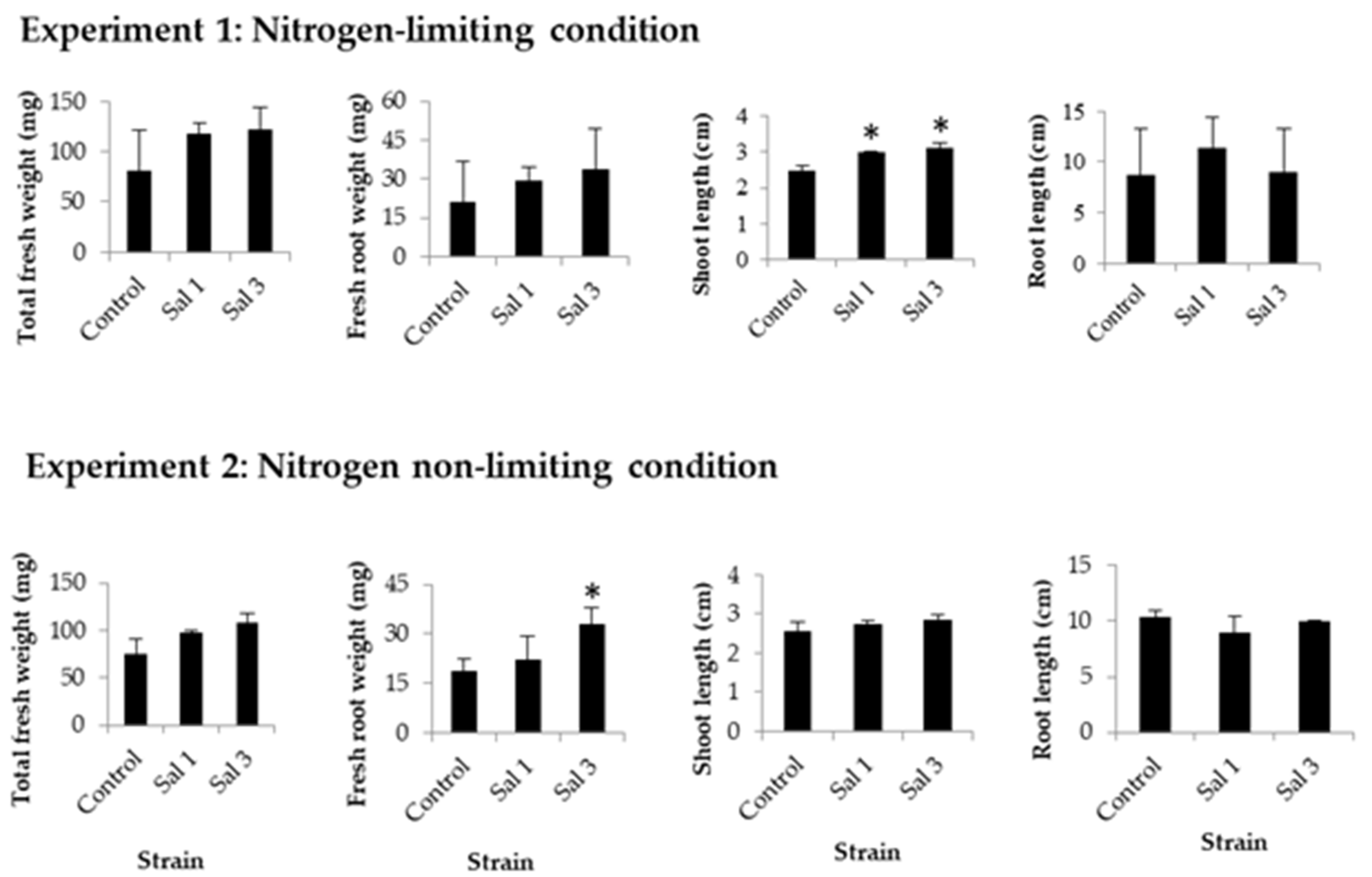
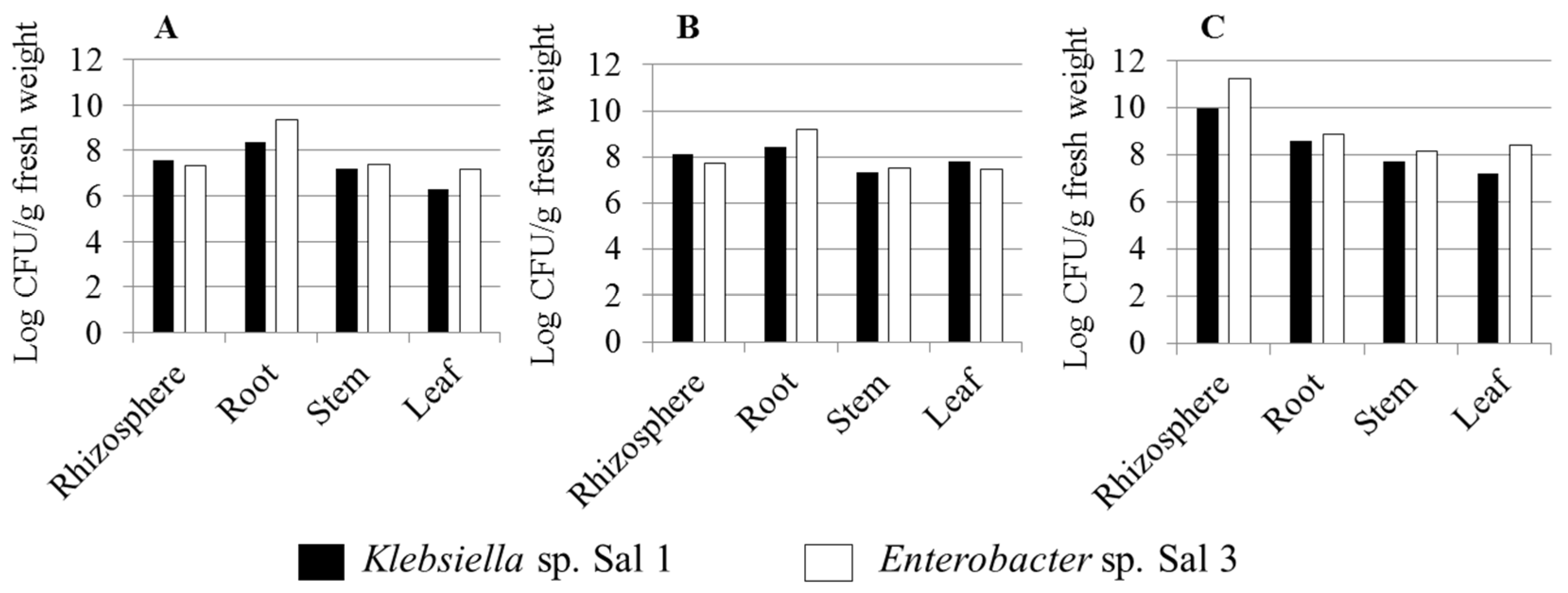
3.3. Effect of Inoculation on Sweet Potato
3.4. Effect of Inoculation on Tomatoes
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Rosenblueth, M.; Martínez-Romero, E. Bacterial endophytes and their interactions with hosts. Am. Phytopathol. Soc. 2006, 19, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Glick, B.R. Mechanisms used by plant growth-promoting bacteria. In Bacteria in Agrobiology: Plant Nutrient Management; Maheshwari, D.K.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 17–47. [Google Scholar]
- Gamalero, E.; Glick, B.R. Plant ethylene modulation by beneficial bacteria. Plant Physiol. 2015, 169, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S.L.; Joubert, P.M.; Doty, S.L. Bacterial endophyte colonization and distribution within plants. Microorganisms 2017, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Hill, W.A.; Dodo, H.; Hahn, S.K.; Mulongoy, K.; Adeyeye, S.O. Sweet potato root and biomass production with and without nitrogen fertilization. Agron. J. 1990, 82, 1120–1122. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda, M.C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Yonebayashi, K.; Katsumi, N.; Nishi, T.; Okazaki, M. Activation of nitrogen-fixing endophytes is associated with the tuber growth of sweet potato. J. Mass Spectrom. 2014, 3, A0032. [Google Scholar] [CrossRef]
- Khan, Z.; Doty, S.L. Characterization of bacterial endophytes of sweet potato plants. Plant Soil 2009, 322, 197–207. [Google Scholar] [CrossRef]
- Dawwam, G.E.; Elbeltagy, A.; Emara, H.M.; Abbas, I.H.; Hassan, M.M. Beneficial effect of plant growth promoting bacteria isolated from the roots of potato plant. Ann. Agric. Sci. 2013, 58, 195–201. [Google Scholar] [CrossRef]
- Souza, R.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, D.; Kaneto, M.; Itoh, K.; Suyama, K.; Gaihre, Y.K.; Pokharel, B. Genetic diversity of soybean-nodulating rhizobia in Nepal in relation to climate and soil properties. Plant Soil 2012, 357, 131–145. [Google Scholar] [CrossRef]
- Adhikari, D.; Itoh, K.; Suyama, K. Genetic diversity of common bean (Phaseolus vulgaris L.) nodulating rhizobia in Nepal. Plant Soil 2013, 368, 341–353. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Gowdaman, V.; Prabagaran, S.R. Culturable and culture-independent bacterial diversity and the prevalence of cold-adapted enzymes from the Himalayan mountain ranges of India and Nepal. Microb. Ecol. 2015, 69, 472–491. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.R.; Dangi, S.; Dhungana, S.A.; Itoh, K. Diversity and plant growth promoting ability of culturable endophytic bacteria in Nepalese sweet potato. Adv. Microbiol. 2018, 8, 734–761. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.A. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Elbeltagy, A.; Nishioka, K.; Sato, T.; Suzuki, H.; Ye, B.; Hamada, T.; Isawa, T.; Mitsui, H.; Minamisawa, K. Endophytic colonization and in planta isolated from wild rice species. Appl. Environ. Microbiol. 2001, 67, 5285–5293. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. J. Plant Physiol. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Othman, R.; Naher, U.A.; Yusoff, S.Z. Effect of urea-N on growth and indoleacetic acid production of Stenotrophomonas maltophilia (Sb16) isolated from rice growing soils in Malaysia. Chil. J. Agric. Res. 2013, 73, 187–192. [Google Scholar] [CrossRef]
- Yin, T.T.; Pin, U.L.; Ghazali, A.H. Influence of external nitrogen on nitrogenase enzyme activity and auxin production in Herbaspirillum seropedicae (Z78). Trop. Life Sci. Res. 2015, 26, 101–110. [Google Scholar]
- Raut, V.; Shaikh, I.; Naphade, B.; Prasar, K.; Adhapure, N. Plant growth promotion using microbial IAA producers inconjunction with Azolla: A novel approach. Chem. Biol. Technol. Agric. 2017, 4. [Google Scholar] [CrossRef]
- Ali, B.; Sabri, A.N.; Ljung, K.; Hasnain, S. Auxin production by plant associated bacteria: Impact on endogenous IAA content and growth of Triticum aestivum L. Lett. Appl. Microbiol. 2009, 48, 542–547. [Google Scholar] [CrossRef]
- Dias, A.C.F.; Costa, F.E.C.; Andreote, F.D.; Lacava, P.T.; Teixeira, M.A.; Assumpcao, L.C.; Araujo, W.L.; Azevedo, J.L.; Melo, I.S. Isolation of Micropropagated strawberry endophytic bacteria and assessment of their potential for plant growth promotion. World J. Microbiol. Biotechnol. 2009, 25, 189–195. [Google Scholar] [CrossRef]
- Egamberdieva, D. Indole-acetic acid production by root associated bacteria and its role in plant growth and development. In Auxins: Structure, Biosynthesis and Functions; Keller, A.H., Fallon, M.D., Eds.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012; pp. 103–122. [Google Scholar]
- Haahtela, K.; Laakso, T.; Nurmiaho-Lassila, E.L.; Korhonen, T.K. Effects of inoculation of Poapratensis and Triticumaestivum with root-associated, N2-fixing Klebsiella, Enterobacter and Azospirillum. Plant Soil 1988, 106, 239–248. [Google Scholar] [CrossRef]
- Lin, L.; Li, Z.; Hu, C.; Zhang, X.; Chang, S.; Yang, L.; Li, Y.; An, Q. Plant growth-promoting nitrogen-fixing Enterobacteria are in association with sugarcane plants growing in Guangxi, China. Microbes Environ. 2012, 27, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Gravel, V.; Antoun, H.; Tweddell, R.J. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonasputida or Trichodermaatroviride: Possible role of indole acetic acid (IAA). Soil Biol. Biochem. 2007, 39, 1968–1977. [Google Scholar] [CrossRef]
- Sharma, A.; Johri, B.N. Growth promoting influence of siderophore-producing Pseudomonas Strains GRP3A and PRS9 in maize (Zeamays L.) under iron limiting conditions. Microbiol. Res. 2003, 158, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.E.; Burlingham, S.K. Production of plant growth substances by Azotobacterchroococcum. J. Gen. Microbiol. 1968, 53, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, D.E.; Refier, D.A.; Gordon, M.P. Cytokinin production by Agrobacterium and Pseudomonas spp. J. Bacteriol. 1987, 169, 4242–4248. [Google Scholar] [CrossRef]
- Plazinski, J.; Rolfe, B.G. Analysis of the pectolytic activity of Rhizobium and Azospirillum strains isolated Trifoliumrepens. J. Plant Physiol. 1985, 120, 181–187. [Google Scholar] [CrossRef]
- Compant, S.; Clement, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef] [Green Version]


| Strain | * Most Similar Genus | Class | Accession Number |
|---|---|---|---|
| Sal 1 | Klebsiella sp. | Gammaproteobacteria | LC389410 |
| Sal 2 | Flavobacterium sp. | Flavobacteriia | LC389415 |
| Sal 3 | Enterobacter sp. | Gammaproteobacteria | LC389433 |
| Sal 4 | Rhizobium sp. | Alphaproteobacteria | LC389434 |
| Sal 5 | Stenotrophomonas sp. | Gammaproteobacteria | LC389439 |
| Sal 6 | Herbasprillum sp. | Betaproteobacteria | LC389442 |
| Sal 7 | Agrobacterium sp. | Alphaproteobacteria | LC389443 |
| Sal 8 | Microbacterium sp. | Actinobacteria | LC389445 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari Dhungana, S.; Adachi, F.; Hayashi, S.; Raj Puri, R.; Itoh, K. Plant Growth Promoting Effects of Nepalese Sweet Potato Endophytes. Horticulturae 2018, 4, 53. https://doi.org/10.3390/horticulturae4040053
Adhikari Dhungana S, Adachi F, Hayashi S, Raj Puri R, Itoh K. Plant Growth Promoting Effects of Nepalese Sweet Potato Endophytes. Horticulturae. 2018; 4(4):53. https://doi.org/10.3390/horticulturae4040053
Chicago/Turabian StyleAdhikari Dhungana, Sabitri, Fumihiko Adachi, Shohei Hayashi, Ramesh Raj Puri, and Kazuhito Itoh. 2018. "Plant Growth Promoting Effects of Nepalese Sweet Potato Endophytes" Horticulturae 4, no. 4: 53. https://doi.org/10.3390/horticulturae4040053
APA StyleAdhikari Dhungana, S., Adachi, F., Hayashi, S., Raj Puri, R., & Itoh, K. (2018). Plant Growth Promoting Effects of Nepalese Sweet Potato Endophytes. Horticulturae, 4(4), 53. https://doi.org/10.3390/horticulturae4040053





