Growth, Water-Use Efficiency, Stomatal Conductance, and Nitrogen Uptake of Two Lettuce Cultivars Grown under Different Percentages of Blue and Red Light
Abstract
1. Introduction
Blue Light Effects on Stomatal Conductance (gs) and the Potential Role with Water Relations
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Lighting Treatments
2.3. Data Collection and Plant Measurements
2.4. Data Analysis
3. Results
3.1. Growth Parameters
3.2. Physiological Responses
4. Discussion
4.1. Growth Parameters
4.2. Physiological Responses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitchell, C.; Burr, J.F.; Dzakovich, M.P.; Gómez, C.; Lopez, R.; Hernández, R.; Kubota, C.; Currey, C.J.; Meng, Q.; Runkle, E.S.; et al. Light-emitting diodes in horticulture. Hort. Rev. 2015, 43, 1–88. [Google Scholar]
- Carvalho, S.D.; Folta, K.M. Environmentally modified organisms—Expanding genetic potential with light. CRC. Crit. Rev. Plant Sci. 2014, 33, 486–508. [Google Scholar] [CrossRef]
- Dougher, T.A.O.; Bugbee, B. Long-term blue light effects on the histology of lettuce and soybean leaves and stems. J. Am. Soc. Hortic. Sci. 2004, 129, 467–472. [Google Scholar]
- Bugbee, B. Toward an optimal spectral quality for plant growth and development: The importance of radiation capture. Acta Hortic. 2016, 1134, 1–12. [Google Scholar] [CrossRef]
- Cope, K.R.; Snowden, M.C.; Bugbee, B. Photobiological interactions of blue light and photosynthetic photon flux: Effects of monochromatic and broad-spectrum light sources. Photochem. Photobiol. 2014, 90, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, L.S.S. Transduction of blue-light signals. Plant Physiol. 1993, 10, 333–337. [Google Scholar] [CrossRef]
- Lin, C.; Ahmad, M.; Cashmore, A.R. Arabidopsis cryptochrome 1 is a soluble protein mediating blue light-dependent regulation of plant growth and development. Plant J. 1996, 10, 893–902. [Google Scholar] [CrossRef] [PubMed]
- McCree, K.J. The action spectrum absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of seven diverse species to blue and green light: Interactions with photon flux. PLoS ONE 2016, 11, e0163121. [Google Scholar] [CrossRef] [PubMed]
- Cope, K.R.; Bugbee, B.; Hill, O.M. Spectral effects of three types of white light-emitting diodes on plant growth and development: Absolute versus relative amounts of blue light. HortScience 2013, 48, 504–509. [Google Scholar]
- Hoenecke, M.E.E.; Bula, R.J.J.; Tibbitts, T.W.W. Importance of “blue” photon levels for lettuce seedlings grown under red-light-emitting diodes. HortScience 1992, 27, 427–430. [Google Scholar] [PubMed]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Poulet, L.; Massa, G.D.; Morrow, R.C.; Bourget, C.M.; Wheeler, R.M.; Mitchell, C.A. Significant reduction in energy for plant-growth lighting in space using targeted LED lighting and spectral manipulation. Life Sci. Sp. Res. 2014, 2, 43–53. [Google Scholar] [CrossRef]
- Smith, H.; Whitelam, G.C. The shade avoidance syndrome: Multiple responses mediated by multiple phytochromes. Plant Cell Environ. 1997, 20, 840–844. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynth 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Assmann, S.M. Enhancement of the stomatal response to blue light by red light, reduced intercellular concentrations of CO2, and low vapor pressure differences. Plant Physiol. 1988, 87, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Boccalandro, H.E.; Giordano, C.V.; Ploschuk, E.L.; Piccoli, P.N.; Bottini, R.; Casal, J.J. Phototropins but not cryptochromes mediate the blue light-specific promotion of stomatal conductance, while both enhance photosynthesis and transpiration under full sunlight. Plant Physiol. 2012, 158, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Talbott, L.D.; Zhu, J.; Han, S.W.; Zeiger, E. Phytochrome and blue light-mediated stomatal opening in the orchid, paphiopedilum. Plant Cell Physiol. 2002, 43, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Goins, G.D.D.; Yorio, N.C.C.; Sanwo, M.M.M.; Brown, C.S.S. Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting. J. Exp. Bot. 1997, 48, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Kim, S.J.; Hahn, E.J.; Heo, J.W.; Paek, K.Y. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Muneer, S.; Kim, E.J.; Park, J.S.; Lee, J.H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef] [PubMed]
- Ieperen, W.V.; Savvides, A.; Fanourakis, D. Red and blue light effects during growth on hydraulic and stomatal conductance in leaves of young cucumber plants. VII Int. Symp. Light Hortic. Syst. 2012, 956, 223–230. [Google Scholar] [CrossRef]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [PubMed]
- Zheng, L.; Van Labeke, M.-C. Long-term effects of red- and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jones, H. Stomatal control of photosynthesis and transpiration. J. Exp. Bot. 1998, 49, 387–398. [Google Scholar] [CrossRef]
- Liu, X.Y.; Guo, S.R.; Xu, Z.G.; Jiao, X.L.; Tezuka, T. Regulation of chloroplast ultrastructure, cross-section anatomy of leaves, and morphology of stomata of cherry tomato by different light irradiations of light-emitting diodes. HortScience 2011, 46, 217–221. [Google Scholar]
- Savvides, A.; Fanourakis, D.; Van Ieperen, W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J. Exp. Bot. 2012, 63, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, J.W. Determination of total Kjeldahl nitrogen by semiautomatedcolorimetry. Environ. Monit. Syst. Lab. 1993, 1–15. [Google Scholar]
- Hernández, R.; Eguchi, T.; Deveci, M.; Kubota, C. Tomato seedling physiological responses under different percentages of blue and red photon flux ratios using LEDs and cool white fluorescent lamps. Sci. Hortic. 2016, 213, 270–280. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K.; Takase, M.; Kon, N.; Fujiwara, K.; Kurata, K. Effect of light quality on growth and vegetable quality in leaf lettuce, spinach and komatsuna. Environ. Control Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef]
- Yanagi, T.; Okamoto, K.; Takita, S. Effect of blue, red, and blue/red lights of two different PPF levels on growth and morphogenesis of lettuce plants. Acta Hortic. 1996, 440, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar]
- Matsuda, R.; Ohashi-Kaneko, K.; Fujiwara, K.; Goto, E.; Kurata, K. Photosynthetic characteristics of rice leaves grown under red light with or without supplemental blue light. Plant Cell Physiol. 2004, 45, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Chaves, I.; Pokorny, R.; Byrdin, M.; Hoang, N.; Ritz, T.; Brettel, K.; Essen, L.-O.; van der Horst, G.T.J.; Batschauer, A.; Ahmad, M. The cryptochromes: Blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 2011, 62, 335–364. [Google Scholar] [CrossRef] [PubMed]
- Borowski, E.; Slawomir, M.; Rubinowska, K.; Hawrylak-Nowak, B.; Grudzinski, W.; Michalek, S.; Rubinowska, K.; Hawrylak-Nowak, B.; Grudzinski, W.; Slawomir, M.; et al. The effects of light quality on photosynthetic parameters and yield of lettuce plants. Acta Sci. Pol. Cultus 2015, 14, 177–188. [Google Scholar]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Beerling, D.J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.F.; Lian, H.L.; Kang, C.Y.; Yang, H.Q. Phytochrome B is involved in mediating red light-induced stomatal opening in Arabidopsis thaliana. Mol. Plant 2010, 3, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Morison, J. Stomatal response to increased CO2 concentration. J. Exp. Bot. 1998, 49, 443–452. [Google Scholar] [CrossRef]
- Poole, I.; Lawson, T.; Weyers, J.D.B.; Raven, J.A. Effect of elevated CO2 on the stomatal distribution and leaf physiology of Alnus glutinosa. New Phytol. 2000, 145, 511–521. [Google Scholar] [CrossRef]
- Folta, K.M.; Maruhnich, S.A. Green light: A signal to slow down or stop. J. Exp. Bot. 2007, 58, 3099–3111. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, P.G.; Mcnaughton, K.G. Stomatal control of transpiration: Scaling up from leaf to region. Adv. Ecol. Res. 1986, 15, 1–49. [Google Scholar] [CrossRef]
- Pieruschka, R.; Huber, G.; Berry, J. Control of transpiration by radiation. Proc. Natl. Acad. Sci. USA 2010, 107, 13372–13377. [Google Scholar] [CrossRef] [PubMed]
- Schuerger, A.C.; Brown, C.S.; Stryjewski, E.C. Anatomical features of pepper plants (Capsicum annuum L.) grown under red light-emitting diodes supplemented with blue or far-red light. Ann. Bot. 1997, 79, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Ingwers, M.W.; McGuire, M.A.; Teskey, R.O. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J. Exp. Bot. 2017, 68, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Park, S.Y.; Oh, M.M. Growth and cell division of lettuce plants under various ratios of red to far-red light-emitting didoes. Hortic. Environ. Biotechnol. 2015, 56, 186–194. [Google Scholar] [CrossRef]
- Pinho, P.; Jokinen, K.; Halonen, L. The influence of the LED light spectrum on the growth and nutrient uptake of hydroponically grown lettuce. Light. Res. Technol. 2017, 49, 866–881. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Growth, photosynthetic and antioxidant parameters of two lettuce cultivars as affected by red, green, and blue light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 639–653. [Google Scholar] [CrossRef]
- Sood, S.; Gupta, V.; Tripathy, B.C. Photoregulation of the greening process of wheat seedlings grown in red light. Plant Mol. Biol. 2005, 59, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Ito, H.; Tanaka, R.; Tanaka, N.K.; Yoshida, K.; Okada, K. Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Plant Biol. 1998, 95, 12719–12723. [Google Scholar] [CrossRef]
- Hepworth, C.; Turner, C.; Landim, M.G.; Cameron, D.; Gray, J.E. Balancing water uptake and loss through the coordinated regulation of stomatal and root development. PLoS ONE 2016, 11, e0156930. [Google Scholar] [CrossRef] [PubMed]
- Hepworth, C.; Doheny-Adams, T.; Hunt, L.; Cameron, D.D.; Gray, J.E. Manipulating stomatal density enhances drought tolerance without deleterious effect on nutrient uptake. New Phytol. 2015, 208, 336–341. [Google Scholar] [CrossRef] [PubMed]
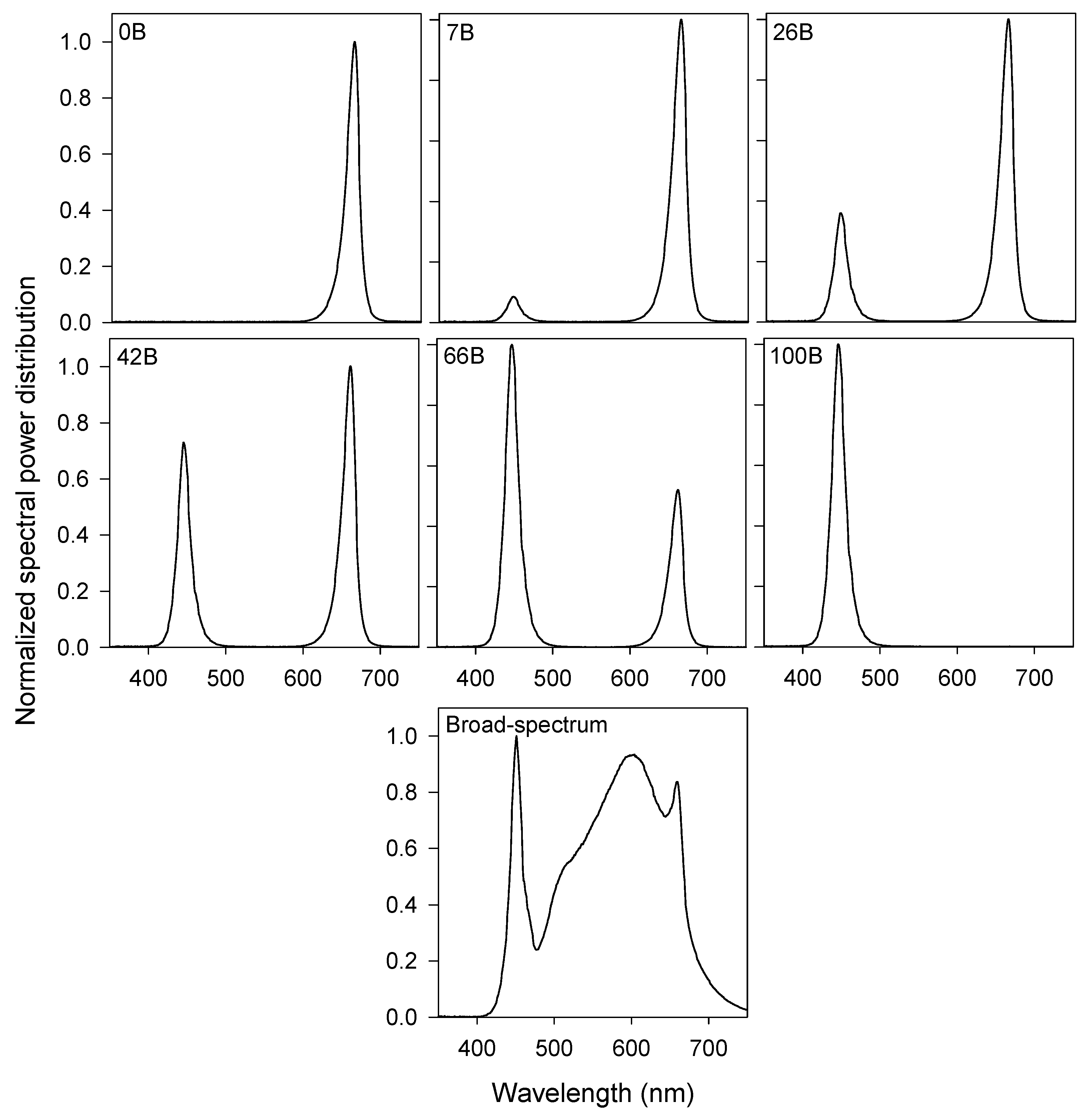
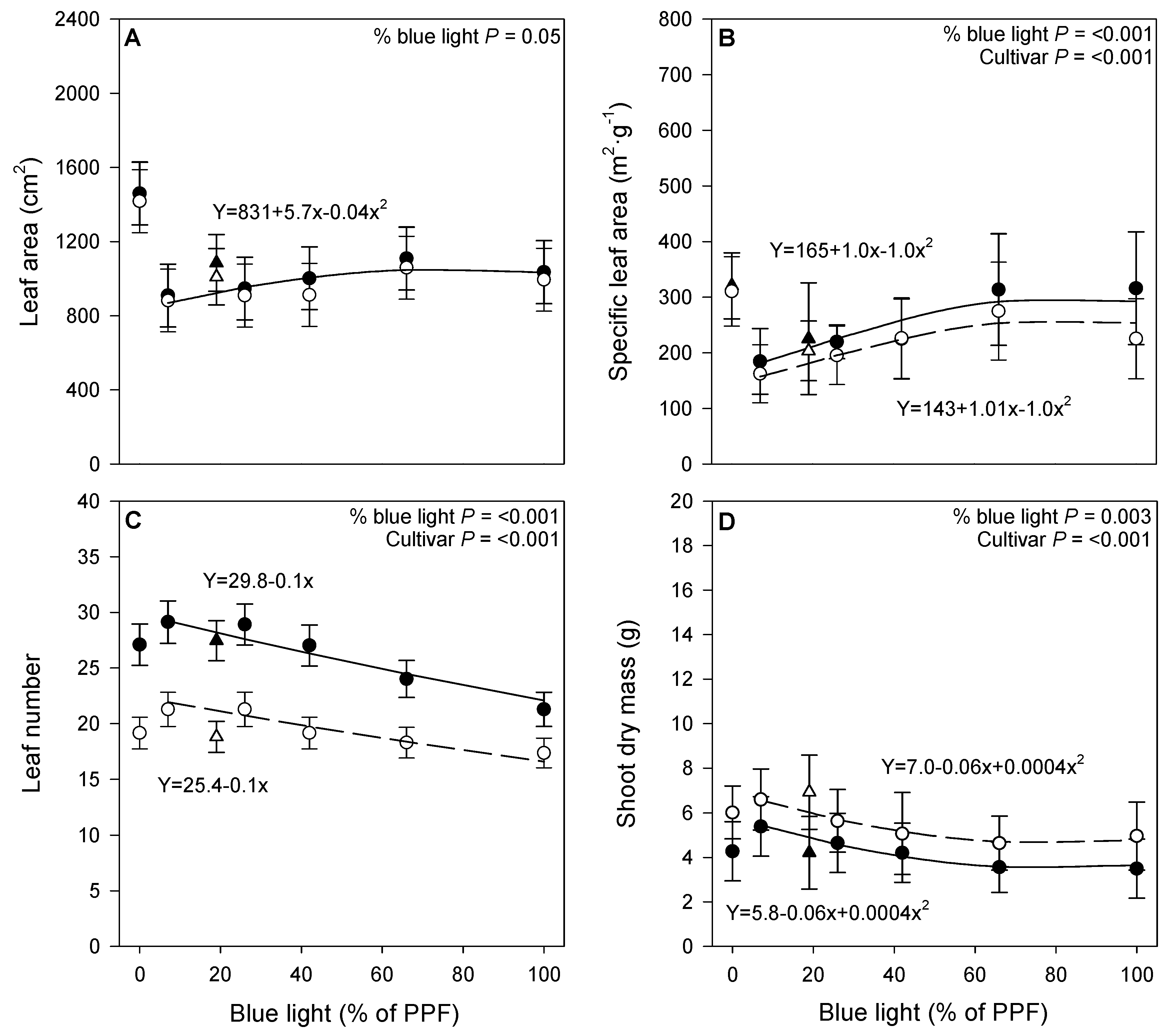
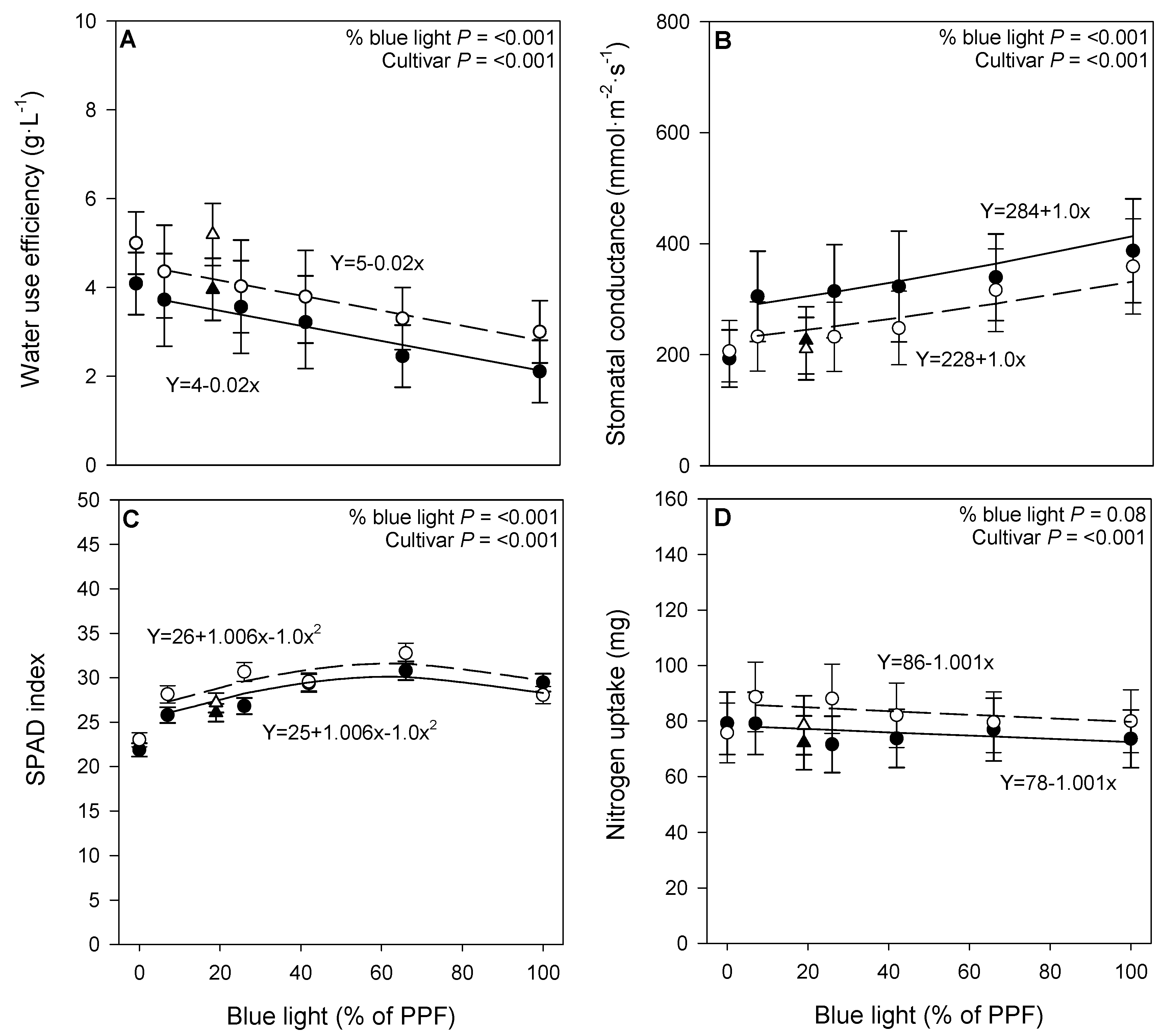
| Treatment | ADT (°C) | ||
|---|---|---|---|
| Rep. 1 | Rep. 2 | Rep. 3 | |
| 0B | 21.7 z | 22.1 | 21.9 |
| 7B | 21.9 | 21.6 | 22.7 |
| 26B | 21.6 | 22.2 | 21.6 |
| 42B | 21.9 | 22.3 | 21.9 |
| 66B | 21.9 | 21.7 | 21.9 |
| 100B | 21.4 | 21.5 | 22.2 |
| Broad-spectrum | 21.1 | 21.5 | 21.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clavijo-Herrera, J.; Van Santen, E.; Gómez, C. Growth, Water-Use Efficiency, Stomatal Conductance, and Nitrogen Uptake of Two Lettuce Cultivars Grown under Different Percentages of Blue and Red Light. Horticulturae 2018, 4, 16. https://doi.org/10.3390/horticulturae4030016
Clavijo-Herrera J, Van Santen E, Gómez C. Growth, Water-Use Efficiency, Stomatal Conductance, and Nitrogen Uptake of Two Lettuce Cultivars Grown under Different Percentages of Blue and Red Light. Horticulturae. 2018; 4(3):16. https://doi.org/10.3390/horticulturae4030016
Chicago/Turabian StyleClavijo-Herrera, Jonathan, Edzard Van Santen, and Celina Gómez. 2018. "Growth, Water-Use Efficiency, Stomatal Conductance, and Nitrogen Uptake of Two Lettuce Cultivars Grown under Different Percentages of Blue and Red Light" Horticulturae 4, no. 3: 16. https://doi.org/10.3390/horticulturae4030016
APA StyleClavijo-Herrera, J., Van Santen, E., & Gómez, C. (2018). Growth, Water-Use Efficiency, Stomatal Conductance, and Nitrogen Uptake of Two Lettuce Cultivars Grown under Different Percentages of Blue and Red Light. Horticulturae, 4(3), 16. https://doi.org/10.3390/horticulturae4030016





