Interannual Variability in Apricot Quality: Role of Calcium and Postharvest Treatments During Cold Storage and Shelf Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Apricot Production and Preharvest and Postharvest Treatments
2.2. Texture and Fruit Color
2.3. Respiration Rate
2.4. Chemical Analysis
2.5. Sensory Evaluation
2.6. Statistical Evaluation
3. Results and Discussion
3.1. Physicochemical Quality Parameters
3.2. Biochemical Composition
3.3. Bioactive Compounds
3.4. Respiration Rate
3.5. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ca | Calcium |
| MAP | Modified-atmosphere packaging |
| 1-MCP | 1-methylcyclopropene |
| TSS | Total soluble solids |
| TA | Total acids |
| calcium nitrate | (Ca(NO3)2) |
| L* | Lightness |
| a* | Red tone intensity |
| b* | Yellow tone intensity |
| C* | Chromaticity |
| ΔE | Color difference |
References
- Mordor Intelligence. Fresh Apricots Market—Size, Share & Forecast (2025–2030). Available online: https://www.mordorintelligence.com/industry-reports/fresh-apricots-market (accessed on 15 January 2025).
- Poyraz, S.; Gül, M. The development of apricot production and foreign trade in the world and in Turkey. Sci. Pap. Ser. Manag. Econom. Eng. Agric. Rural Dev. 2022, 22, 601–616. [Google Scholar]
- Zhebentyayeva, T.; Ledbetter, C.; Burgos, L.; Llácer, G. Apricot. In Fruit Breeding; Badenes, M., Byrne, D., Eds.; Springer: Boston, MA, USA, 2012; Volume 8, pp. 415–459. [Google Scholar]
- Kafkaletou, M.; Velliou, A.; Christopoulos, M.V.; Ouzounidou, G.; Tsantili, E. Impact of Cold Storage Temperature and Shelf Life on Ripening Physiology, Quality Attributes, and Nutritional Value in Apricots—Implication of Cultivar. Plants 2023, 12, 2875. [Google Scholar] [CrossRef]
- Leccese, A.; Bureau, S.; Reich, M.; Renard, M.G.C.C.; Audergon, J.M.; Mennone, C.; Bartolini, S.; Viti, R. Pomological and Nutraceutical Properties in Apricot Fruit: Cultivation Systems and Cold Storage Fruit Management. Plant Foods Hum. Nutr. 2010, 65, 112–120. [Google Scholar] [CrossRef]
- Fratianni, F.; Ombra, M.N.; d’Acierno, A.; Cipriano, L.; Nazzaro, F. Apricots: Biochemistry and functional properties. Curr. Opin. Food Sci. 2018, 19, 23–29. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Day, K.R.; Johnson, R.S.; Garner, D. Influence of In-Season Foliar Calcium Sprays on Fruit Quality and Surface Discoloration Incidence of Peaches and Nectarines. J. Am. Pomol. Soc. 2000, 54, 118–122. [Google Scholar]
- Gerasopoulos, D.; Drogoudi, P.D. Summer-pruning and preharvest calcium chloride sprays affect storability and low temperature breakdown incidence in kiwifruit. Postharvest Biol. Technol. 2005, 36, 303–308. [Google Scholar] [CrossRef]
- Abdrabboh, A.G. Effect of Some Preharvest Treatments on Quality of Canino Apricot Fruits Under Cold Storage Conditions. Postharvest Biol. Technol. 2012, 4, 227–234. [Google Scholar]
- Poovaiah, B.W. Role of calcium in ripening and senescence. Commun. Soil Sci. Plant Anal. 1979, 10, 83–88. [Google Scholar] [CrossRef]
- Chéour, F.; Souiden, Y. Calcium delays the postharvest ripening and related membrane-lipid changes of tomato. J. Nutr. Food Sci. 2015, 5, 1. [Google Scholar]
- Jia, K.; Wang, W.; Zhang, Q.; Jia, W. Cell wall integrity signaling in fruit ripening. Int. J. Mol. Sci. 2023, 24, 4054. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Vasilakakis, M.; Mignani, I.; Diamantidis, G.; Tzavella-Klonari, K. The effect of preharvest calcium sprays on quality attributes, physicochemical aspects of cell wall components and susceptibility to brown rot of peach fruits (Prunus persica L. cv. Andross). Sci. Hortic. 2005, 107, 43–50. [Google Scholar] [CrossRef]
- Wojcik, P. “Dabrowicka prune” fruit quality as influenced by calcium spraying. J. Plant Nutr. 2001, 24, 1229–1241. [Google Scholar] [CrossRef]
- Milović, M.; Magazin, N.; Kevrešan, Ž.; Keserović, Z.; Mastilović, J.; Milić, B.; Kalajdžić, J.; Popara, G.; Kovač, R.; Bajić, A.; et al. Promena čvrstine ploda kajsije tokom skladištenja u zavisnosti od primene tretmana pre i nakon berbe. In Proceedings of the VIII Conference “Innovations in Fruit Growing”, Online, 1–2 August 2023; University of Belgrade, Faculty of Agriculture: Belgrade, Serbia, 2023; pp. 105–113. [Google Scholar]
- Özkaya, O.; Şener, A.; Saridaş, M.A.; Ünal, Ü.; Valizadeh, A.; Dündar, Ö. Influence of Fast Cold Chain and Modified Atmosphere Packaging Storage on Postharvest Quality of Early Season Harvested Sweet Cherries. J. Food Process. Preserv. 2015, 39, 2119–2128. [Google Scholar] [CrossRef]
- Ozturk, B.; Uzun, S.; Karakaya, O. Combined effects of aminoethoxyvinylglycine and MAP on the fruit quality. Sci. Hortic. 2019, 251, 209–214. [Google Scholar] [CrossRef]
- Sisler, E.C.; Serek, M. Inhibitors of ethylene responses in plants at the receptor level: Recent developments. Physiol. Plant. 1997, 100, 577–582. [Google Scholar] [CrossRef]
- Sisler, E.C.; Serek, M. Compounds Interacting with the Ethylene Receptor in Plants. Plant Biol. 2003, 5, 473–480. [Google Scholar] [CrossRef]
- Fan, X.; Argenta, L.; Mattheis, J.P. Inhibition of ethylene action by 1 methylcyclopropene prolongs storage life of apricots. Postharvest Biol. Technol. 2000, 20, 135–142. [Google Scholar] [CrossRef]
- Kluge, R.A.; Jacomino, A.P. Shelf life of peaches treated with 1-methylcyclopropene. Sci. Agric. 2002, 59, 69–72. [Google Scholar] [CrossRef]
- Argenta, L.; Fan, X.; Mattheis, J.P. Influence of 1-methylcyclopropene on Ripening, Storage Life, and Volatile Production by d’Anjou cv. Pear Fruit. J. Agric. Food Chem. 2003, 51, 3858–3864. [Google Scholar] [CrossRef]
- McArtney, S.M.; Obermiller, J.D.; Hoyt, T.; Parker, M.L. Law Rome’, Golden Delicious’ apples differ in their response to preharvest and postharvest 1-methylcyclo-propene treatment combinations. J. Hortic. Sci. 2009, 44, 1632–1636. [Google Scholar]
- Dong, L.; Lurie, S.; Zhou, H.W. Effect of 1-methylcyclopropene on ripening of ‘Canino’ apricots and ‘Royal Zee’ plums. Postharvest Biol. Technol. 2002, 24, 135–145. [Google Scholar] [CrossRef]
- Khasawneh, A.E.R.; Alsmairat, N.; Othman, Y.A.; Ayad, J.Y.; Al-Hajaj, H.; Qrunfleh, I.M. Controlled-release nitrogen fertilizers for improving yield and fruit quality of young apricot trees. Sci. Hortic. 2022, 303, 111233. [Google Scholar] [CrossRef]
- Miodragović, M.; Magazin, N.; Keserović, Z.; Milić, B.; Popović, B.; Blagojević, B.; Kalajdžić, J. The early performance and fruit properties of apricot cultivars grafted on Prunus spinosa L. interstock. Sci. Hortic. 2019, 250, 199–206. [Google Scholar] [CrossRef]
- Keserović, Z.; Magazin, N.; Milić, B.; Igić, M.; Miodragović, M.; Tarlanović, J. New apricot cultivar—“Buda”. J. Pomol. 2018, 52, 27–31. [Google Scholar]
- Montanaro, G.; Dichio, B.; Lang, A.; Mininni, A.N.; Xiloyannis, C. Fruit calcium accumulation coupled and uncoupled from its transpiration in kiwifruit. J. Plant Physiol. 2006, 163, 1203–1210. [Google Scholar] [CrossRef]
- Taylor, M.D.; Locascio, S.J. Blossom-end rot: A calcium deficiency. J. Plant Nutr. 2004, 27, 123–139. [Google Scholar] [CrossRef]
- Kevrešan, Ž.S.; Magazin, N.P.; Mastilović, J.S.; Milović, M.Đ.; Kovač, R.M.; Kalajdžić, J.D.; Milić, B.M.; Barać, G.N. Effect of N, Ca, MAP, and 1-MCP Treatments on Ethylene–IAD index interactions during apricot storage and shelf life. Food Feed Res. 2025, 52, 181–191. [Google Scholar] [CrossRef]
- Kovač, R.; Kevrešan, Ž.; Mastilović, J.; Magazin, N.; Milić, B.; Milović, M.; Bajić, A.; Kalajdžić, J.; Barać, G.; Keserović, Z. IAD values of apricot (Prunus armeniaca L.) at harvest in relation to fruit quality and sensory properties during cold storage and shelf life. N. Z. J. Crop Hortic. Sci. 2022, 50, 191–205. [Google Scholar] [CrossRef]
- Kevrešan, Ž.; Milić, B.; Rajić, A.; Kovač, R.; Milović, M.; Kalajdžić, J.; Barać, G. Does application of naphthenic acids in early fruit development stage result in prolonged effect on cold storage and shelf life of apricot fruit? Food Feed Res. 2022, 49, 139–153. [Google Scholar] [CrossRef]
- Milović, M.; Kevrešan, Ž.; Mastilović, J.; Kovač, R.; Kalajdžić, J.; Magazin, N.; Bajić, A.; Milić, B.; Barać, G.; Keserović, Z. Could an early treatment with GA and BA impact prolonged cold storage and shelf life of apricot? Horticulturae 2022, 8, 1220. [Google Scholar] [CrossRef]
- Andreu-Coll, L.; Burló, F.; Galindo, A.; García-Brunton, J.; Vigueras-Fernández, J.; Blaya-Ros, P.J.; Signes-Pastor, A.J. Enhancing ‘Mirlo Rojo’Apricot (Prunus armeniaca L.) Quality Through Regulated Deficit Irrigation: Effects on Antioxidant Activity, Fatty Acid Profile, and Volatile Compounds. Horticulturae 2024, 10, 1253. [Google Scholar] [CrossRef]
- Lanauskas, J.; Uselis, N.; Kviklys, D.; Kviklienė, N.; Buskienė, L. Rootstock effect on the performance of sweet cherry cv. Lapins. Hortic. Sci. 2012, 39, 55–60. [Google Scholar] [CrossRef]
- Prasad, B.; Dimri, D.C.; Bora, L. Effect of pre-harvest foliar spray of calcium and potassium on fruit quality of Pear cv. Pathernakh. Sci. Res. Essays 2015, 10, 376–380. [Google Scholar] [CrossRef]
- Stanley, J.; Feng, J.; Olsson, S. Crop load and harvest maturity effects on consumer preferences for apricots. J. Sci. Food Agric. 2015, 95, 752–763. [Google Scholar] [CrossRef]
- Ali, S.; Masud, T.; Abbasi, K.S.; Mahmood, T.; Hussain, I. Influence of CaCl on Biochemical Composition, Antioxidant and Enzymatic Activity of Apricot at Ambient Storage. Pak. J. Nutr. 2013, 12, 476–483. [Google Scholar] [CrossRef]
- Khorshidi, S.; Davarynejad, G.; Tehranifar, A. Effect of modified atmosphere packaging on chemical composition, antioxidant activity, anthocyanin, and total phenolic content of cherry fruits. Hortic. Environ. Biotechnol. 2011, 52, 471–481. [Google Scholar] [CrossRef]
- Muftuoğlu, F.; Ayhan, Z.; Esturk, O. Modified Atmosphere Packaging of Kabaaşı Apricot (Prunus armeniaca L. ‘Kabaaşı’): Effect of Atmosphere, Packaging Material Type and Coating on the Physicochemical Properties and Sensory Quality. Food Bioprocess Technol. 2012, 5, 1601–1611. [Google Scholar] [CrossRef]
- Singh, S.P.; Pal, R.K. Controlled atmosphere storage of guava (Psidium guajava L.) fruit. Postharvest Biol. Technol. 2008, 47, 296–306. [Google Scholar] [CrossRef]
- Selcuk, N.; Erkan, M. The effects of modified and palliflex controlled atmosphere storage on postharvest quality and composition of ‘Istanbul’ medlar fruit. Postharvest Biol. Technol. 2015, 99, 9–19. [Google Scholar] [CrossRef]
- Mphahlelea, R.R.; Fawolea, O.A.; Opara, U.L. Influence of packaging system and long term storage on physiological attributes, biochemical quality, volatile composition and antioxidant properties of pomegranate fruit. Sci. Hortic. 2016, 211, 140–151. [Google Scholar] [CrossRef]
- Akbulut, M.; Artik, N. Phenolic compounds profile of apricot and wild apricot fruits and their changes during the process. In Proceedings of the 7th Food Congress, Ankara, Turkey, 22–24 May 2002; pp. 57–64. [Google Scholar]
- Hamauzu, Y. Role and evolution of fruit phenolic compounds during ripening and storage. Stewart Postharvest Rev. 2006, 2, 1–7. [Google Scholar]
- Shin, Y.; Ryu, J.A.; Liu, R.H.; Nock, J.F.; Watkins, C.B. Harvest maturity, storage temperature and relative humidity affect fruit quality, antioxidant contents and activity, and inhibition of cell proliferation of strawberry fruits. Postharvest Biol. Technol. 2008, 49, 201–209. [Google Scholar] [CrossRef]
- Dragović-Uzelac, V.; Levaj, B.; Mrkić, V.; Bursać, D.; Boraš, M. The content of polyphenols and carotenoids in three apricot cultivars depending on stage of maturity and geographical region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Bartolini, S.; Leccese, A.; Viti, R. Quality and Antioxidant Properties of Apricot Fruits at Ready-to-eat: Influence of the Weather Conditions under Mediterranean Coastal Area. J. Food Process. Technol. 2015, 7, 1–6. [Google Scholar]
- Fallahi, E.; Colt, M.W.; Baird, C.R.; Fallahi, B.; Chun, I.J. Influence of Nitrogen and Bagging on Fruit Quality and Mineral Concentrations of ‘BC-2 Fuji’ apple. HortTechnology 2001, 11, 462–466. [Google Scholar] [CrossRef]
- Din, K.U.; Hashmi, M.S.; Ahmad, A.; Khan, M.R.; Qazi, I.M.; Muhammad, A.; Badshah, S. Hypobaric and salicylic acid treatments prevent tissue softening and browning in apricot fruit. J. Food Meas. Charact. 2025, 19, 1–15. [Google Scholar] [CrossRef]
- Watkins, C.B.; Nock, J.F.; Whitaker, B.D. Responses of early, mid and late season apple cultivars to postharvest application of 1-methylcyclopropene (1-MCP) under air and controlled atmosphere storage conditions. Postharvest Biol. Technol. 2000, 19, 17–32. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, J.; Long, L.E. Quality and physiological responses of two late-season sweet cherry cultivars ‘Lapins’ and ‘Skeena’ to modified atmosphere packaging (MAP) during simulated long distance ocean shipping. Postharvest Biol. Technol. 2015, 110, 1–8. [Google Scholar] [CrossRef]
- Fatima, T.; Bashir, O.; Gani, G.; Bhat, T.; Jan, N. Nutritional and health benefits of apricots. Int. J. Unani Integr. Med. 2018, 2, 5–9. [Google Scholar] [CrossRef]
- Mangaraj, S.; Goswami, T.K. Modified atmosphere packaging of fruits and vegetables for extending shelf-life: A review. Fresh Prod. 2009, 3, 1–31. [Google Scholar]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
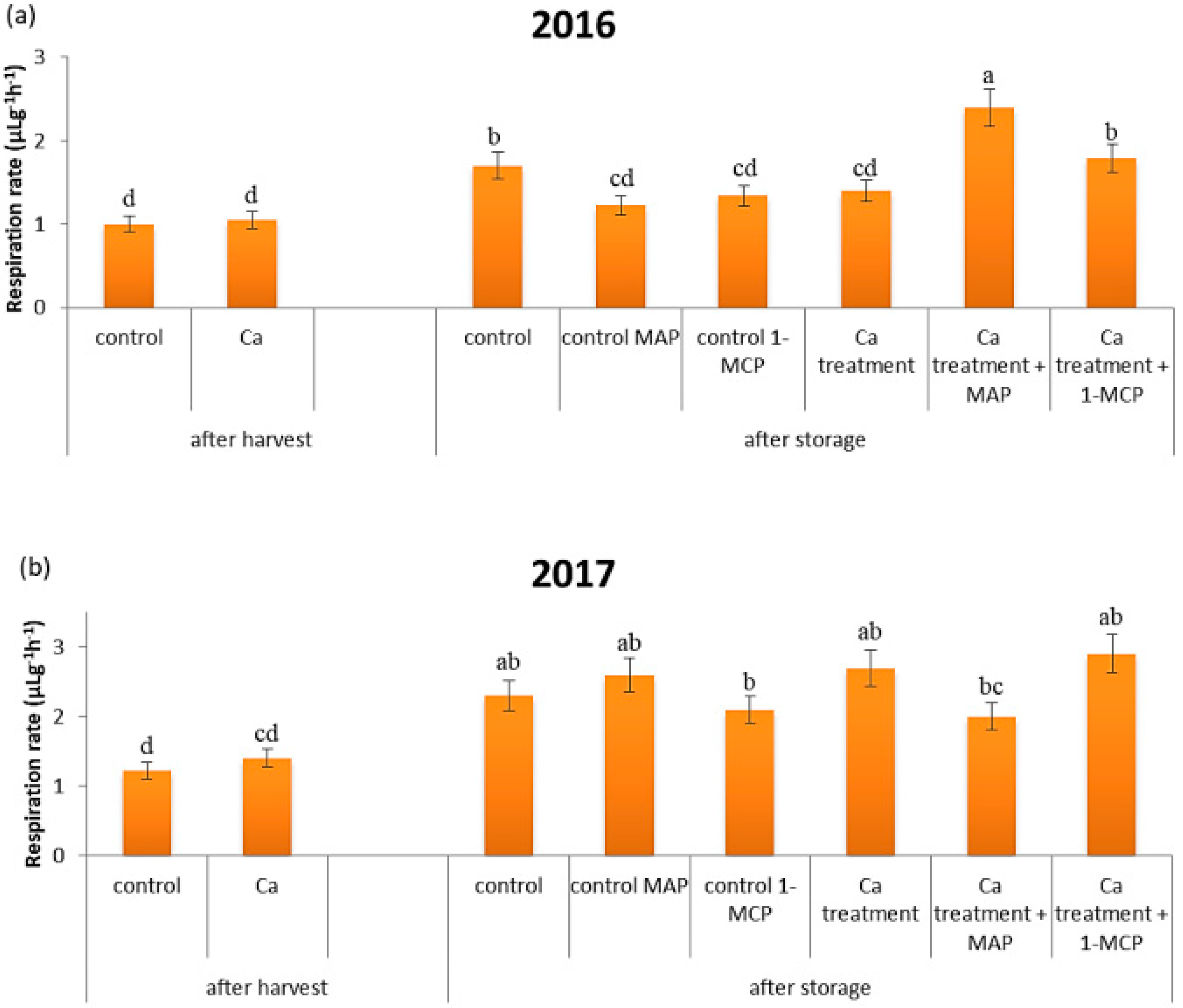
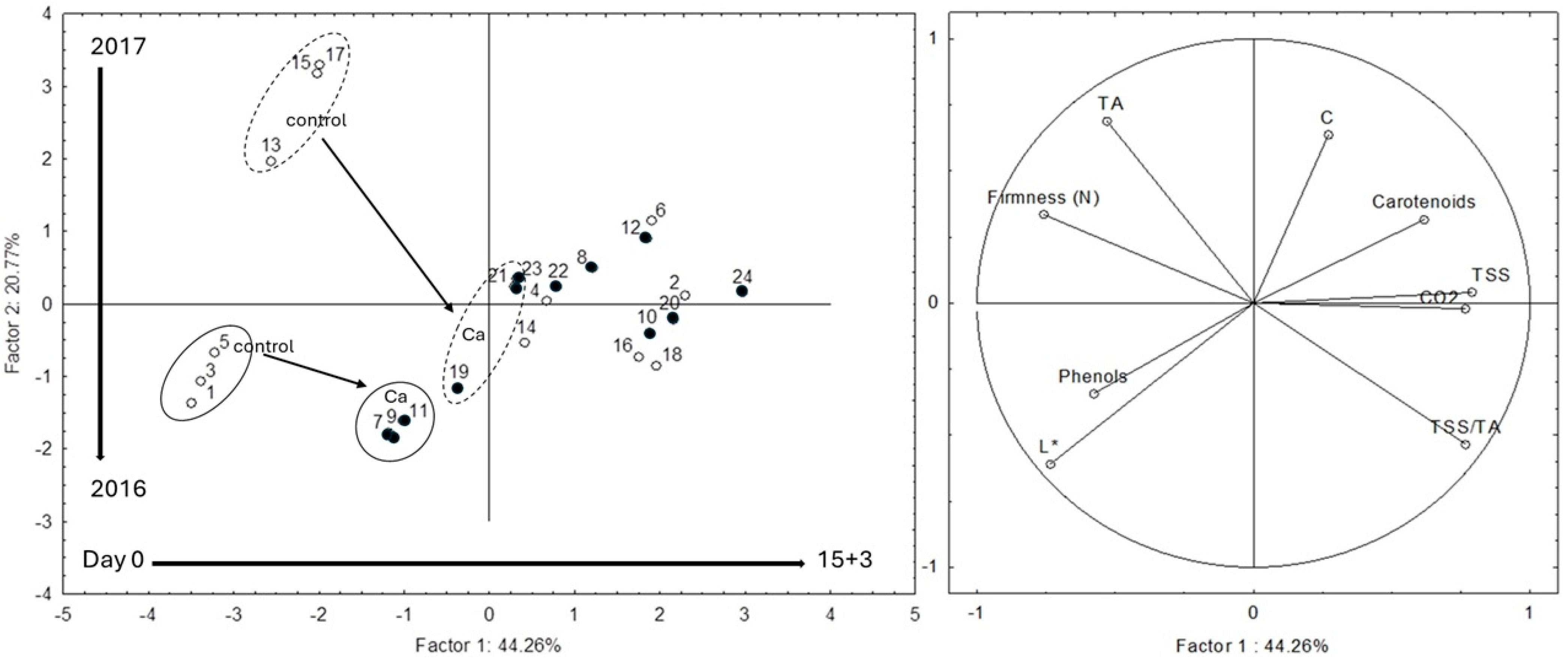
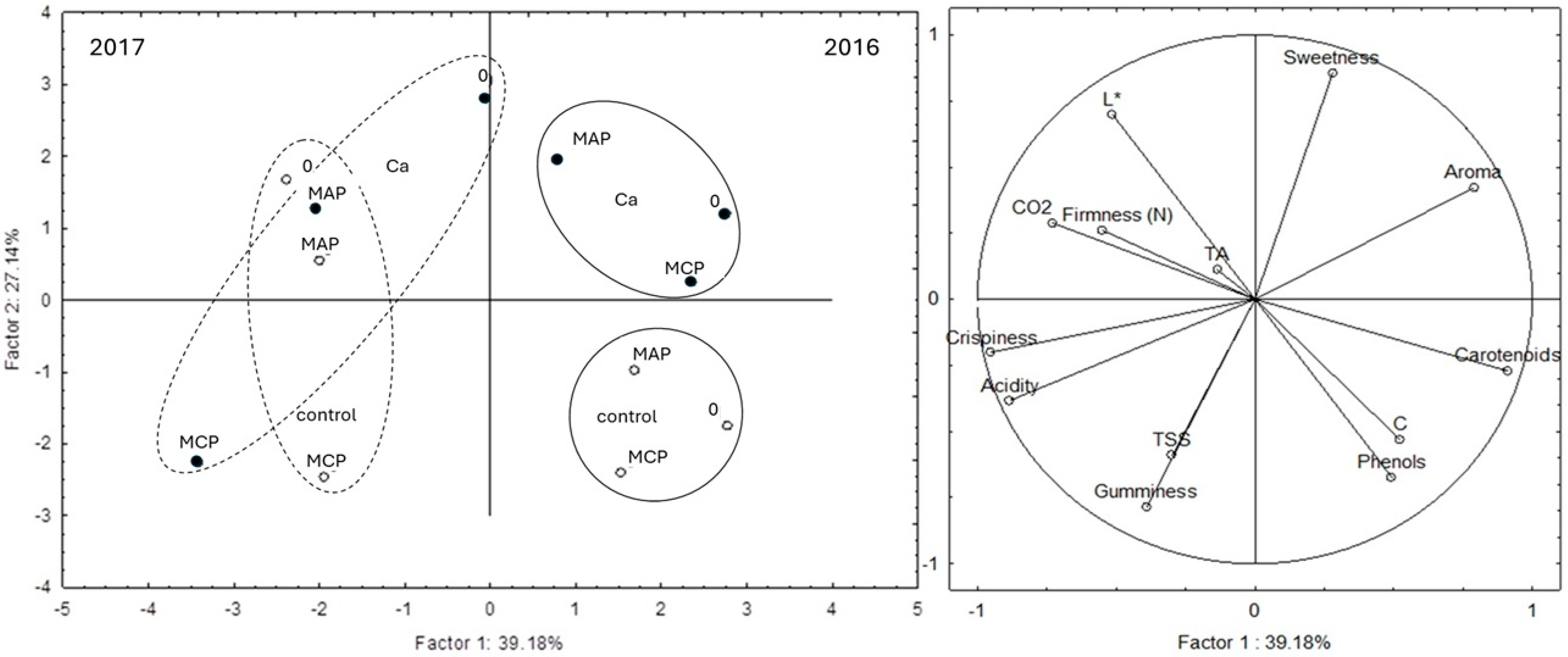
| Preharvest Treatment | Postharvest Treatment | Storage (Days) | L* | C* | hue° | ∆E |
|---|---|---|---|---|---|---|
| 2016 | ||||||
| control | control | 0 | 63.81 a | 32.95 f | 77.83 ab | 3.88 |
| 15 + 3 | 55.40 f | 37.90 b | 67.88 efg | |||
| MAP | 0 | 63.53 a | 34.25 ef | 78.45 ab | 3.57 | |
| 15 + 3 | 56.52 def | 38.11 b | 69.05 efg | |||
| 1-MCP | 0 | 62.96 a | 35.78 cde | 77.0 bc | 4.22 | |
| 15 + 3 | 55.46 f | 40.89 a | 66.61 efg | |||
| Ca treatment | control | 0 | 63.96 a | 35.77 cde | 83.93 a | 4.45 |
| 15 + 3 | 57.06 cdef | 37.68 b | 71.57 cde | |||
| MAP | 0 | 63.54 a | 35.30 cde | 81.77 ab | 4.56 | |
| 15 + 3 | 56.49 def | 33.39 f | 68.53 efg | |||
| 1-MCP | 0 | 62.49 a | 35.49 cde | 77.74 ab | 4.18 | |
| 15 + 3 | 56.14 ef | 39.77 a | 66.99 efg | |||
| 2017 | ||||||
| control | control | 0 | 59.13 bc | 35.19 de | 82.14 ab | 4.12 |
| 15 + 3 | 58.79 bcd | 35.64 cde | 70.67 def | |||
| MAP | 0 | 56.90 cdef | 39.64 a | 75.80 bcd | 1.76 | |
| 15 + 3 | 57.10 cdef | 34.09 ef | 67.60 efg | |||
| 1-MCP | 0 | 57.00 cdef | 40.36 a | 79.30 ab | 5.33 | |
| 15 + 3 | 57.14 cdef | 37.07 bc | 64.53 fg | |||
| Ca treatment | control | 0 | 59.91 b | 35.58 cde | 80.34 ab | 4.24 |
| 15 + 3 | 58.49 bcde | 36.68 bcd | 68.51 efg | |||
| MAP | 0 | 56.80 cdef | 40.42 a | 77.88 abc | 3.59 | |
| 15 + 3 | 58.03 bcde | 35.85 cde | 67.30 efg | |||
| 1-MCP | 0 | 56.72 def | 40.87 a | 76.03 bcd | 4.71 | |
| 15 + 3 | 56.18 ef | 36.79 bcd | 62.65 g | |||
| Statistical significance | ||||||
| Year (Y) | ** | ** | ns | |||
| Ca treatment (Ca) | ns | ns | ns | |||
| Postharvest treatments (PT) | ** | ** | ** | |||
| Storage (S) | ** | ns | ** | |||
| Y × Ca | ns | * | * | |||
| Y × PT | * | ** | ns | |||
| Ca × PT | ns | * | ns | |||
| Y × S | ** | ** | ns | |||
| Ca × S | ns | ** | ns | |||
| PT × S | ns | ** | ns | |||
| Y × Ca × PT | ns | ** | ns | |||
| Y × Ca × S | ns | ** | ns | |||
| Y × PT × S | ns | ** | ns | |||
| Ca × PT × S | ns | ns | ns | |||
| Y × Ca × PT × S | ns | ** | ns | |||
| Preharvest Treatment | Postharvest Treatment | Sweetness | Acidity | Aroma | Foreign Taste | Crispiness | Gumminess |
|---|---|---|---|---|---|---|---|
| 2016 | |||||||
| control | control | 27 def | 28 def | 39 ab | 0 a | 6.5 c | 24.5 ab |
| MAP | 28 def | 40 bcde | 29.5 bc | 2.5 a | 18.5 c | 22.5 ab | |
| 1-MCP | 24 ef | 41 bcde | 28 bc | 2.5 a | 20 c | 34.5 ab | |
| Ca treatment | control | 45 abc | 16 f | 52 a | 0 a | 7 c | 18.5 ab |
| MAP | 38.5 abcde | 24.5 cde | 51 a | 5 a | 9.5 c | 21 ab | |
| 1-MCP | 47.5 ab | 21 ef | 55 a | 0 a | 10 c | 24 ab | |
| 2017 | |||||||
| control | control | 35 bcdef | 59 ab | 19 c | 0 a | 64 ab | 16.5 b |
| MAP | 30 cdef | 46 bcd | 32 bc | 10 a | 49 b | 34 ab | |
| 1-MCP | 22 f | 56 b | 17 c | 8 a | 56 b | 44.5 a | |
| Ca treatment | control | 54 a | 31 cdef | 38.5 ab | 0 a | 18 c | 13 b |
| MAP | 42.5 abcd | 53 bc | 19 c | 2 a | 50 b | 26 b | |
| 1-MCP | 21 f | 77.5 a | 13 c | 12 a | 75 a | 36 ab | |
| Statistical significance | |||||||
| Year (Y) | ** | ** | ** | ns | ** | ns | |
| Ca treatment (Ca) | ** | ns | ** | ns | * | ns | |
| Postharvest treatments (PT) | ** | * | ns | ns | ** | * | |
| Y × Ca | ns | ns | ** | ns | ns | ns | |
| Y × PT | ** | ns | ns | ns | ns | ns | |
| Ca × PT | ns | ns | ns | ns | ** | ns | |
| Y × Ca × PT | ns | * | * | ns | ** | ns | |
| Preharvest Treatment | Postharvest Treatment | Storage (Days) | Firmness (N) | Phenols (mg/100 g) | Carotenoids (mg/100 g FW) | TSS (%) | TA (g Malic Acid/ 100 g) | Ripening Index (TSS/TA) (%) |
|---|---|---|---|---|---|---|---|---|
| 2016 | ||||||||
| control | control | 0 | 24.3 c | 47.0 a | 0.85 h | 8.64 j | 1.78 b | 79 cde |
| 15 + 3 | 2.93 h | 45.0 b | 2.00 a | 11.14 d | 1.47 b | 87 a | ||
| MAP | 15 + 3 | 7.70 fg | 45.2 b | 1.69 c | 9.82 i | 1.44 b | 85 a | |
| 1-MCP | 15 + 3 | 6.76 fgh | 39.9 d | 1.80 b | 10.90 e | 1.53 b | 86 a | |
| Ca treatment | control | 0 | 19.20 d | 46.1 ab | 1.06 fg | 10.58 f | 1.50 b | 86 a |
| 15 + 3 | 8.06 fg | 39.7 d | 1.89 ab | 10.12 h | 1.55 b | 85 a | ||
| MAP | 15 + 3 | 5.03 gh | 32.9 fg | 1.39 e | 10.20 gh | 1.51 b | 85 a | |
| 1-MCP | 15 + 3 | 5.08 gh | 39.9 d | 1.51 d | 11.3 c | 1.66 b | 85 a | |
| 2017 | ||||||||
| control | control | 0 | 50.03 a | 39.4 d | 1.16 fg | 9.90 i | 2.38 a | 76 e |
| 15 + 3 | 14.54 e | 37.0 e | 1.12 fg | 9.48 j | 1.48 b | 84.5 ab | ||
| MAP | 15 + 3 | 11.33 ef | 34.2 f | 1.15 fg | 10.55 f | 1.43 b | 86.5 a | |
| 1-MCP | 15 + 3 | 8.14 fg | 42.7 c | 1.15 fg | 12.09 b | 1.41 b | 88.5 a | |
| Ca treatment | control | 0 | 36.9 b | 40.6 d | 1.04 g | 10.27 g | 1.31 b | 87 a |
| 15 + 3 | 4.62 gh | 32.7 fg | 1.18 f | 11.0 e | 1.55 b | 86 a | ||
| MAP | 15 + 3 | 13.20 e | 34.1 f | 1.16 fg | 10.51 f | 1.69 b | 84 abc | |
| 1-MCP | 15 + 3 | 5.36 gh | 36.1 e | 1.11 gf | 13.04 a | 1.71 b | 87 a | |
| Statistical significance | ** | ** | ** | ** | ns | ns | ||
| Year (Y) | ** | ** | * | ** | ** | ** | ||
| Ca treatment (Ca) | ns | ** | ** | ** | ns | ns | ||
| Postharvest treatments (PT) | ** | ** | ** | ** | ** | ** | ||
| Storage (S) | ** | ** | ns | ** | * | ** | ||
| Y × Ca | ns | ** | ** | ** | ns | ns | ||
| Y × PT | ns | ns | ns | ** | ns | ns | ||
| Ca × PT | ** | ** | ** | ns | ns | ns | ||
| Y × S | ** | ** | ** | ** | ** | ns | ||
| Ca × S | ns | ** | ** | ** | ns | ns | ||
| PT × S | ** | ** | ns | ** | ns | ns | ||
| Y × Ca × PT | ns | ** | * | ** | ** | ns | ||
| Y × Ca × S | ns | ** | * | ** | ns | ns | ||
| Y × PT × S | ns | ns | ns | ** | ns | ns | ||
| Ca × PT × S | ** | ** | ns | ** | ns | ns | ||
| Y × Ca × PT × S | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milović, M.; Magazin, N.; Mastilović, J.; Kevrešan, Ž.; Kalajdžić, J.; Milić, B.; Kovač, R.; Barać, G. Interannual Variability in Apricot Quality: Role of Calcium and Postharvest Treatments During Cold Storage and Shelf Life. Horticulturae 2025, 11, 1140. https://doi.org/10.3390/horticulturae11091140
Milović M, Magazin N, Mastilović J, Kevrešan Ž, Kalajdžić J, Milić B, Kovač R, Barać G. Interannual Variability in Apricot Quality: Role of Calcium and Postharvest Treatments During Cold Storage and Shelf Life. Horticulturae. 2025; 11(9):1140. https://doi.org/10.3390/horticulturae11091140
Chicago/Turabian StyleMilović, Maja, Nenad Magazin, Jasna Mastilović, Žarko Kevrešan, Jelena Kalajdžić, Biserka Milić, Renata Kovač, and Gordana Barać. 2025. "Interannual Variability in Apricot Quality: Role of Calcium and Postharvest Treatments During Cold Storage and Shelf Life" Horticulturae 11, no. 9: 1140. https://doi.org/10.3390/horticulturae11091140
APA StyleMilović, M., Magazin, N., Mastilović, J., Kevrešan, Ž., Kalajdžić, J., Milić, B., Kovač, R., & Barać, G. (2025). Interannual Variability in Apricot Quality: Role of Calcium and Postharvest Treatments During Cold Storage and Shelf Life. Horticulturae, 11(9), 1140. https://doi.org/10.3390/horticulturae11091140






