1. Introduction
Urbanization and rapid industrialization have intensified environmental degradation, prompting a growing emphasis on introducing ornamental and native plants into urban areas with the aim of improving aesthetic environments and restoring ecosystems [
1,
2]. As urban gardens can be established even in relatively small spaces, they serve as an effective greening strategy in cities where it is challenging to expand green areas, also contributing to increased urban biodiversity [
3].
Native herbaceous plants of Northeast Asia, such as
Pseudolysimachion linariifolium (Pall. ex Link) Holub, are widely utilized in urban gardens due to their adaptability and ecological value [
4,
5]. However, recent research has primarily focused on the identification of bioactive compounds [
6,
7], while direct assessments of their responses to environmental stress remain limited. In contrast, physiological and morphological studies on closely related Veronica species and other herbaceous plants have yielded valuable insights into adaptation mechanisms under flood stress [
8,
9,
10,
11,
12]. These findings highlight the necessity for targeted investigations on
P. linariifolium to comprehensively evaluate its potential as a resilient species for urban greening and sustainable landscape management under changing climatic conditions.
In rapidly urbanized regions, such as South Korea, soil impermeability due to surface sealing reduces the drainage capacity, leading to unstable soil moisture conditions and increasing the risk of both droughts and floods [
13]. Given South Korea’s climatic conditions, it is challenging to maintain the moisture of urban garden plants because of the frequent occurrence of intermittent droughts in spring and autumn, heavy summer rainfall, and flood damage caused by typhoons [
14].
Water is one of the most critical environmental factors that influence plant growth, although it is often limited in availability. Throughout their growth, plants frequently encounter either an excess or deficiency of water [
15]. Water stress caused by climate change has emerged as a significant challenge [
16]. Initial responses to water stress are known to involve mechanisms that enhance the conservation of water and the plant’s efficient use of water, such as leaf expansion, stem elongation, and stomatal closure influenced by turgor pressure [
17]. Under severe stress, photosynthetic activity decreases, osmotic regulation is lost, and metabolic dysfunction occurs, potentially causing permanent damage [
18,
19,
20,
21]. Prolonged soil waterlogging also drastically reduces soil oxygen levels [
22] and impairs root respiration, which inhibits nutrient and water uptake [
23,
24]. This leads to stomatal closure and reduced stomatal conductance, which significantly reduces physiological processes such as photosynthesis [
25,
26,
27]. Plants employ various survival strategies that involve the interaction of multiple physiological and morphological mechanisms to cope with water stress [
12,
23,
28].
Recent advances in research on abiotic stress have led to the implementation of non-destructive vegetation index analysis using leaf spectral reflectance, as well as remote sensing techniques based on multispectral and hyperspectral imagery [
29,
30,
31]. Vegetation indices, such as the Normalized Difference Vegetation Index (NDVI) and Green Normalized Difference Vegetation Index (GNDVI), indirectly reflect photosynthetic activity and chlorophyll content, making them valuable tools for assessing plant growth status and for diagnosing stress [
32,
33]. Accordingly, vegetation indices have been proposed as important indicators to monitor the effects of water stress on plant cover and growth conditions [
34,
35,
36].
NDVI and GNDVI are widely used vegetation indices that effectively capture variations in chlorophyll content and photosynthetic activity. NDVI has been extensively validated as a robust indicator of plant health and biomass, while GNDVI offers improved sensitivity to changes in chlorophyll concentration under stress conditions [
37,
38,
39,
40]. In addition, BGI, VARI, GLI, and NGRDI were selected due to their sensitivity to specific vegetation traits or environmental variations, providing complementary insights into chlorophyll content, leaf coloration, and stress-induced changes, as supported by recent studies [
41,
42,
43,
44]. Collectively, these indices have demonstrated utility in detecting water stress across various plant species, making them especially suitable for evaluating the physiological responses of
Pseudolysimachion linariifolium under waterlogging in this study.
The Fourth Industrial Revolution has spurred drone use tailored to user objectives to acquire high-spatial-resolution imagery of targeted small areas [
45]. This technological progress has led to a surge in vegetation studies that employ drones, particularly those equipped with multispectral sensors [
46,
47]. By integrating drone-based multispectral imaging, monitoring techniques originally developed for satellite platforms have been directly applied to vegetation research at finer spatial scales [
48,
49,
50]. The evolution of remote sensing technologies using multispectral drones and integrating diverse imaging types such as digital (RGB), thermal, and hyperspectral imagery has enabled comprehensive analytical approaches. UAVs combining these image types have become increasingly common in vegetation research; multi-sensor fusion now facilitates more comprehensive, real-time vegetation monitoring and analysis [
46,
51,
52,
53,
54].
Despite these advances, little is known about the physiological response of P. linariifolium to waterlogging stress, especially in urban garden settings. Our study aims to evaluate these physiological responses using non-destructive UAV-based multispectral vegetation indices. This research seeks to provide foundational data to establish principles for water level maintenance and growth monitoring of garden plants in future urban greening projects.
2. Materials and Methods
2.1. Plant Materials and Pre-Treatment Conditions
We conducted a waterlogging stress experiment on
P. linariifolium (Pall. ex Link) Holub in a greenhouse at the Hankyong National University Experimental Farm (37°0′42.31″ N, 127°19′12.48″ E). The plant materials were purchased from a nursery in June 2023 and subsequently were transplanted into pots in the university greenhouse.
Figure 1 displays the site—the field compartment and the plant samples for the experiments—of the groups of
P. linariifolium plants subject to treatment with waterlogging stress.
Rectangular pots measuring 143 × 143 × 160 mm were used for transplantation. After potting, the plants were acclimatized to a uniform mode of fertilization and irrigation conditions for 30 days before the waterlogging stress experiment was initiated. At the beginning of this period, each pot was fertilized once with Perfect All, a commercial fertilizer for floriculture, at a rate of 0.5 g per pot (corresponding to 15-15-15 N-P2O5-K2O per 10 are). During the 30-day period, each pot was irrigated daily with 600 mL of water to maintain uniform conditions.
2.2. Experimental Plot Preparation
2.2.1. Experimental Soil
The soil used in this study was collected from the top 10 cm layer of the upland field at the Hankyong National University Experimental Farm. The collected soil was air-dried for one week to remove residual moisture. After air-drying, the soil samples were passed through a 2 mm soil sieve to separate particles larger than 2 mm, such as stones, minerals, and agricultural debris. The pre-treated soil was then mixed with sand in a ratio of 2:1 (soil:sand) to enhance permeability and aeration. This mixture was used as the experimental soil for the waterlogging stress treatments.
2.2.2. Physical Properties of Experimental Soil
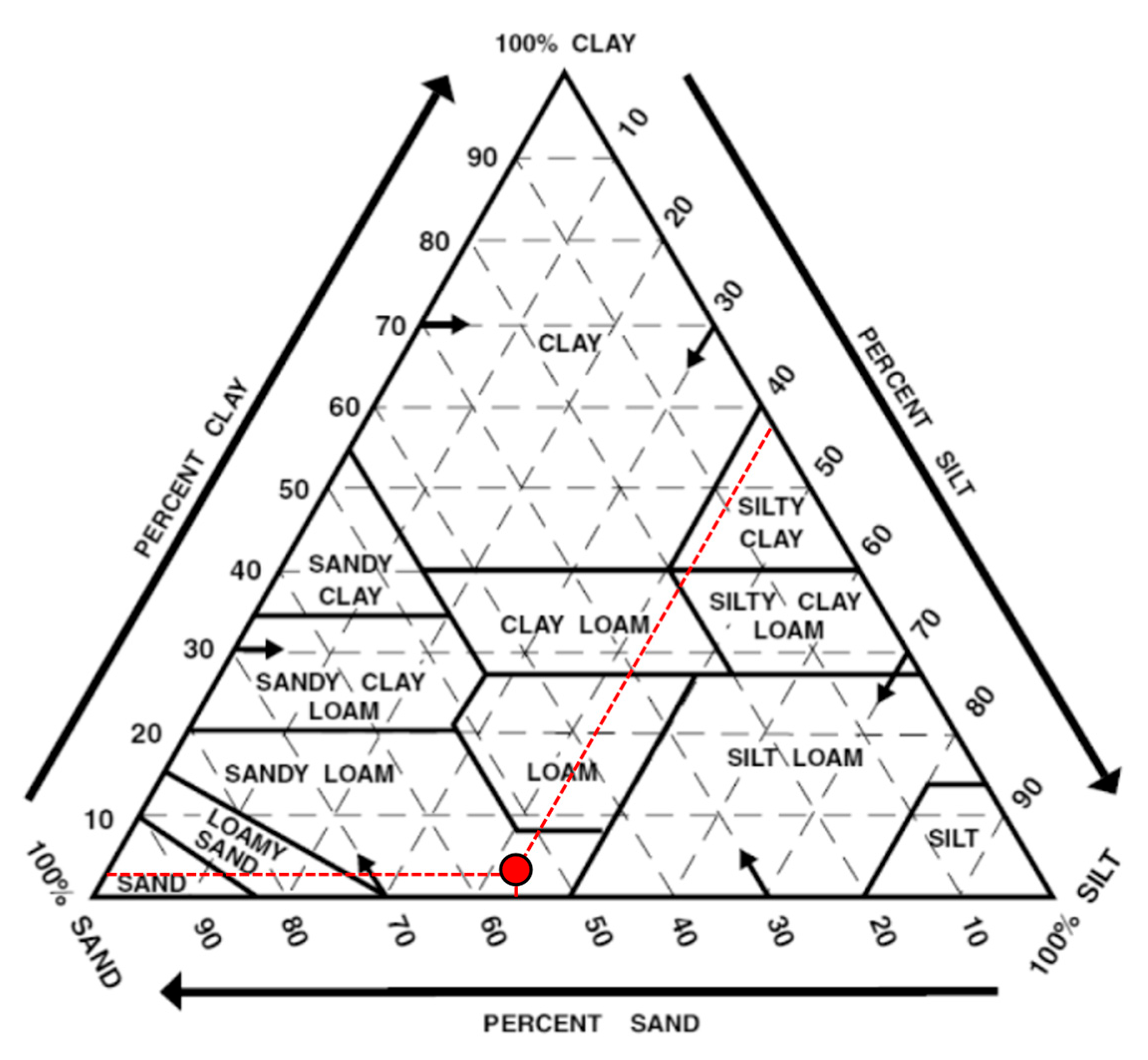
The soil texture of the samples used in this experiment was analyzed using the micro-pipette method and classified according to the USDA soil texture triangle [
55]. The analysis indicates that the soil consists of 0.6% clay, 43% silt, and 56% sand.
As shown in
Figure 2, the experimental soil falls in the sandy loam category on the USDA soil texture triangle on the basis of the above values.
2.2.3. Waterlogging Stress Treatment and Water Management
After plant acclimatization, the experiment was conducted using two treatment groups: a uniformly irrigated control group and a waterlogging treatment group, which was maintained in saturated conditions through surface waterlogging.
Angle-type drippers were used for irrigation by installing one dripper in each pot to deliver water directly to the soil through drip irrigation. During the acclimatization period, each potted plant was irrigated daily with 600 mL of water for 30 days. Waterlogging stress treatments were initiated once acclimatization was achieved following transplantation.
For the control group, we maintained the same irrigation regime used during plant acclimatization, supplying 600 mL of water daily per pot to sustain optimal moisture conditions. For the waterlogging treatment group, as shown in
Figure 1b, each pot was placed inside a larger plastic container (referred to as a “living box”) to enable controlled waterlogging. Water was added to each container until it reached and slightly covered the soil surface of the pot, ensuring continuous soil saturation.
The water level was visually checked daily, and if it dropped below the soil surface due to evaporation or drainage, water was replenished as necessary to maintain surface saturation throughout the 30-day treatment period.
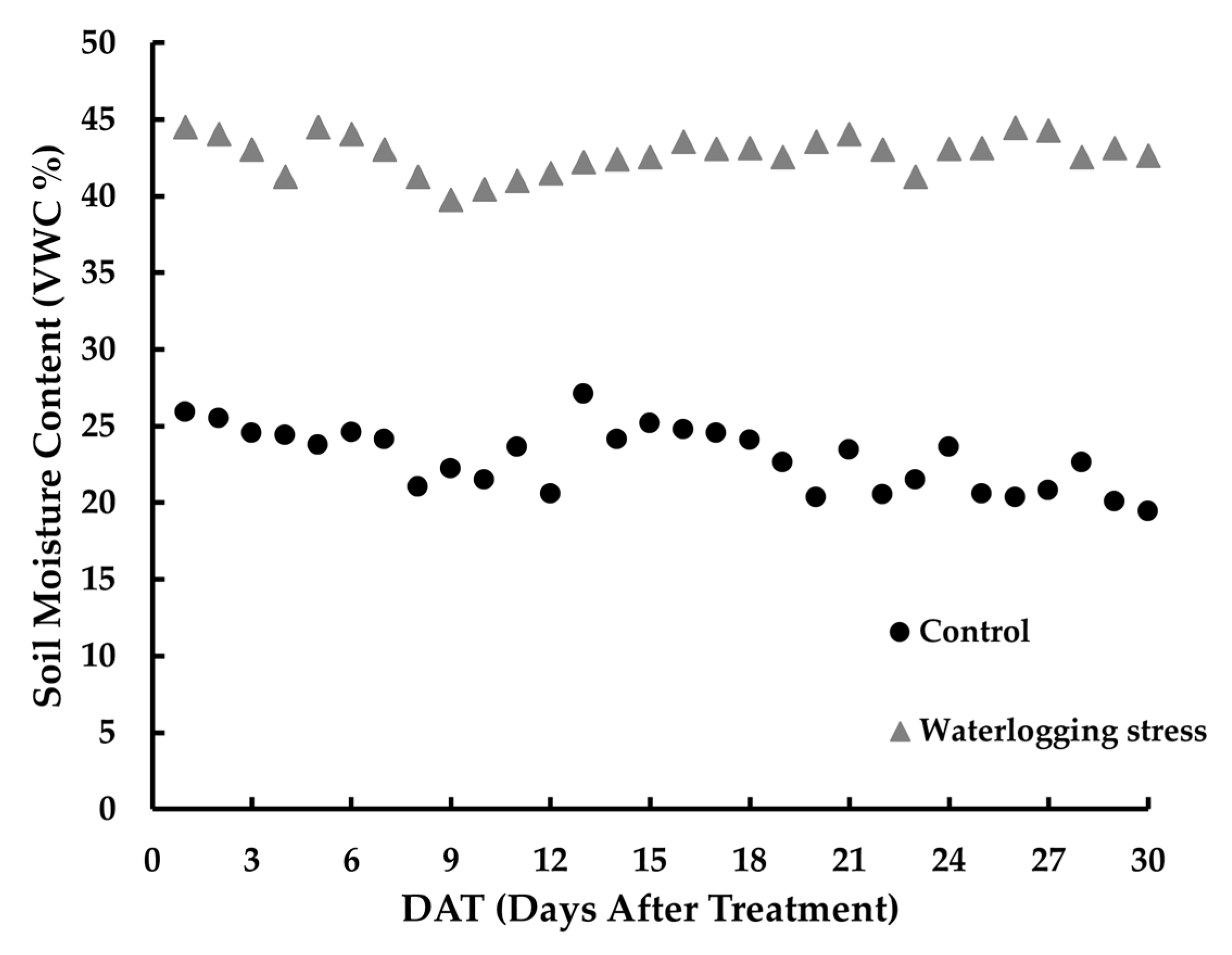
In each treatment group, two out of the four replicate pots (n = 2) were equipped with soil moisture sensors (WaterScout SM 100, Spectrum Technologies, Inc., Aurora, IL 60504, USA) inserted at a depth of 10 cm from the soil surface. Volumetric water content was recorded at one-hour intervals.
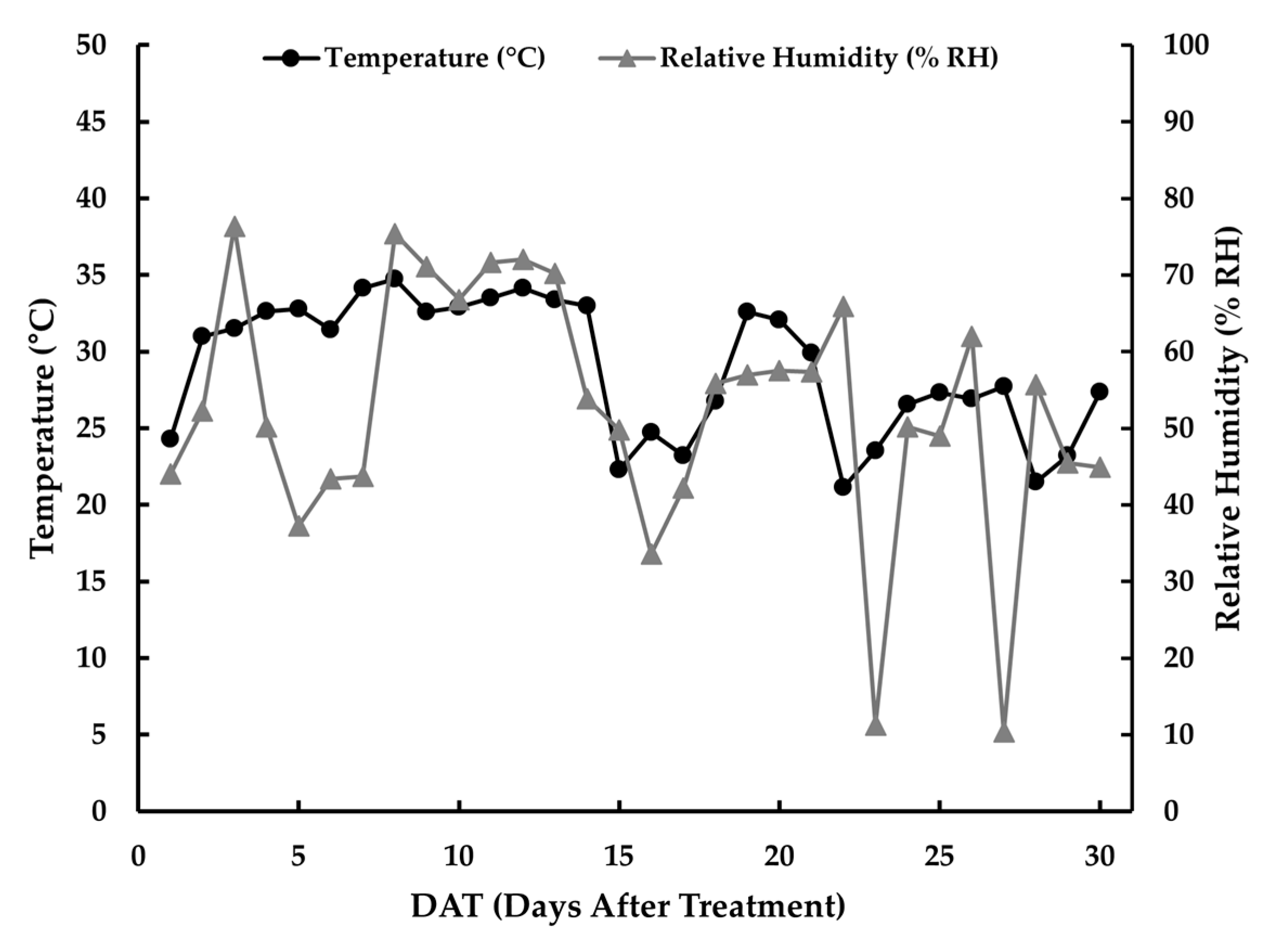
The moisture data that were recorded to track changes in soil moisture content were stored in a data logger (WatchDog 1000 Series Watermark Irrigation Micro Stations, Spectrum Technologies, Inc., Aurora, IL 60504, USA). Additionally, a digital thermo-hygrometer was used to monitor the temperature and the relative humidity inside the greenhouse at one-hour intervals (Testo 174 H, Titisee-Neustadt, Germany).
2.3. Data Measurement Methods
Multispectral Drone Imagery
A multispectral drone (Mavic 3 Multispectral, DJI Enterprise, Shenzhen, China) equipped with both an RGB camera and a multispectral camera was used to monitor changes in response to waterlogging stress among the experimental groups of the target plants. The RGB camera collected digital images in three bands: R445 (blue), R545 (green), and R650 (red). For the same image area, the multispectral camera acquired grayscale images at R560 ± 16 nm (green), R650 ± 16 nm (red), R730 ± 16 nm (red-edge), and R860 ± 26 nm (near-infrared). Five image replicates were acquired for each treatment group at a height of 3 m for the waterlogging stress experiment at 3, 6, 9, 12, 15, 20, and 30 days after treatment (DAT).
2.4. Image Data Processing
2.4.1. RGB Image Processing
RGB images collected through multispectral drone measurements were analyzed using the R software (ver. 4.3.2) environment [
56]. Image preprocessing was performed using the R packages ‘FIELDimageR’ and ‘FIELDimageR.Extra’. The ‘fieldview’ function was used to assign the EPSG:4326 geographic coordinate system to the images. The region of interest (ROI) was defined for subsequent analysis.
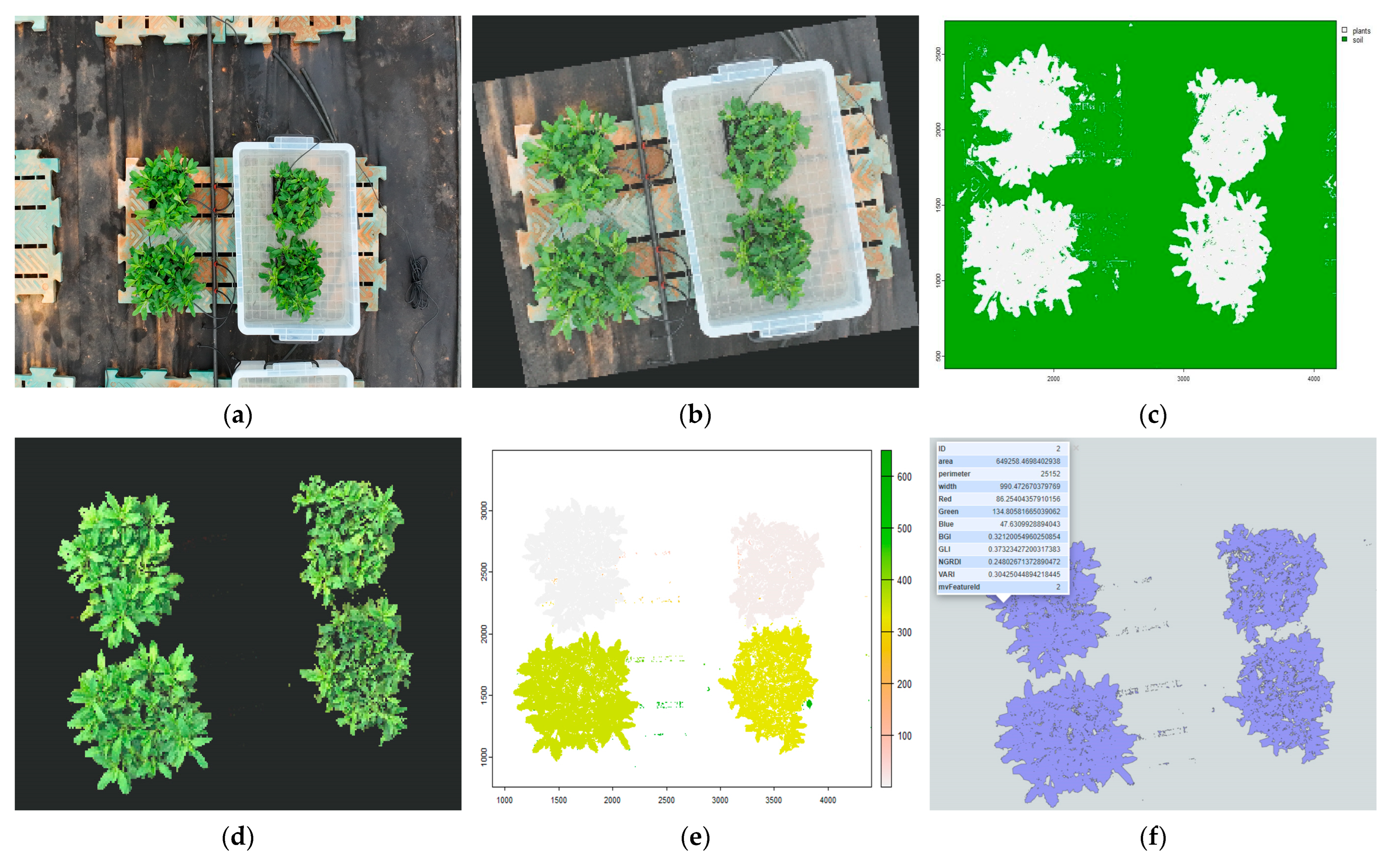
We implemented a supervised classification approach based on a random forest model to distinguish plant objects from the background. Representative training samples for both soil (background) and plant classes were manually selected from the mosaic image using the ‘fieldView’ function, with 1000 random points sampled for each class and labeled accordingly. The training samples were combined into a single dataset.
The ‘fieldSegment’ function was then applied to the mosaic image using the prepared training samples to perform supervised segmentation through the random forest algorithm. This process generated a classification model and predicted class labels for each pixel, resulting in a classified raster in which plant objects and background soil were clearly distinguished. The ‘fieldView’ and ‘plot’ functions visually inspected and verified the classification results.
Following background removal, the ‘fieldCount’ function was used to mask individual plant objects to enable the calculation of mean RGB image vegetation indices (RGB-VIs) for each object, including BGI, GLI, NGRDI, and VARI (
Table 1). The overall workflow of the image processing procedure is illustrated in
Figure 3.
2.4.2. Multispectral Image Processing
Multispectral images acquired with the multispectral camera were processed using R (ver. 4.3.2) and the Quantum Geographical Information System (QGIS ver. 3.42.1) [
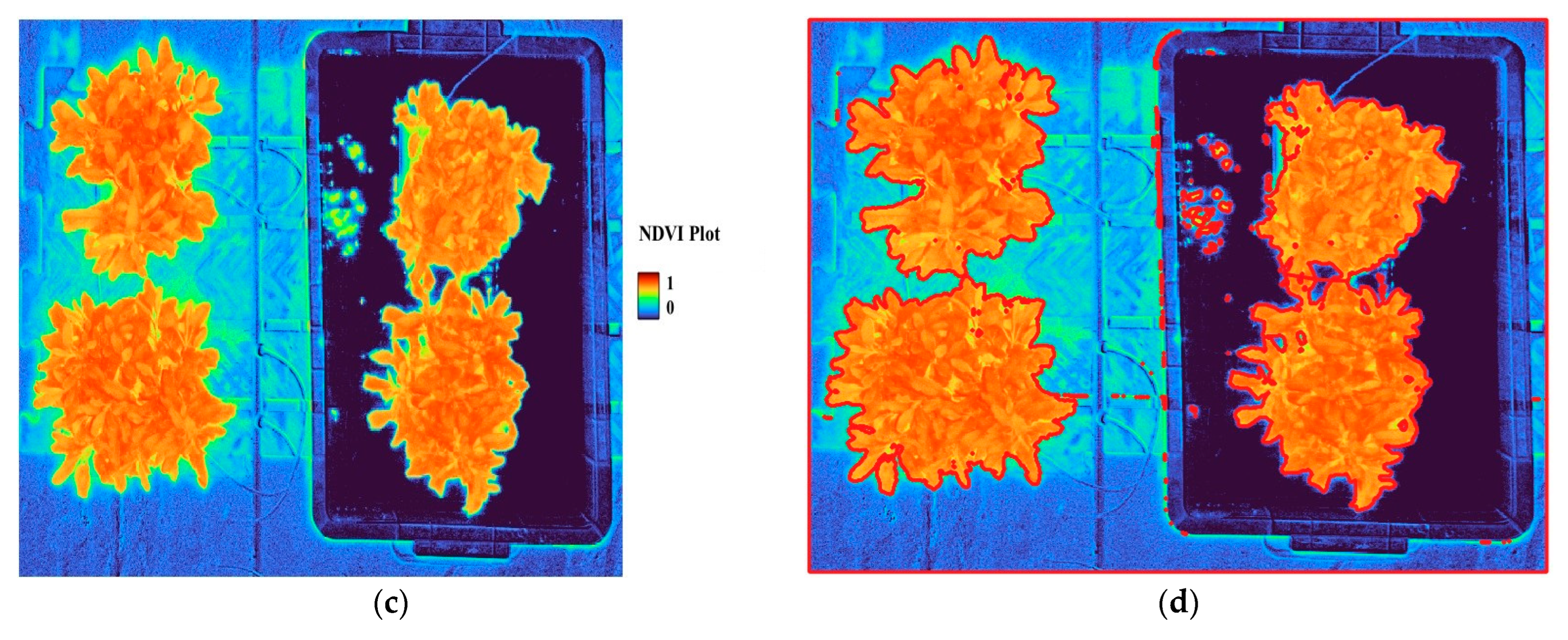
61]. Georeferencing was used to align the positions and orientations of the grayscale images for each wavelength. The aligned images were then used to calculate vegetation indices—specifically, NDVI and GNDVI—using the ‘raster calculator’ function in QGIS.
The resulting raster images were converted to vector format to delineate plant object boundaries as polygons. Using both the preprocessed raster and the vector images, we performed zonal statistics to extract vegetation index values (NDVI, GNDVI) for each plant object, including mean, variance, maximum, and minimum values (
Table 2).
Furthermore, following the DJI Enterprise image processing guide [
64], the raw grayscale image digital number (DN) values were radiometrically corrected using sensor metadata. Pixel values were converted to reflectance. The R packages ‘terra’ and ‘exifr’ were used to extract metadata for each band, including black level, gain, exposure time, and irradiance. Radiometric correction was performed using the following equations:
Radiance calculation for each pixel:
Through this radiometric correction process, pixel values for each band were converted to actual reflectance values that were then used to calculate vegetation indices. The workflow for multispectral image processing is illustrated in
Figure 4.
2.5. Statistical Analysis
All statistical analyses were performed using R software (version 4.3.2). To evaluate and predict waterlogging stress responses in P. linariifolium, one-way analysis of variance (ANOVA) was conducted to compare spectral vegetation indices, image-based indices (RGB-VIs), and soil moisture content among treatments. Duncan’s multiple range test was used to assess the significance of differences between treatments. Soil moisture content data were normalized using min–max scaling and analyzed based on cumulative values over the experimental period.
Correlation analysis and principal component analysis (PCA) were performed to identify optimal predictive variables among those displaying significant differences. The response patterns of control and waterlogging-treated groups were classified with hierarchical clustering analysis. Gower distance was calculated to handle mixed data types (categorical and continuous variables), and Ward’s method was used to merge clusters to minimize within-cluster variance. The optimal number of clusters was determined using the elbow method based on within-cluster sum of squares (WSS), and the results were visualized in a two-dimensional space. Silhouette analysis was used to evaluate the final clusters to see if they were adequate.
Based on the selected optimal variables, multiple regression analysis was performed to assess the relative impact of independent variables (spectral vegetation indices and image-based indices) on the dependent variable (cumulative volumetric soil moisture content) under conditions of waterlogging stress. The significance of each regression coefficient (p-value) was tested to identify independent variables with statistically significant effects on the dependent variable. Multicollinearity was assessed using the variance inflation factor (VIF). Variables with VIF greater than 10 were excluded from the model.
Model performance metrics and residual analysis were used to evaluate the overall fit of the multiple regression model. The normality of residuals was checked using a Q–Q plot, and homoscedasticity was examined using a residuals vs. fitted plot. All hypothesis tests were conducted at a significance level of 0.05.
4. Discussion
Our study quantitatively evaluated the physiological responses of
P. linariifolium to the stress of waterlogging using a non-destructive, image-based approach and identified key predictive variables. Various vegetation indices were calculated from RGB and multispectral imagery acquired by drones. The vegetation indices were then analyzed in combination with soil moisture data recorded by sensors. Unlike conventional physiological and biochemical analyses, this approach allows for consistent monitoring and offers potential applicability in field conditions. The results reaffirm the effectiveness of image-based evaluation methods [
65,
66,
67].
Waterlogging is representative of environmental stress that cuts off the oxygen supply to the soil and suppresses root respiration [
68,
69,
70,
71], leading to an overall decline in physiological function [
56,
57,
58]. In the present study, we observed a gradual decline in the growth status of
P. linariifolium with the increasing duration of waterlogging. This indicates progressive accumulation of the physiological effects of waterlogging over time. A significant reduction in the aboveground traits, such as leaf area and chlorophyll content—indirectly inferred from changes in vegetation indices—suggest the potential of image-based monitoring to evaluate stress-induced physiological responses and to confirm the validity of image-derived quantification.
Soil moisture measurements showed that the control maintained an average moisture content of 22.91%, whereas the waterlogging treatment consistently maintained high levels close to saturation, which averaged 42.77%. These contrasting moisture conditions likely functioned as a clear physical stress factor that is potentially related to inhibited root respiration and oxygen deprivation [
23,
24,
72,
73]. Distinct physiological differences between the control and the treatment groups appeared after 6 DAT, indicating that the stress response accumulates over time before manifesting [
22].
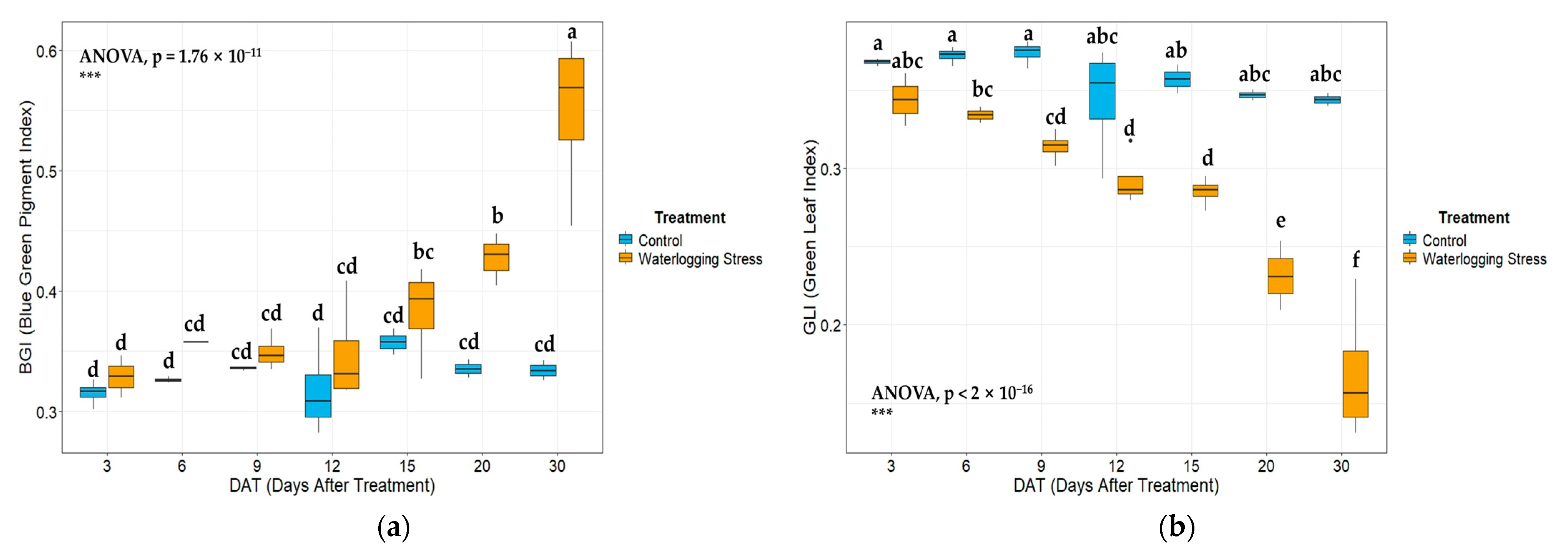
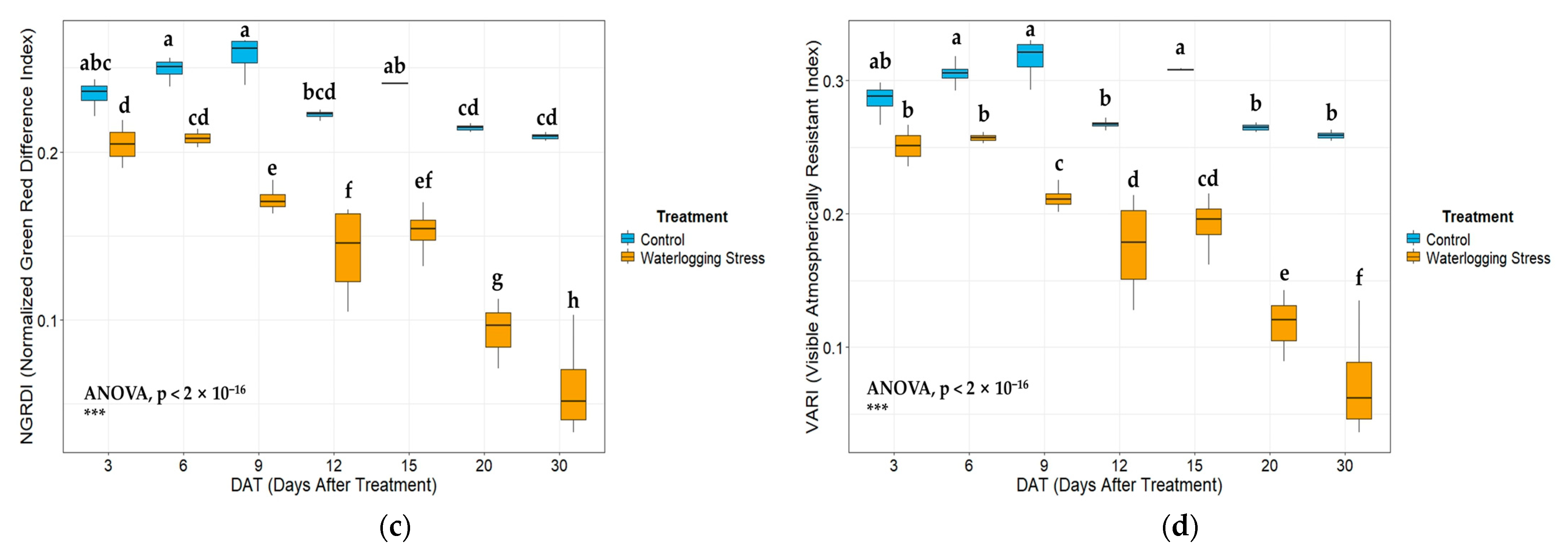
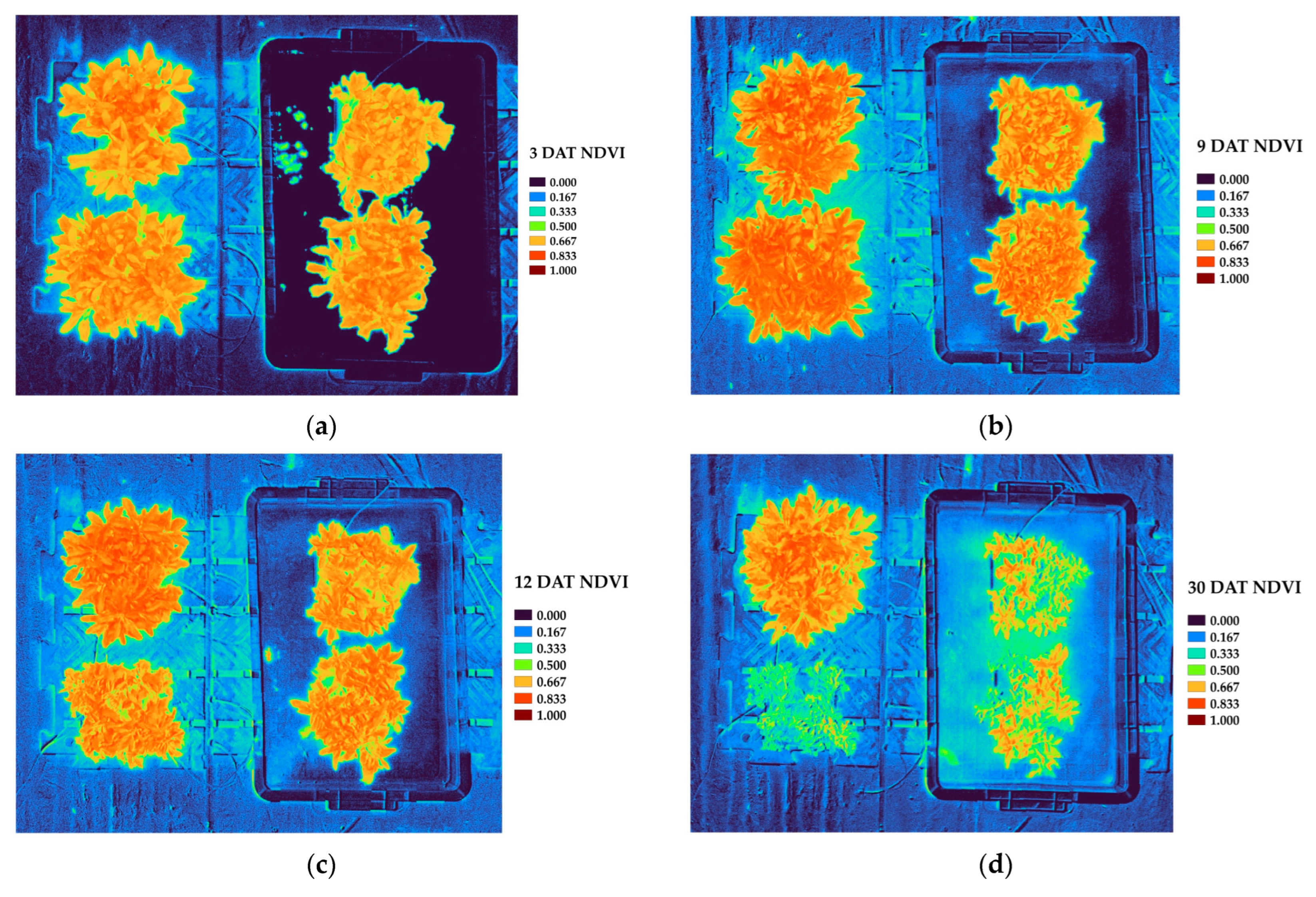
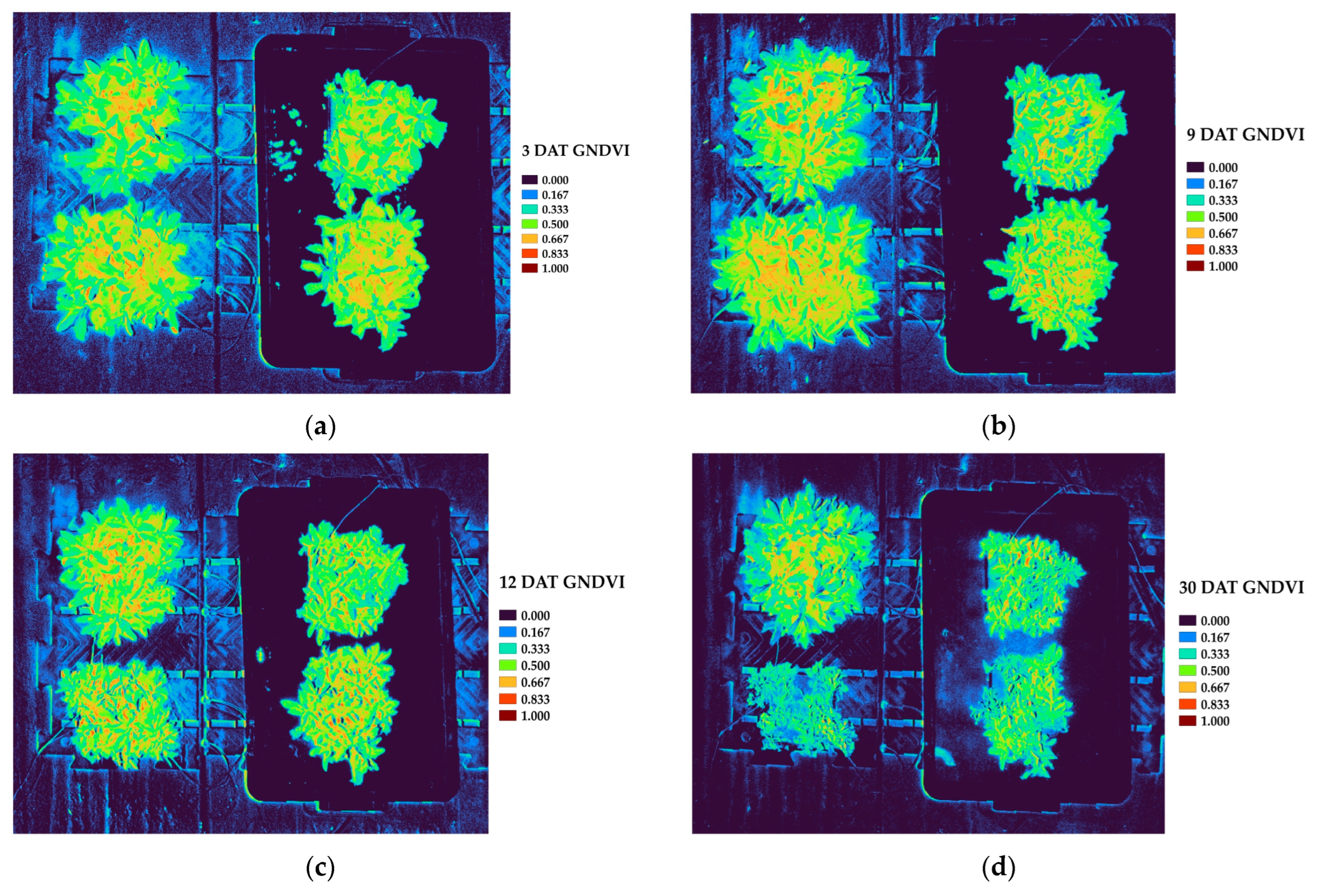
In the vegetation index analysis, key indices that include NDVI, GNDVI, GLI, VARI, and NGRDI exhibited declining trends under waterlogged conditions. The physiological suppression induced by stress is effectively reflected by the significantly low values of NDVI and GNDVI in waterlogged plots, which are closely correlated with photosynthetic activity and chlorophyll content [
63,
74,
75].
NDVI and GNDVI are widely recognized for their sensitivity to chlorophyll concentration and photosynthetic capacity, making them reliable indicators of plant vigor and stress-induced functional decline [
76,
77,
78]. RGB-based indices such as GLI, NGRDI, and VARI assess changes in reflectance within the visible spectrum, capturing alterations in leaf coloration associated with chlorophyll degradation, chlorosis, and yellowing, thus providing early, non-destructive markers of physiological stress [
79,
80]. Conversely, BGI quantifies increases in brownish pigments related to tissue senescence and damage [
57], reflecting advanced stages of waterlogging stress.
The gradual decline in vegetation index values over time under continuous waterlogging is consistent with previous findings of excess moisture that negatively affects plant growth [
81,
82,
83,
84,
85]. Our findings reinforce the utility of image-based remote sensing as a tool to capture the temporal and spatial dynamics of plant stress.
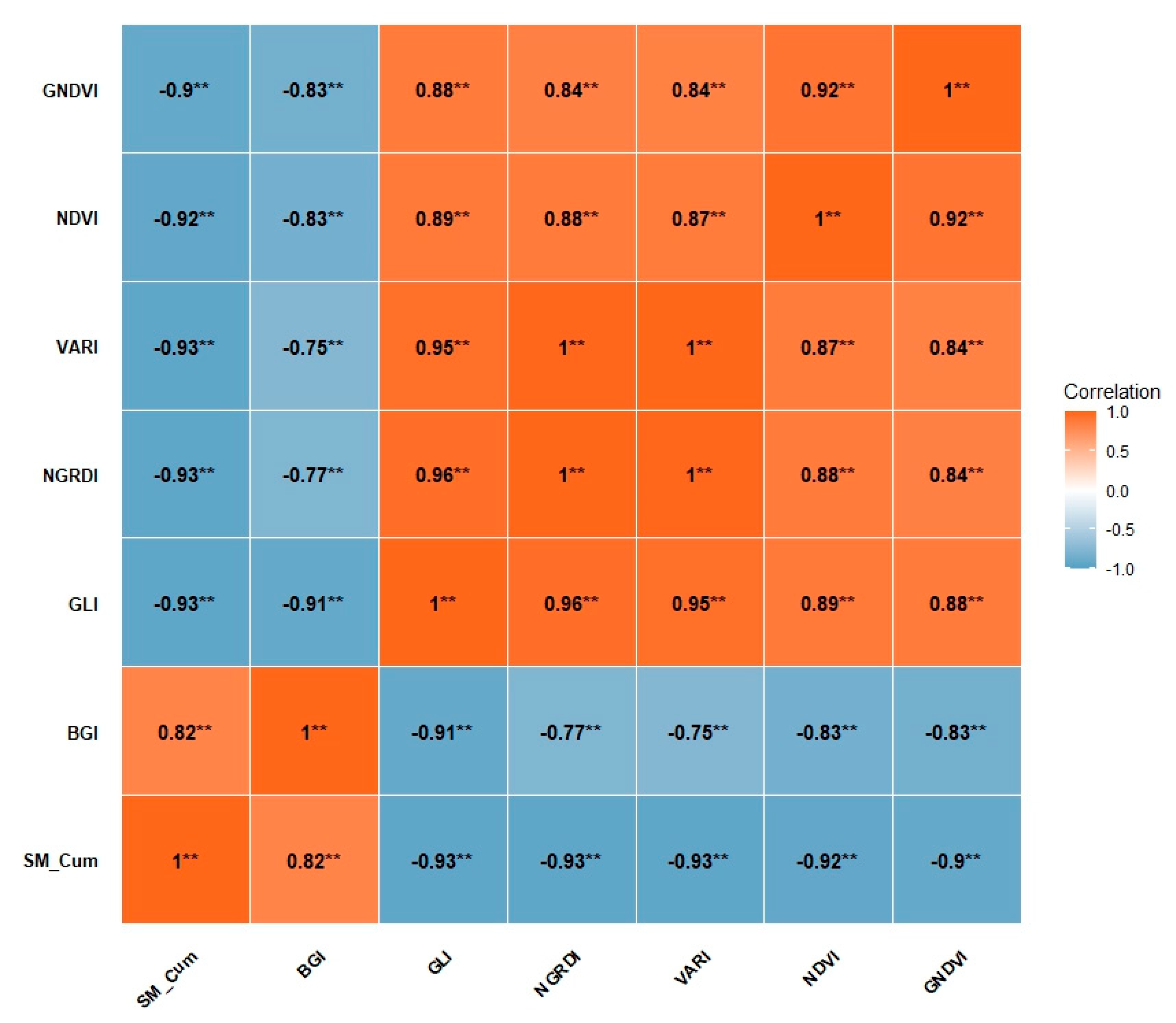
Correlation analysis indicated significant negative relationships between cumulative volumetric soil moisture and key vegetation indices. This result suggests a strong link between soil water levels and plant health status [
86,
87,
88], emphasizing the importance of water management as a factor that directly influences plant growth.
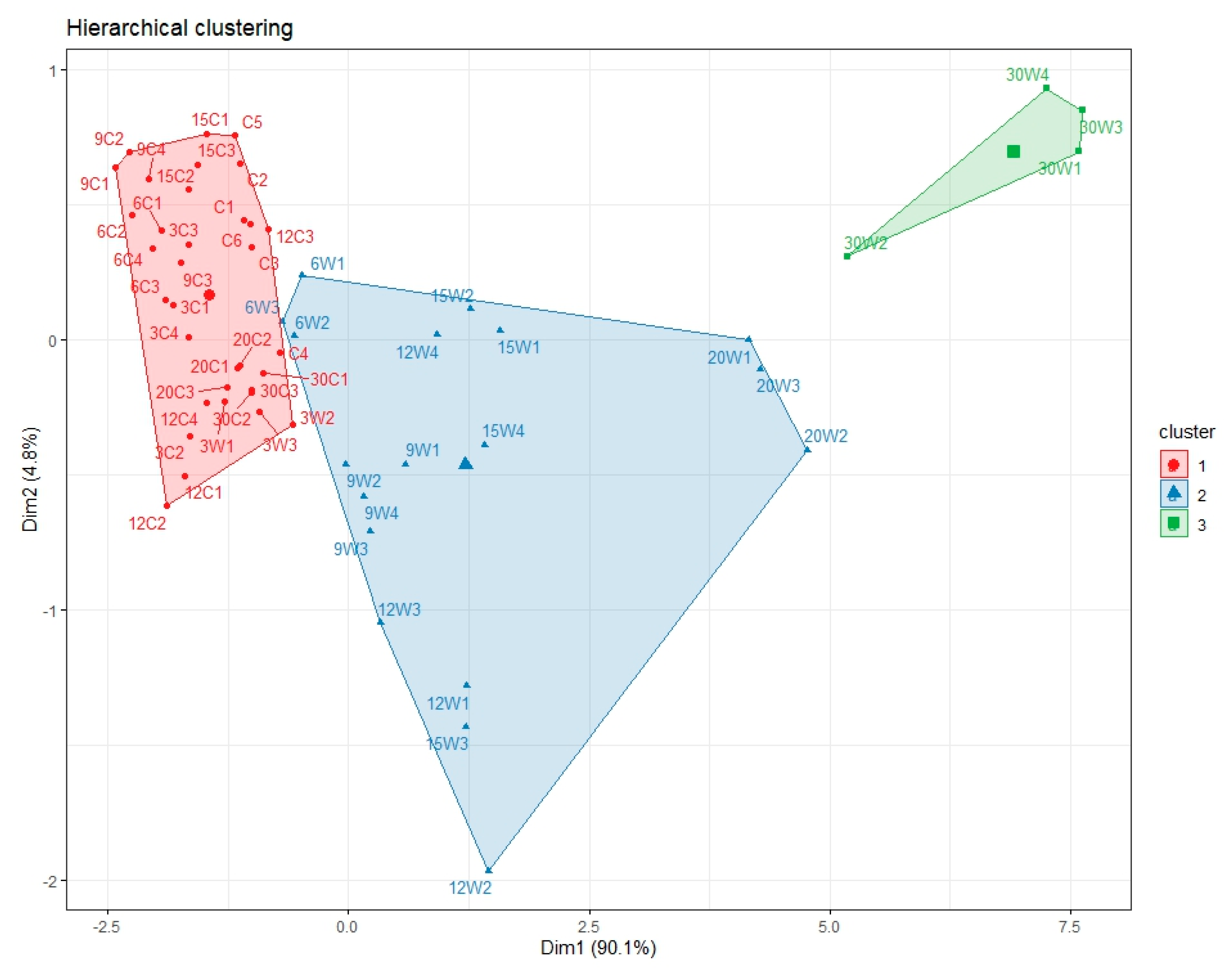
Principal component analysis (PCA) results showed that major vegetation indices were strongly associated with the first principal component (PC1) and were clearly separated according to waterlogging treatment. High values of indices, such as NDVI, GNDVI, and VARI, were representative of the control group cluster, and the high values of BGI and accumulated soil moisture were associated with the waterlogged group cluster. These results confirm that the vegetation indices show distinct patterns in a multidimensional space under stress conditions [
89], indicating their usefulness as classification tools to distinguish stress environments.
Hierarchical clustering analysis identified three main clusters corresponding to three distinct stress levels over time. Early- and late-stage stress groups were clearly differentiated from the control, with cluster boundaries becoming more distinct after 9 DAT. This suggests that physiological responses to waterlogging are cumulative over time and may become irreversible beyond a certain point.
The final multiple regression model, using only NDVI and GNDVI as predictors, explained 89.7% of the cumulative volumetric water content. The multiple regression model fulfilled all the assumptions of linear regression, including normality and homoscedasticity. This highlights the potential to construct high-quality predictive models using non-destructive image-derived data, which contributes to the automation and refinement of field monitoring systems [
90]. Furthermore, since NDVI and GNDVI are widely applicable across both satellite and UAV platforms, their compatibility supports broad implementation in future monitoring frameworks with improved spatial resolution and sensor diversity.
Despite the promising predictive ability of the model, it is recognized that the model’s structure and variables included have limitations. Multicollinearity was carefully addressed by excluding variables with high VIF values, and the final model retained NDVI and GNDVI with acceptable VIFs. However, other potentially influential environmental or physiological variables were not included due to data constraints. Future improvements should be explored by incorporating additional relevant variables and advanced modeling techniques to enhance prediction accuracy and robustness under diverse field conditions.
Our study provides baseline data to quantify the stress response of ornamental or non-crop species like P. linariifolium through image-based methods. Our findings may serve as a helpful reference to develop management systems for urban green spaces or garden plants. Nevertheless, our study has the following limitations: (1) a semi-controlled greenhouse, where soil moisture was precisely controlled to simulate waterlogging stress, while ambient environmental conditions were relatively natural; (2) physiological metrics like leaf temperature or stomatal conductance were not simultaneously measured; and (3) only specific growth stages were evaluated. Future research should focus on repetitive experiments under outdoor conditions and adopt strategies that integrate multiple stress factors and developmental stages.
We acknowledge that urban environments present complex challenges—notably, varying microclimates, background noise, and other abiotic stressors—that were not fully replicated in our semi-controlled greenhouse setup. These limitations are addressed in the manuscript. Future research will focus on long-term, repetitive field experiments in diverse urban settings to enhance model robustness and applicability under multiple stresses. Moreover, strategic integration of multiple stress factors and developmental stages will be critical for comprehensive assessments.
5. Conclusions
Our study utilized a non-destructive analysis of multispectral drone imagery to quantitatively assess changes in vegetation indices of P. linariifolium under the stress of waterlogging in a greenhouse environment. As the duration of the stress of waterlogging increased, we observed significant decreases in key spectral vegetation indices, such as NDVI and GNDVI, reflecting reduced photosynthetic capacity and chlorophyll content.
Importantly, our research demonstrates that waterlogging responses in urban ornamental forbs like P. linariifolium can be rapidly monitored using drone-based multispectral imagery. Our findings offer valuable foundational data that can form the basis for technologies that advance urban green space management and garden plant maintenance.
However, our study is limited by its focus on short-term waterlogging treatments under semi-controlled greenhouse conditions and the absence of long-term monitoring throughout the entire growing period. Future research should address these limitations with repetitive experiments in open-field conditions and incorporate a wide range of environmental factors and physiological indicators for comprehensive, long-term assessment.