Effect of Explant Physiology and Media Composition on Callogenesis of Vitellaria paradoxa Leaf Explants
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishment of Mother Plants
2.2. Assessment of Explant Physiology
2.3. Histological Studies
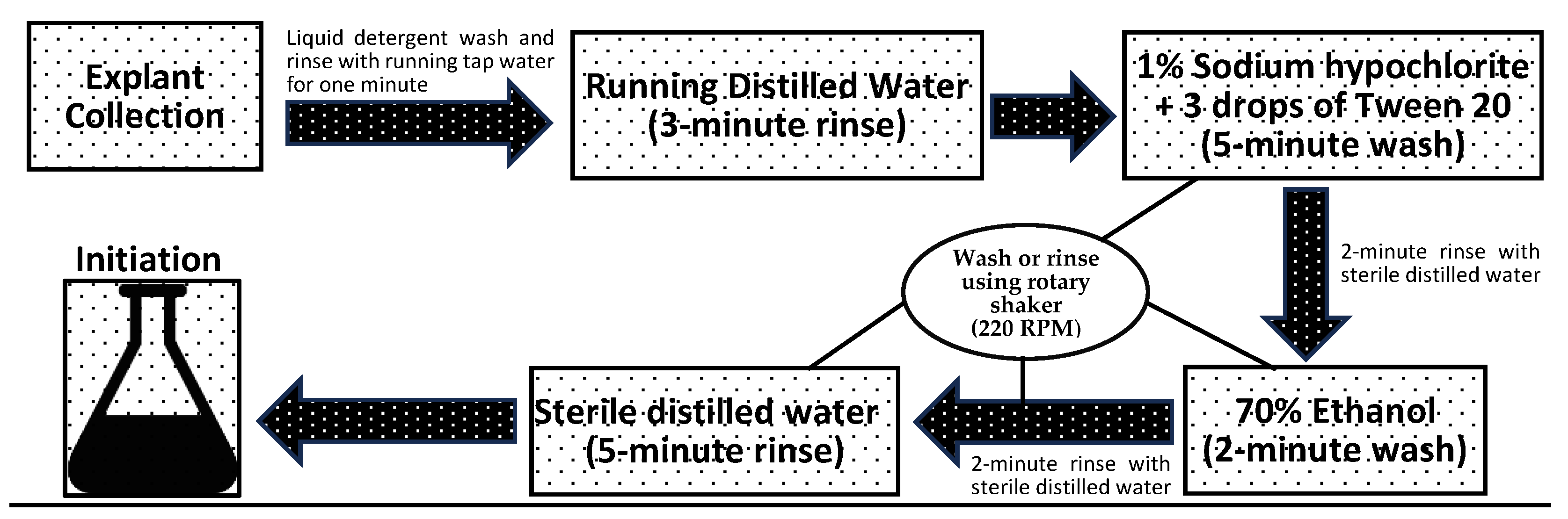
2.4. Explant Preparation
2.5. Culture Conditions and Callus Induction
2.6. Experimental Design and Data Analysis
3. Results
3.1. Growth Trend of V. paradoxa Leaf Explants: A 30-Day Assessment
3.2. Effect of Media Composition on Callogenesis
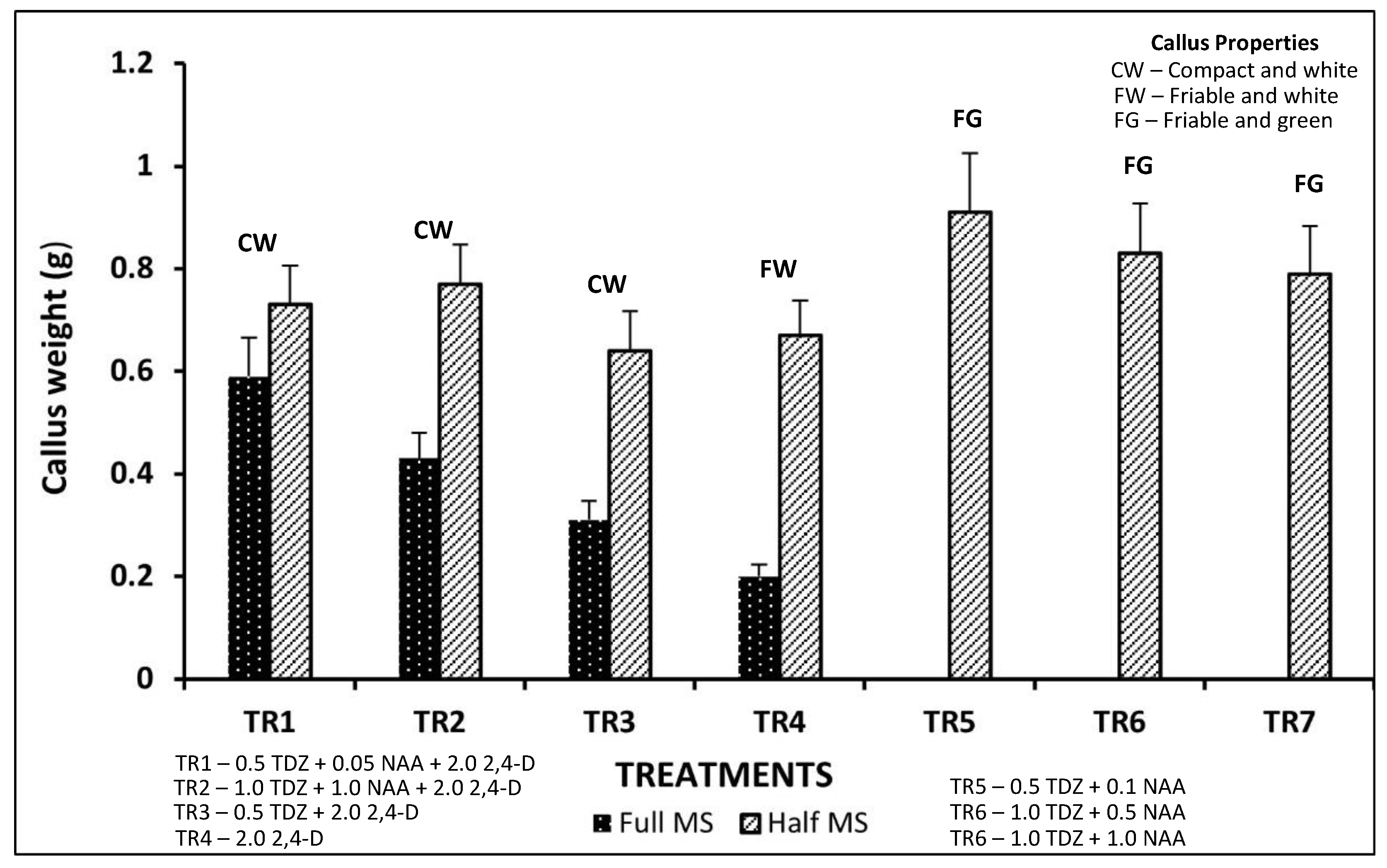
3.3. Effect of Media Composition on Callus Growth at Various Phases of Development

3.4. Effect of Media Composition on Growth of T1 and T2 Calli
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2,4-D | 2,4-Dichlorophenoxyacetic acid |
| BAP | 6-Benzylaminopurine |
| CIM | Callus induction medium |
| HCl | Hydrochloric acid |
| HSD | Honestly significant difference |
| KOH | Potassium hydroxide |
| NAA | Naphthalene acetic acid |
| TDZ | Thidiazuron |
References
- Choungo Nguekeng, P.B.; Hendre, P.; Tchoundjeu, Z.; Kalousová, M.; Tchanou Tchapda, A.V.; Kyereh, D.; Masters, E.; Lojka, B. The current state of knowledge of Shea butter tree (Vitellaria paradoxa cf gaertner.) for nutritional value and tree improvement in West and Central Africa. Forests 2021, 12, 1740. [Google Scholar] [CrossRef]
- Jepsen, T.; Stopponi, G.; Jørgensen, N.O. Shea tree (Vitellaria paradoxa CF Gaertn.) agroforestry systems in Northern Ghana: Population structure, management of trees and impact of below canopy microclimate. Agrofor. Syst. 2024, 98, 1493–1506. [Google Scholar] [CrossRef]
- Martínez, M.T.; Vieitez, A.M.; Corredoira, E. Improved secondary embryo production in Quercus alba and Q. rubra by activated charcoal, silver thiosulphate and sucrose: Influence of embryogenic explant used for subculture. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 121, 531–546. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, S.; Wu, T.; Wu, K.; Li, Y.; Zhang, X.; da Silva, J.A.T.; Zeng, S.; Ma, G. Shoot organogenesis and somatic embryogenesis from leaf and petiole explants of endangered Euryodendron excelsum. Sci. Rep. 2022, 12, 20506. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Asthana, P.; Rai, M.K.; Jaiswal, U. Somatic embryogenesis and plant regeneration from suspension cultures of Sapindus trifoliatus. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 157, 36. [Google Scholar] [CrossRef]
- Afful, N.T.; Abdulai, I.; Azu, E.; Elegba, W.; Annor, C.; Akama, C.; Asare, K.; Dentey, J.; Amoatey, H.M. In vitro regeneration of Vitellaria paradoxa from shoot tip explants. BioTechnologia 2022, 103, 71. [Google Scholar] [CrossRef]
- Attikora, A.J.P.; Silue, S.; Kone, M.; Silue, N.; Kwibuka, Y.; Yao, S.D.M.; De Clerck, C.; Him, S.L.; Diarrassouba, N.; Alabi, T.; et al. Efficient in vitro regeneration and genetic fidelity analysis of shea tree (Vitellaria paradoxa Gaertn) using ISSR markers. Electron. J. Biotechnol. 2025, 75, 28–38. [Google Scholar] [CrossRef]
- Afolabi, J.O.; Olorode, E.M.; Olomola, D.B.; Fasakin, Y.O.; Adekunle, E.A. Effects of different media strengths and hormone concentrations on in-vitro regeneration of Vitellaria paradoxa CF Gaertn. Niger. J. Biotechnol. 2020, 37, 150–158. [Google Scholar] [CrossRef]
- Lovett, P.N.; Haq, N. Progress in developing in vitro systems for shea tree (Vitellaria paradoxa CF Gaertn.) propagation. For. Trees Livelihoods 2013, 22, 60–69. [Google Scholar] [CrossRef]
- Adu-Gyamfi, P.K.K.; Barnor, M.; Dadzie, A.M.; Lowor, S.; Opoku, S.Y.; Opoku-Ameyaw, K.; Bissah, M.; Padi, F.K. Preliminary investigation on somatic embryogenesis from immature cotyledon explants of shea (Vitellaria paradoxa G.). J. Agric. Sci. Technol. B 2012, 2, 1171. [Google Scholar]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant Propagation by Tissue Culture, 3rd ed.; The Back Ground; Springer: Dordrecht, The Netherlands, 2008; pp. 65–175. [Google Scholar]
- Read, P.E.; Bavougian, C.M. In Vitro Rejuvenation of Woody Species. In Protocols for Micropropagation of Selected Economically-Important Horticultural Plants; Lambardi, M., Ozudogru, E., Jain, S., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 994. [Google Scholar] [CrossRef]
- Grzelak, M.; Pacholczak, A.; Nowakowska, K. Challenges and insights in the acclimatization step of micropropagated woody plants. Plant Cell Tiss. Organ. Cult. 2024, 159, 72. [Google Scholar] [CrossRef]
- Lv, Z.; Zhao, W.; Kong, S.; Li, L.; Lin, S. Overview of molecular mechanisms of plant leaf development: A systematic review. Front. Plant Sci. 2023, 14, 1293424. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F.A. Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ding, A.M.; Xu, C.T.; Xie, Q.; Zhang, M.J.; Yan, N.; Dai, C.B.; Lv, J.; Cui, M.; Wang, W.; Sun, Y. ERF4 interacts with antagonizes TCP15 in regulating endoreduplication cell growth in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 1673–1689. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Schmidt, S.; Beveridge, C. Plants in Action A Resource for Teachers and Students of Plant Science (II); Austrlaian and New Zealand Societes of Plant Sciences: 2010. Available online: https://rseco.org/ (accessed on 1 February 2025).
- Hayta, S.; Smedley, M.A.; Li, J.; Harwood, W.A.; Gilmartin, P.M. Plant regeneration from leaf-derived callus cultures of primrose (Primula vulgaris). HortScience 2016, 51, 558–562. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lin, M.Z.; Huang, B.; Chung, H.H.; Chen, J.T. Thidiazuron enhanced somatic embryogenesis is from callus lines of Arabica coffee and subsequent plant regeneration. Acta Biol. Cracoviensia s. Bot. 2018, 60, 35–44. [Google Scholar]
- Chung, H.H.; Ouyang, H.Y. Use of thidiazuron for high-frequency callus induction and organogenesis of wild strawberry (Fragaria vesca). Plants 2020, 10, 67. [Google Scholar] [CrossRef]
- Rezali, N.I.; Sidik, N.J.; Saleh, A.; Osman, N.I.; Adam, N.A.M. The effects of different strength of MS media in solid and liquid media on in vitro growth of Typhonium flagelliforme. Asian Pac. J. Trop. Biomed. 2017, 7, 151–156. [Google Scholar] [CrossRef]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. Vitr. Cell. Dev. Biol.-Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Mosoh, D.A.; Khandel, A.K.; Verma, S.K.; Vendrame, W.A. Optimizing callus induction and indirect organogenesis in non-dormant corm explants of Gloriosa superba (L.) via media priming. Front. Hortic. 2024, 3, 1378098. [Google Scholar] [CrossRef]
- Ndoumou, D.O.; Tchinda, N.D. Comparaison des Premières Étapes de L’embryogenèse Somatique chez Baillonella toxisperma et Vitellaria paradoxa (Sapotacées). Biotechnol. Agron. Soc. Environ. 2008, 12, 131–138. [Google Scholar]
- Aisagbonhi, E.P.; Isalar, C.E.; Odenore, V.D.; Ogbebor, J.U.; Eke, C.R.; Asemota, O.; Shittu, H.O. The interplay between explant developmental stages and phytohormone type in callogenesis of shea tree (Vitellaria paradoxa CF Gaertn). Euro. Int. J. Sci. Tech. 2015, 4, 50–57. [Google Scholar]
- Hurný, A.; Cuesta, C.; Cavallari, N.; Ötvös, K.; Duclercq, J.; Dokládal, L.; Montesinos, J.C.; Gallemí, M.; Semerádová, H.; Rauter, T.; et al. SYNERGISTIC ON AUXIN AND CYTOKININ 1 positively regulates growth and attenuates soil pathogen resistance. Nat. Commun. 2020, 11, 2170. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Strader, L.C. Interplay of auxin and cytokinin in lateral root development. Int. J. Mol. Sci. 2019, 20, 486. [Google Scholar] [CrossRef] [PubMed]
- Romanov, G.A. Perception, transduction and crosstalk of auxin and cytokinin signals. Int. J. Mol. Sci. 2022, 23, 13150. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. The components of plant tissue culture media I: Macro-and micro-nutrients. In Plant Propagation by Tissue Culture: Volume 1. The Background; Springer: Dordrecht, The Netherlands, 2008; pp. 65–113. [Google Scholar]
- Bar, M.; Ori, N. Leaf development and morphogenesis. Development 2014, 141, 4219–4230. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Seo, P.J. Varying auxin levels induce distinct pluripotent states in callus cells. Front. Plant Sci. 2018, 9, 1653. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK21054/ (accessed on 1 February 2025).
- Lee, J.; Kang, M.H.; Kim, J.Y.; Lim, P.O. The role of light and circadian clock in regulation of leaf senescence. Front. Plant Sci. 2021, 12, 669170. [Google Scholar] [CrossRef]
- Fehér, A. Callus, dedifferentiation, totipotency, somatic embryogenesis: What these terms mean in the era of molecular plant biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef]
- Watts, S.; Kariyat, R. Morphological characterization of trichomes shows enormous variation in shape, density and dimensions across the leaves of 14 Solanum species. AoB Plants 2021, 13, plab071. [Google Scholar] [CrossRef]
- Herman, E.B. Recent Advances in Plant Tissue Culture, X.X.I. Media and Techniques for Growth, Regeneration and Storage: 2011–2015; Agritech Consultants Inc.: Shrub Oak, NY, USA, 2015; 148p. [Google Scholar]
- Corredoira, E.; San-José, M.C.; Vieitez, A.M. Induction of somatic embryogenesis from different explants of shoot cultures derived from young Quercus alba trees. Trees Struct. Funct. 2012, 26, 881–891. [Google Scholar] [CrossRef]
- Kaur, S.; Boora, R.S.; Singh, D. Propagation studies in Sapodilla [Manilkara zapota (L.) P. Royen]: A Review. Agric. Rev. 2020, 41, 356–363. [Google Scholar] [CrossRef]
- Amghar, I.; Ibriz, M.; Ibrahimi, M.; Boudra, A.; Gaboun, F.; Meziani, R.; Iraqi, D.; Mazri, M.A.; Diria, G.; Abdelwahd, R. In Vitro Root Induction from Argan (Argania spinosa (L.) Skeels) Adventitious Shoots: Influence of Ammonium Nitrate, Auxins, Silver Nitrate and Putrescine, and Evaluation of Plantlet Acclimatization. Plants 2021, 10, 1062. [Google Scholar] [CrossRef]
- Akkenapally, S.; Mudalkar, S.; Bodiga, S.; Sundaram, R.; Bokam, A.K. In vitro callus generation somatic embryogenesis from leaf explant of Madhuca longifolia var latifolia (Roxb), A. Chev. Vegetos 2024, 1–7. [Google Scholar] [CrossRef]
- Coskun, Y.; Duran, R.E.; Kilic, S. Striking effects of melatonin on secondary metabolites produced by callus culture of rosemary (Rosmarinus officinalis L.). Plant Cell Tissue Organ Cult. (PCTOC) 2019, 138, 89–95. [Google Scholar] [CrossRef]
- Conrado, R.; Gomes, T.C.; Roque, G.S.C.; De Souza, A.O. Overview of bioactive fungal secondary metabolites: Cytotoxic and antimicrobial compounds. Antibiotics 2022, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer effects of rosemary (Rosmarinus officinalis L.) extract and rosemary extract polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- Adetunde, L.A.; Awo, O.; Imoro, A.W.M. Antimicrobial activities of the extract of shea tree (Vitellaria paradoxa Gaertn. F.) leaf and bark on some selected clinical pathogens. J. Med. Plants Res. 2023, 17, 338–344. [Google Scholar] [CrossRef]
- Mohammed, I.H.; Halilu, M.E.; Atinga, V.; Sabo, I.; Aliyu, W.A.; Yusuf, A.A.; Suleiman, A.U.; Zainab, M.; Garba, I.; Avosuahi, O.R. Comparative phytochemistry of Vitellaria paradoxa: Towards establishing a chemotaxonomic marker. Malays. J. Pharm. Sci. 2024, 22, 61–76. [Google Scholar] [CrossRef]


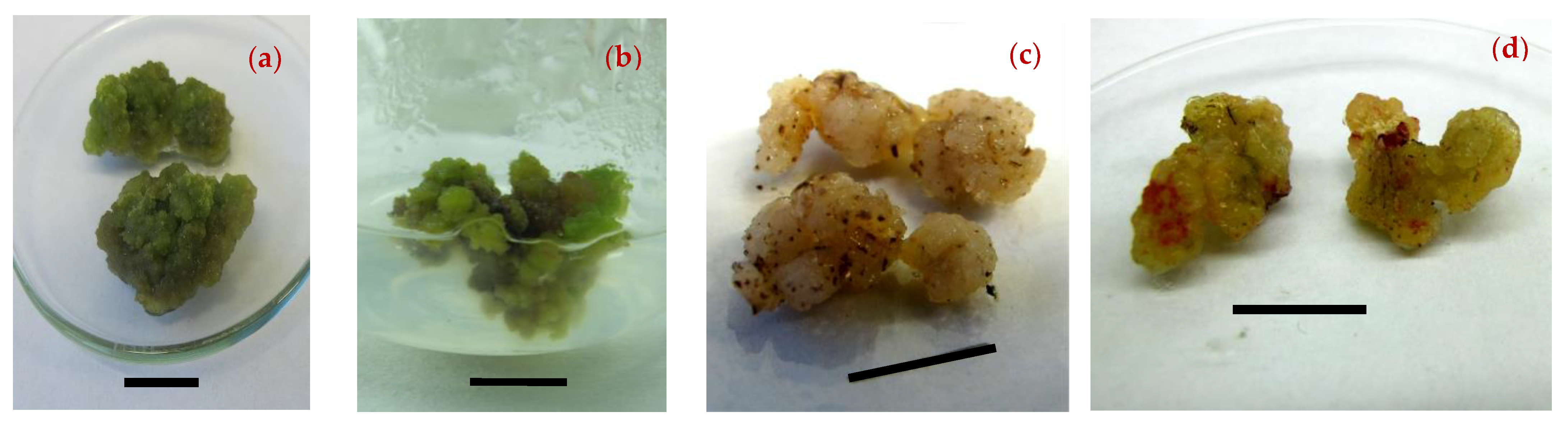
| Stage of Growth | Age (Days) | Leaf Color and Appearance |
|---|---|---|
| I | 0–5 | Pink/brown |
| II | 6–10 | Brown with green patches |
| III | 11–15 | Green with dark patches |
| IV | 16–20 | Dark green |
| V | 21–25 | Light green |
| VI | 26–30 | Light green |
| MS Media Strength | Media Formulations (mg/L) | Average Callusing at Six Stages of Growth (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TDZ | NAA | 2,4-D | BAP | I | II | III | IV | V | VI | |
| Full strength | 0.5 | 0.05 | 2.0 | 0 | 41.2 | 90.0 | 54.7 | 48.8 | 35.3 | |
| 0.5 | 0.05 | 2.0 | 0.5 | 0 | 31.1 | 31.1 | 50.8 | 33.2 | 0.0 | |
| 1.0 | 1.0 | 2.0 | 0 | 35.3 | 63.4 | 52.7 | 41.2 | 26.6 | ||
| 0.5 | 2.0 | 0 | 50.8 | 75.0 | 63.4 | 45.0 | 28.9 | |||
| 2.0 | 0 | 54.7 | 61.1 | 71.6 | 50.8 | 37.3 | ||||
| 0.5 | 0.1 | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| 1.0 | 0.5 | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| 1.0 | 1.0 | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| Half strength | 0.5 | 0.05 | 2.0 | 0 | 48.8 | 90.0 | 90.0 | 52.7 | 35.3 | |
| 1.0 | 1.0 | 2.0 | 0 | 45.0 | 79.5 | 90.0 | 54.7 | 31.1 | ||
| 0.5 | 2.0 | 0 | 58.9 | 90.0 | 90.0 | 54.7 | 26.6 | |||
| 2.0 | 0 | 63.4 | 90.0 | 61.1 | 50.8 | 43.1 | ||||
| 0.5 | 0.1 | 0 | 0.0 | 35.3 | 28.9 | 0.0 | 0.0 | |||
| 1.0 | 0.5 | 0 | 90.0 | 46.9 | 43.1 | 28.9 | 0.0 | |||
| 1.0 | 1.0 | 0 | 61.1 | 56.8 | 39.2 | 24.1 | 18.4 | |||
| 0.5 | 0.1 | 0.5 | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Grand average callusing (%) | 0.0 c | 36.3 ab | 50.6 a | 46.0 a | 30.3 ab | 17.7 bc | ||||
| Rank of callus formation | − | ++ | ++++ | ++++ | +++ | + | ||||
| Legend: (−) No callusing; (+) Less than 10% of explant callused; (++) 10–30% explant transformation; (+++) 31–50% of explant callused; (++++) 51–79% explant transformation; (+++++) More than 80% of the leaf callused. Different letters indicate significantly different means at (p ≤ 0.05). Values shown are angularly transformed percentages. Colors were used to represent percentage ranges of callus induction for ease of visual comparison across treatments and growth stages. | ||||||||||
| Color code (percentage range) | 0–20 | 21–40 | 41–60 | 61–80 | 81–100 | |||||
| Source of Callus (CIM) | Compositions of Transfer Media (Half-Strength MS) | Mean Callus Weight (g) | Callus Ranking | |||
|---|---|---|---|---|---|---|
| TDZ | NAA | BAP | GA3 | |||
| T1 Calli | 1.0 | 1.0 | 2.30 ± 0.19 | +++++ | ||
| 0.5 | 0.1 | 2.0 | 0.5 | 3.28 ± 0.26 | +++++ | |
| 0.5 | 0.5 | 0.40 ± 0.05 | − | |||
| 2.0 | 0.5 | 0.53 ± 0.06 | + | |||
| 0.5 | 2.0 | 0.5 | 1.90 ± 0.20 | ++++ | ||
| 0.5 | 2.0 | 3.05 ± 0.29 | +++++ | |||
| 0.5 | 3.0 | 1.08 ± 0.11 | +++ | |||
| T2 Calli | 1.0 | 1.0 | 1.38 ± 0.17 | +++ | ||
| 0.5 | 0.1 | 2.0 | 0.5 | 1.74 ± 0.16 | ++++ | |
| 0.5 | 0.5 | 1.05 ± 0.12 | +++ | |||
| 2.0 | 0.5 | 0.92 ± 0.11 | ++ | |||
| 0.5 | 2.0 | 0.5 | 1.53 ± 0.16 | +++ | ||
| 0.5 | 2.0 | 1.97 ± 0.17 | ++++ | |||
| 0.5 | 3.0 | 1.82 ± 0.16 | ++++ | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okao, M.; Bharati, R.; Fernández-Cusimamani, E. Effect of Explant Physiology and Media Composition on Callogenesis of Vitellaria paradoxa Leaf Explants. Horticulturae 2025, 11, 1127. https://doi.org/10.3390/horticulturae11091127
Okao M, Bharati R, Fernández-Cusimamani E. Effect of Explant Physiology and Media Composition on Callogenesis of Vitellaria paradoxa Leaf Explants. Horticulturae. 2025; 11(9):1127. https://doi.org/10.3390/horticulturae11091127
Chicago/Turabian StyleOkao, Moses, Rohit Bharati, and Eloy Fernández-Cusimamani. 2025. "Effect of Explant Physiology and Media Composition on Callogenesis of Vitellaria paradoxa Leaf Explants" Horticulturae 11, no. 9: 1127. https://doi.org/10.3390/horticulturae11091127
APA StyleOkao, M., Bharati, R., & Fernández-Cusimamani, E. (2025). Effect of Explant Physiology and Media Composition on Callogenesis of Vitellaria paradoxa Leaf Explants. Horticulturae, 11(9), 1127. https://doi.org/10.3390/horticulturae11091127







