Effects of Melatonin and Bacillus amyloliquefaciens MPA 1034 on the Postharvest Quality of Potato Tubers
Abstract
1. Introduction
2. Materials and Methods
2.1. Melatonin Preparation
2.2. Fungal Pathogen
2.3. Preparation of BCA Isolate Suspension
2.4. In Vitro Screening of Melatonin and B. amyloliquefaciens Against F. oxysporum
2.5. In Vivo Screening of Melatonin and B. amyloliquefaciens Against F. oxysporum
- Moderately severe p ≤ 5 mm
- Severe 5 mm < p < 7 mm
- Highly severe p ≥ 7 mm
2.6. Scanning Electron Microscopy Studies of the Interactions of B. amyloliquefaciens Isolate with F. oxysporum
2.7. Determination of the Effects of Melatonin and Bacillus Amyloliquefaciens on the Postharvest Quality of Potatoes
Preparation of Samples
2.8. Determination of Phenolic Content
2.9. Determination of Ascorbic Acid Content
2.10. Determination of Antioxidant Activity [DPPH (2, 2-Diphenyl-1-Picrylhydrazyl)]
2.11. Determination of Protein Content
2.12. Statistical Analysis
3. Results
3.1. Effect of Melatonin Integrated with Bacillus amyloliquefaciens on Mycelial Growth of F. oxysporum
3.2. Effect of Melatonin Integrated with Bacillus amyloliquefaciens Against F. oxysporum in Potato Tubers
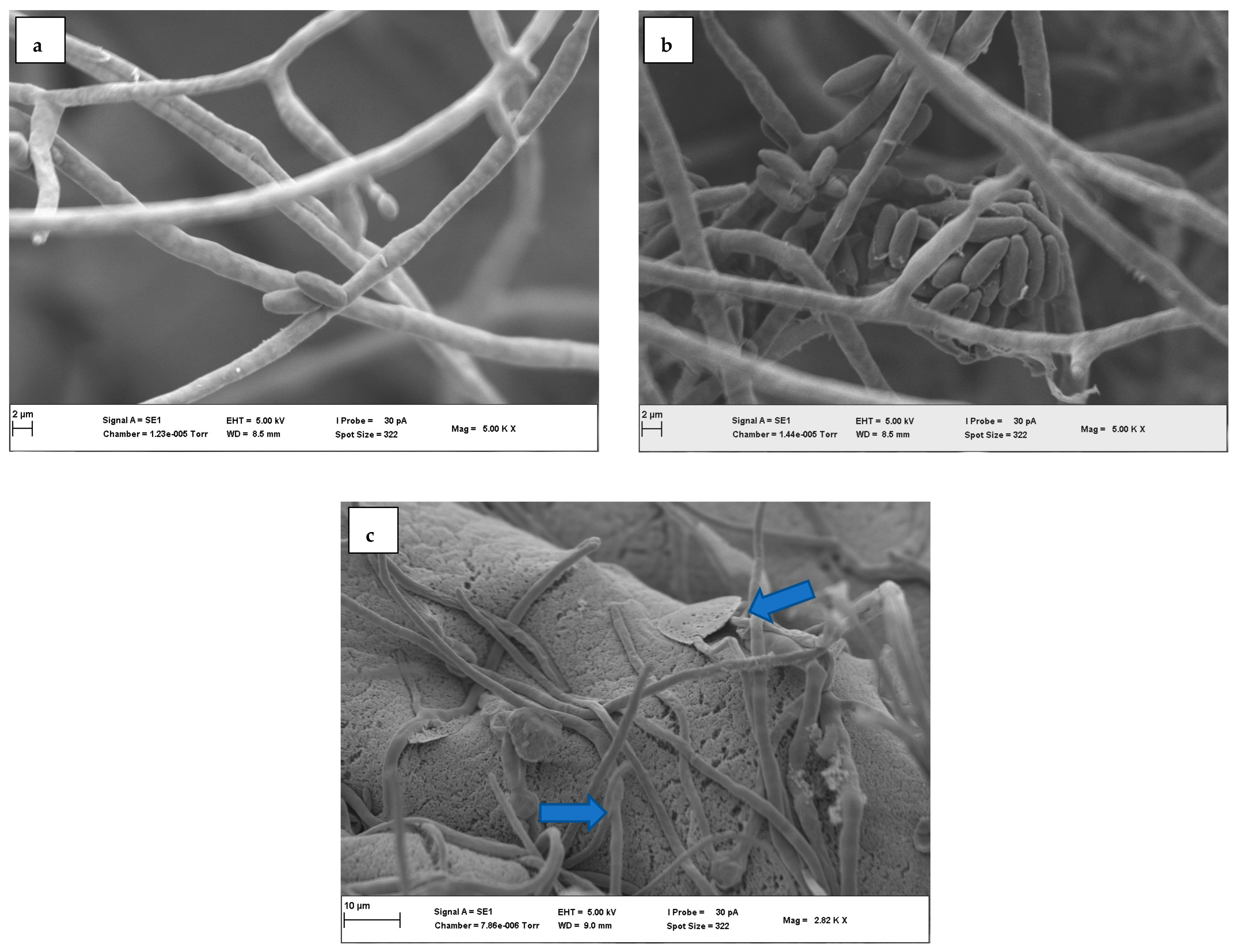
3.3. Scanning Electron Microscopy
3.4. Phenolic Content
3.5. Ascorbic Acid
3.6. Antioxidant Activity [DPPH (2, 2-Diphenyl-1-Picrylhydrazyl)]
3.7. Protein Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. World Food and Agriculture—Statistical Yearbook 2022; FAO: Rome, Italy, 2022. [Google Scholar]
- Devaux, A.; Goffart, J.-P.; Petsakos, A.; Kromann, P.; Gatto, M.; Okello, J.; Suarez, V.; Hareau, G. Global Food Security, Contributions from Sustainable Potato Agri-Food Systems. In The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Campos, H., Ortiz, O., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–35. [Google Scholar]
- Westermann, D.T. Nutritional requirements of potatoes. Am. J. Potato Res. 2005, 82, 301–307. [Google Scholar] [CrossRef]
- Zaheer, K.; Akhtar, M.H. Potato production, usage, and nutrition—A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 711–721. [Google Scholar] [CrossRef]
- Burgos, G.; Zum Felde, T.; Andre, C.; Kubow, S. The potato and its contribution to the human diet and health. In The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Campos, H., Ortiz, O., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 37–74. [Google Scholar]
- Visvanathan, R.; Jayathilake, C.; Chaminda Jayawardana, B.; Liyanage, R. Health-beneficial properties of potato and compounds of interest. J. Sci. Food Agric. 2016, 96, 4850–4860. [Google Scholar] [CrossRef]
- Raigond, P.; Jayanty, S.S.; Parmar, V.; Dutt, S.; Changan, S.S.; Luthra, S.K.; Singh, B. Health-Promoting Compounds In Potatoes: Tuber Exhibiting Great Potential For Human Health. Food Chem. 2023, 424, 136368. [Google Scholar] [CrossRef]
- Ngceni, X. Effect of Storage Temperatures on the Postharvest Performance and Sprouting of Selected Potato Cultivars; University of KwaZulu-Natal: Pietermaritzburg, South Africa, 2019. [Google Scholar]
- Bojanowski, A.; Avis, T.J.; Pelletier, S.; Tweddell, R.J. Management of potato dry rot. Postharvest Biol. Technol. 2013, 84, 99–109. [Google Scholar] [CrossRef]
- Fravel, D.; Deahl, K.; Stommel, J. Compatibility of the biocontrol fungus Fusarium oxysporum strain CS-20 with selected fungicides. Biol. Control 2005, 34, 165–169. [Google Scholar] [CrossRef]
- El-Nagar, A.; Mazrou, Y.S.; Elzaawely, A.A.; Makhlouf, A.H.; Hassan, M.; El-Zahaby, H.M.; Xuan, T.D. Potential of Three Plant Extracts in Suppressing Potato Dry Rot Caused by Fusarium incarnatum Under Normal and Cold Storage. Agronomy 2025, 15, 593. [Google Scholar] [CrossRef]
- Jawed, S.; Wang, H.-F.; Cheng, X.-L.; Mehmood, A.; Kaleri, A.H.; Jatoi, Z.A.; Kaleri, A.A.; Kubar, A.A.; Nizamani, M.M. Efficacy of different fungicides and bio control agents against Fusarium oxysporum, causal agent of potato dry rot. Indian J. Sci. Technol. 2019, 12, 7. [Google Scholar] [CrossRef]
- Nguyen, P.-A.; Strub, C.; Fontana, A.; Schorr-Galindo, S. Crop molds and mycotoxins: Alternative management using biocontrol. Biol. Control 2017, 104, 10–27. [Google Scholar] [CrossRef]
- Pal, K.K.; Gardener, B.M. Biological control of plant pathogens. Plant Health Instr. 2006, 2, 1117–1142. [Google Scholar] [CrossRef]
- Li, Y.; Xia, X.; Zhao, Q.; Dong, P. The biocontrol of potato dry rot by microorganisms and bioactive substances: A review. Physiol. Mol. Plant Pathol. 2022, 122, 101919. [Google Scholar] [CrossRef]
- Recep, K.; Fikrettin, S.; Erkol, D.; Cafer, E. Biological control of the potato dry rot caused by Fusarium species using PGPR strains. Biol. Control 2009, 50, 194–198. [Google Scholar] [CrossRef]
- Lastochkina, O.; Baymiev, A.; Shayahmetova, A.; Garshina, D.; Koryakov, I.; Shpirnaya, I.; Pusenkova, L.; Mardanshin, I.d.; Kasnak, C.; Palamutoglu, R. Effects of endophytic Bacillus subtilis and salicylic acid on postharvest diseases (Phytophthora infestans, Fusarium oxysporum) development in stored potato tubers. Plants 2020, 9, 76. [Google Scholar] [CrossRef]
- Khedher, S.B.; Mejdoub-Trabelsi, B.; Tounsi, S. Biological potential of Bacillus subtilis V26 for the control of Fusarium wilt and tuber dry rot on potato caused by Fusarium species and the promotion of plant growth. Biol. Control 2021, 152, 104444. [Google Scholar] [CrossRef]
- Zang, H.; Ma, J.; Wu, Z.; Yuan, L.; Lin, Z.-Q.; Zhu, R.; Bañuelos, G.S.; Reiter, R.J.; Li, M.; Yin, X. Synergistic effect of melatonin and selenium improves resistance to postharvest gray mold disease of tomato fruit. Front. Plant Sci. 2022, 13, 903936. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar] [CrossRef]
- Kolář, J.; Macháčková, I. Melatonin in higher plants: Occurrence and possible functions. J. Pineal Res. 2005, 39, 333–341. [Google Scholar] [CrossRef]
- He, X.; Yin, B.; Zhang, J.; Zhou, S.; Li, Z.; Zhang, X.; Xu, J.; Liang, B. Exogenous melatonin alleviates apple replant disease by regulating rhizosphere soil microbial community structure and nitrogen metabolism. Sci. Total Environ. 2023, 884, 163830. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B. Non-vertebrate melatonin. J. Pineal Res. 2003, 34, 233–241. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, L.; Gu, P.; Zhan, X.; Zhang, Y.; Hou, C.; Wu, Z.; Wu, Y.F.; Wang, Q.C. Exogenous application of melatonin improves plant resistance to virus infection. Plant Pathol. 2019, 68, 1287–1295. [Google Scholar] [CrossRef]
- Chen, L.; Wang, M.R.; Li, J.W.; Feng, C.H.; Cui, Z.H.; Zhao, L.; Wang, Q.C. Exogenous application of melatonin improves eradication of apple stem grooving virus from the infected in vitro shoots by shoot tip culture. Plant Pathol. 2019, 68, 997–1006. [Google Scholar] [CrossRef]
- Wei, Y.; Hu, W.; Wang, Q.; Zeng, H.; Li, X.; Yan, Y.; Reiter, R.J.; He, C.; Shi, H. Identification, transcriptional and functional analysis of heat-shock protein 90s in banana (Musa acuminata L.) highlight their novel role in melatonin-mediated plant response to Fusarium wilt. J. Pineal Res. 2017, 62, e12367. [Google Scholar] [CrossRef]
- Mbatha, L. The Evaluation of the Effects of Biological Control Agents and Melatonin Against Fusarium Oxysporum Infecting Potatoes; University of KwaZulu-Natal: Pietermaritzburg, South Africa, 2024. [Google Scholar]
- Mejdoub-Trabelsi, B.; Jabnoun-Khiareddine, H.; Daami-Remadi, M. Interactions between four Fusarium species in potato tubers and consequences for fungal development and susceptibility assessment of five potato cultivars under different storage temperature. J. Plant Pathol. Microbiol. 2015, 6, 2. [Google Scholar] [CrossRef]
- Lapwood, D.; Read, P.; Spokes, J. Methods for assessing the susceptibility of potato tubers of different cultivars to rotting by Erwinia carotovora subspecies atroseptica and carotovora. Plant Pathol. 1984, 33, 13–20. [Google Scholar] [CrossRef]
- Wang, B.-N.; Liu, H.F.; Zheng, J.B.; Fan, M.T.; Cao, W. Distribution of phenolic acids in different tissues of jujube and their antioxidant activity. J. Agric. Food Chem. 2011, 59, 1288–1292. [Google Scholar] [CrossRef]
- Boonkasem, P.; Sricharoen, P.; Techawongstein, S.; Chanthai, S. Determination of ascorbic acid and total phenolics related to the antioxidant activity of some local tomato (Solanum lycopersicum) varieties. Der Pharma Chem. 2015, 7, 66–70. [Google Scholar]
- Rocchetti, G.; Miragoli, F.; Zacconi, C.; Lucini, L.; Rebecchi, A. Impact of cooking and fermentation by lactic acid bacteria on phenolic profile of quinoa and buckwheat seeds. Food Res. Int. 2019, 119, 886–894. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nguvo, K.J.; Gao, X. Weapons hidden underneath: Bio-control agents and their potentials to activate plant induced systemic resistance in controlling crop Fusarium diseases. J. Plant Dis. Prot. 2019, 126, 177–190. [Google Scholar] [CrossRef]
- Xue, H.; Liu, Q.; Yang, Z. Pathogenicity, Mycotoxin Production, and Control of Potato Dry Rot Caused by Fusarium spp.: A Review. J. Fungi 2023, 9, 843. [Google Scholar] [CrossRef]
- Mandal, M.K.; Suren, H.; Ward, B.; Boroujerdi, A.; Kousik, C. Differential roles of melatonin in plant-host resistance and pathogen suppression in cucurbits. J. Pineal Res. 2018, 65, e12505. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria × anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, H.; Chen, S.; Yu, D.; Reiter, R.J. Phytomelatonin: An emerging regulator of plant biotic stress resistance. Trends Plant Sci. 2021, 26, 70–82. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Shi, X.-C.; Wang, R.; Wang, H.-L.; Liu, F.; Laborda, P. Melatonin in fruit production and postharvest preservation: A review. Food Chem. 2020, 320, 126642. [Google Scholar] [CrossRef]
- Li, R.; Bi, R.; Cai, H.; Zhao, J.; Sun, P.; Xu, W.; Zhou, Y.; Yang, W.; Zheng, L.; Chen, X.L. Melatonin functions as a broad-spectrum antifungal by targeting a conserved pathogen protein kinase. J. Pineal Res. 2023, 74, e12839. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as biological control agents of plant diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef] [PubMed]
- Anckaert, A.; Arguelles Arias, A.; Hoff, G.; Calonne-Salmon, M.; Declerck, S.; Ongena, M. The Use of Bacillus spp. as Bacterial Biocontrol Agents to Control Plant Diseases. Cambridge. 2021. Available online: https://library.oapen.org/handle/20.500.12657/61511 (accessed on 1 September 2023).
- Soliman, S.A.; Khaleil, M.M.; Metwally, R.A. Evaluation of the antifungal activity of Bacillus amyloliquefaciens and B. velezensis and characterization of the bioactive secondary metabolites produced against plant pathogenic fungi. Biology 2022, 11, 1390. [Google Scholar] [CrossRef]
- Yuan, J.; Raza, W.; Shen, Q.; Huang, Q. Antifungal activity of Bacillus amyloliquefaciens NJN-6 volatile compounds against Fusarium oxysporum f. sp. cubense. Appl. Environ. Microbiol. 2012, 78, 5942–5944. [Google Scholar] [CrossRef]
- Minerdi, D.; Bossi, S.; Gullino, M.L.; Garibaldi, A. Volatile organic compounds: A potential direct long-distance mechanism for antagonistic action of Fusarium oxysporum strain MSA 35. Environ. Microbiol. 2009, 11, 844–854. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Xu, J.-D.; Gao, M.-Z.; Huang, T.-X.; Zheng, Y.; Zhang, Y.-Y.; Wang, Y.-P.; Luo, Y.-M.; Zhang, Y.; Hu, Y.-H. Exploring plant growth promoting rhizobacteria potential for green agriculture system to optimize sweet potato productivity and soil sustainability in northern Jiangsu, China. Eur. J. Agron. 2023, 142, 126661. [Google Scholar] [CrossRef]
- WoldemariamYohannes, K.; Wan, Z.; Yu, Q.; Li, H.; Wei, X.; Liu, Y.; Wang, J.; Sun, B. Prebiotic, probiotic, antimicrobial, and functional food applications of Bacillus amyloliquefaciens. J. Agric. Food Chem. 2020, 68, 14709–14727. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.M.; Hassan, A.A.; Mansour, E.H.; Fahmy, H.A.; El-Bedawey, A.E.-F.A. Melatonin, phenolics content and antioxidant activity of germinated selected legumes and their fractions. J. Saudi Soc. Agric. Sci. 2019, 18, 294–301. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M.; Sikorska, A.; Mystkowska, I.; Baranowska, A.; Niewęgłowski, M.; Dołęga, H. The effect of herbicides and biostimulants on polyphenol content of potato (Solanum tuberosum L.) tubers and leaves. J. Saudi Soc. Agric. Sci. 2019, 18, 102–106. [Google Scholar] [CrossRef]


| Treatments | 5 DPI | 7 DPI | 9 DPI | |||
|---|---|---|---|---|---|---|
| MMG (mm) | MGI (%) | MMG (mm) | MGI (%) | MMG (mm) | MGI (%) | |
| Bamy + MEL0 | 45.00 d | 13.46 | 60.67 c | 13.33 | 68.67 c | 13.08 |
| Bamy + MEL1 | 36.67 c | 29.49 | 41.67 b | 40.48 | 45.67 b | 42.19 |
| Bamy + MEL10 | 35.00 bc | 32.69 | 39.33 b | 43.81 | 43.33 b | 45.15 |
| Bamy + MEL15 | 31.67 ab | 39.10 | 34.33 a | 50.95 | 34.67 a | 56.12 |
| Bamy + MEL50 | 31.67 ab | 39.10 | 34.00 a | 51.43 | 35.33 a | 55.27 |
| Bamy + MEL100 | 30.00 a | 42.31 | 31.00 a | 55.71 | 31.67 a | 59.92 |
| Control | 52.00 e | 0.00 | 70.00 d | 0.00 | 79.00 d | 0.00 |
| p-value | <0.001 | - | <0.001 | - | <0.001 | - |
| LSD | 4.586 | - | 4.407 | - | 5.170 | - |
| CV (%) | 7.0 | - | 5.7 | - | 6.1 | - |
| Treatments | Pathogen Penetration (mm) | Mean Lesion Diameter (mm) | Disease Severity (%) |
|---|---|---|---|
| Bamy + MEL100 | 6.39 a | 14.06 a | 50.61 |
| Bamy + MEL50 | 8.20 ab | 16.59 b | 59.72 |
| Bamy + MEL15 | 9.23 b | 21.66 c | 77.97 |
| Control | 9.70 b | 31.02 d | |
| p-value | 0.019 | <0.001 | - |
| CV | 12.70% | 7.90% | - |
| Treatment | Mean Phenolic Content (mg GAE/g DW) | ||
|---|---|---|---|
| Day 0 | Day 7 | Day 14 | |
| Bamy | 81.28 ab | 119.0 ab | 87.3 a |
| MEL100 | 75.16 a | 83.3 a | 91.1 a |
| Bamy + MEL100 | 88.42 b | 119.8 ab | 144.1 b |
| Control | 81.74 ab | 135.6 b | 104.4 a |
| p-value | 0.135 | 0.079 | 0.02 |
| CV (%) | 7.30 | 18.4 | 17.3 |
| Treatment | Mean Ascorbic Acid (mg AAE/100 g DM) | ||
|---|---|---|---|
| Day 0 | Day 7 | Day 14 | |
| Bamy | 3.49 a | 2.14 a | 2.70 a |
| MEL100 | 4.29 b | 5.28 b | 5.48 b |
| Bamy + MEL100 | 4.62 b | 5.83 b | 3.62 ab |
| Control | 4.13 b | 4.89 b | 4.09 ab |
| p-value | 0.009 | 0.046 | 0.060 |
| CV (%) | 7.0 | 30.5 | 26.1 |
| Treatment | Antioxidant Activity (mg/g DM) | ||
|---|---|---|---|
| Day 0 | Day 7 | Day 14 | |
| Bamy | 0.5863 | 0.5863 | 0.5859 |
| MEL100 | 0.5863 | 0.5863 | 0.5863 |
| Bamy + MEL100 | 0.5863 | 0.5863 | 0.5863 |
| Control | 0.5863 | 0.5863 | 0.5863 |
| p-value | 0.798 | 0.862 | 0.405 |
| CV (%) | 0 | 0 | 0.1 |
| Treatment | Mean Protein Content (mg/g DM) | ||
|---|---|---|---|
| Day 0 | Day 7 | Day 14 | |
| Bamy | 55.78 b | 56.73 | 50.88 |
| MEL100 | 56.10 b | 52.56 | 51.69 |
| Bamy + MEL100 | 53.97 a | 51.88 | 64.81 |
| Control | 55.28 b | 54.73 | 50.06 |
| p-value | 0.016 | 0.978 | 0.579 |
| CV (%) | 1.2 | 28.1 | 26.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbatha, L.A.; Mbili, N.C. Effects of Melatonin and Bacillus amyloliquefaciens MPA 1034 on the Postharvest Quality of Potato Tubers. Horticulturae 2025, 11, 1119. https://doi.org/10.3390/horticulturae11091119
Mbatha LA, Mbili NC. Effects of Melatonin and Bacillus amyloliquefaciens MPA 1034 on the Postharvest Quality of Potato Tubers. Horticulturae. 2025; 11(9):1119. https://doi.org/10.3390/horticulturae11091119
Chicago/Turabian StyleMbatha, Londeka Akhona, and Nokwazi Carol Mbili. 2025. "Effects of Melatonin and Bacillus amyloliquefaciens MPA 1034 on the Postharvest Quality of Potato Tubers" Horticulturae 11, no. 9: 1119. https://doi.org/10.3390/horticulturae11091119
APA StyleMbatha, L. A., & Mbili, N. C. (2025). Effects of Melatonin and Bacillus amyloliquefaciens MPA 1034 on the Postharvest Quality of Potato Tubers. Horticulturae, 11(9), 1119. https://doi.org/10.3390/horticulturae11091119







