The Effect of Alternative Nutrient Supplements on Histological Traits and Postharvest Water Loss in Pepper Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Site Description
2.2. Plant Material
2.3. Experimental Design
2.4. Soil Sampling and Analysis
2.5. Samplings for Macromorphological and Micromorphometric Analyses of Fruits
2.6. Measurement of Water Loss Rate of Fruit
2.7. Statistical Analysis
3. Results
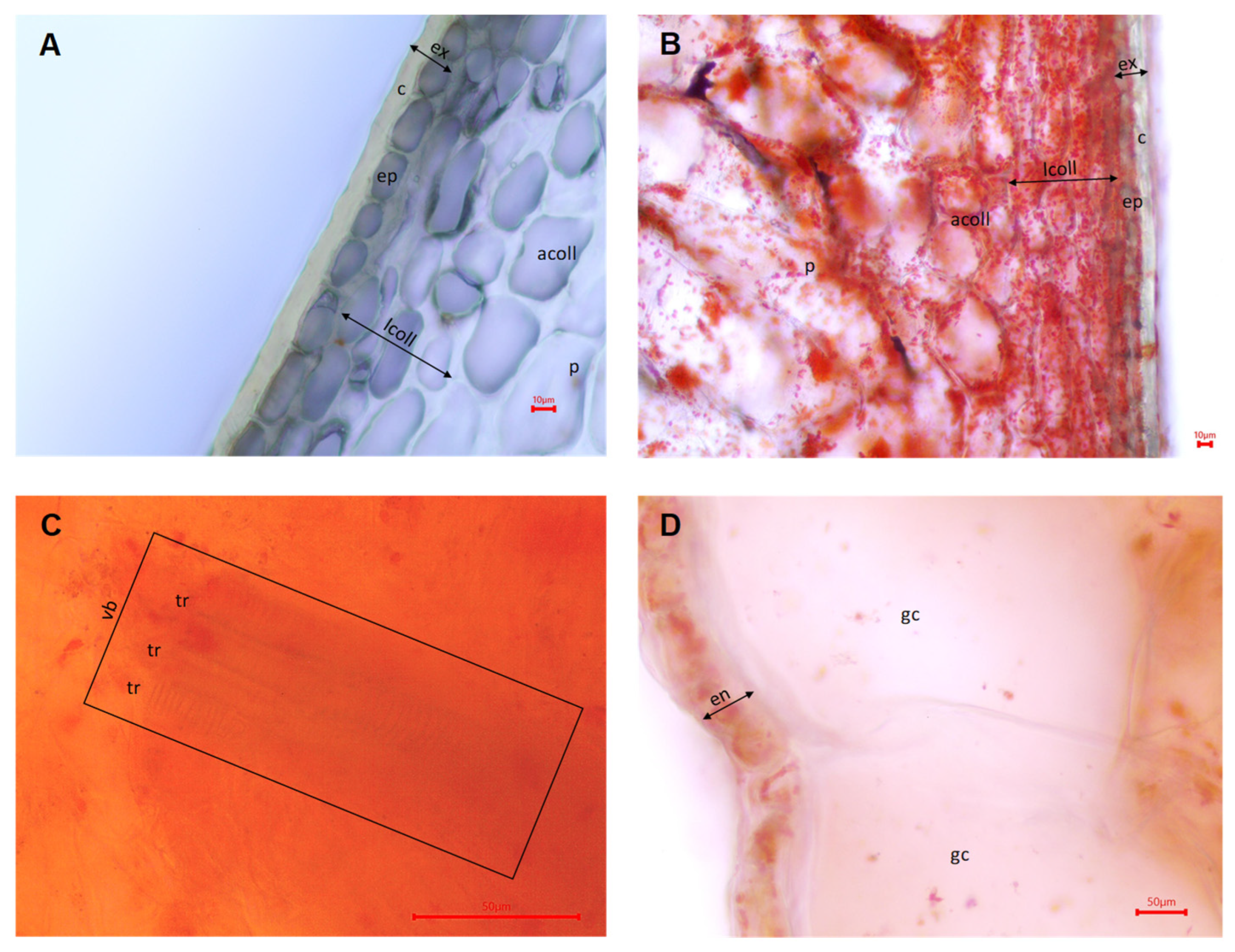
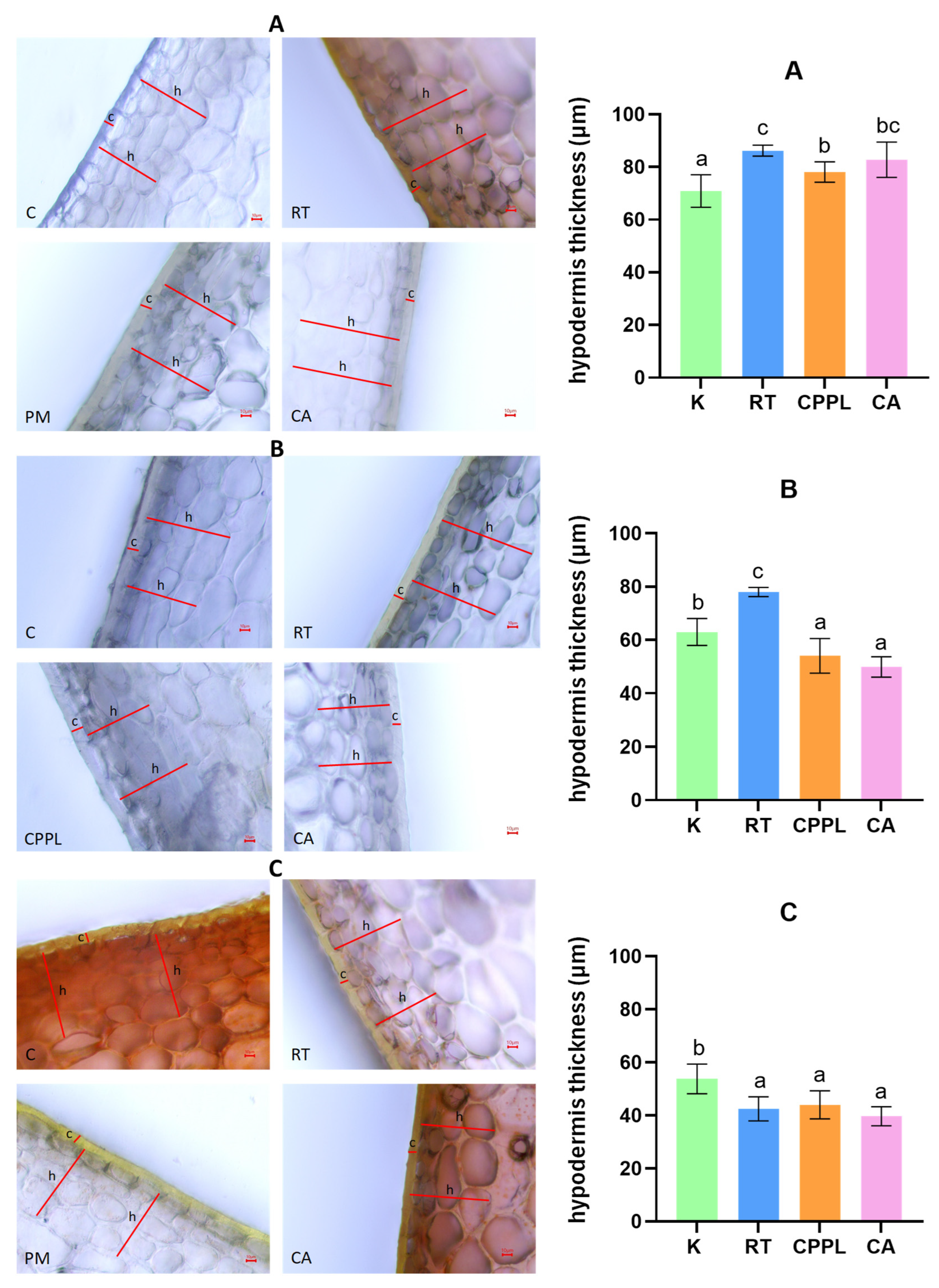
3.1. Microanatomical Structure of the Fruit Pericarp in the Hungarian Wax Pepper Cultivar ‘Tizenegyes’
3.2. Ripening Stage and Treatment-Dependent Changes in the Fruit Pericarp Microanatomy of the ‘Tizenegyes’ Pepper Cultivar
3.2.1. Effects of Ripening Stage on the Characteristics of the Fruit Pericarp
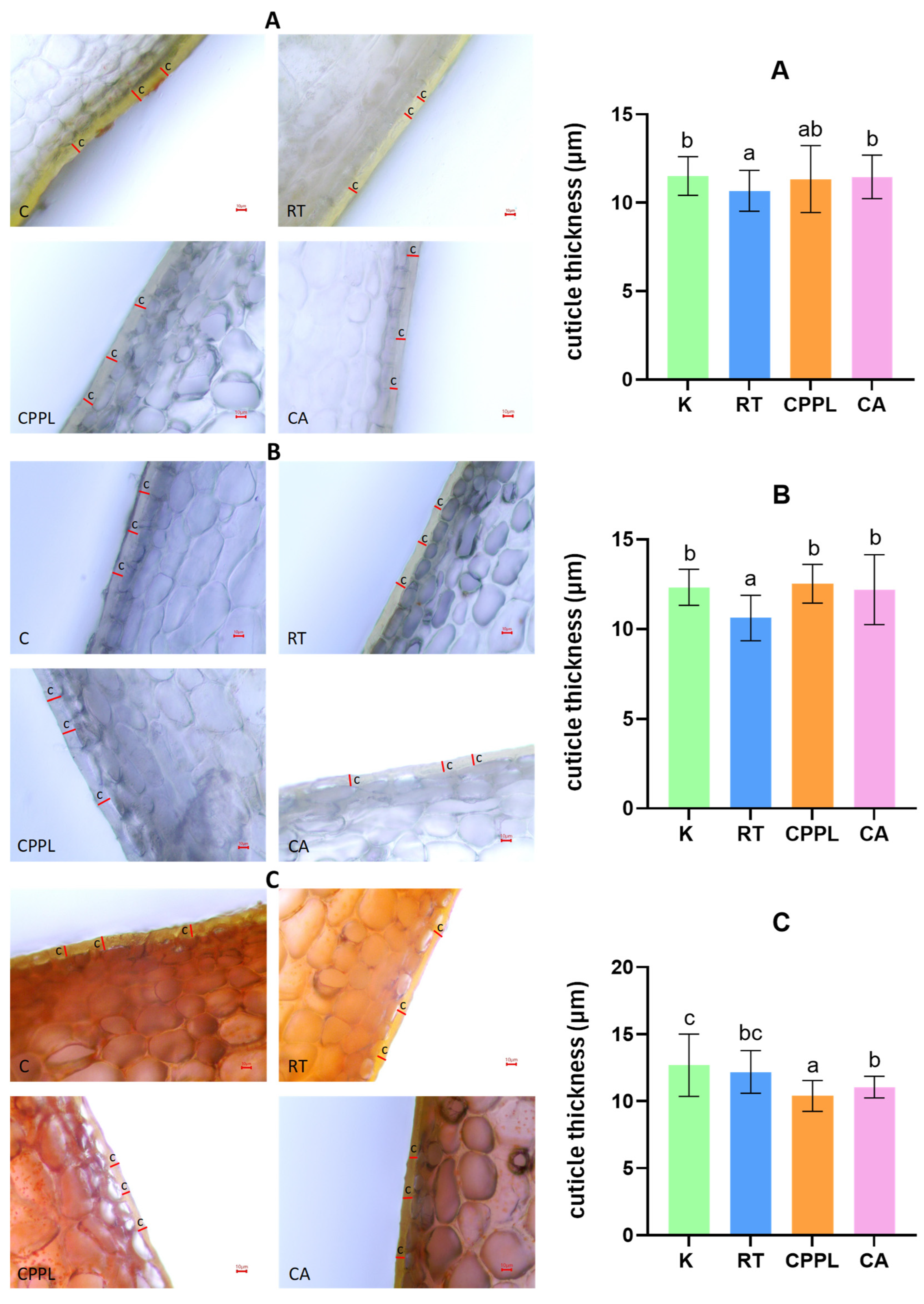
3.2.2. Effects of Treatments on the Characteristics of the Fruit Pericarp
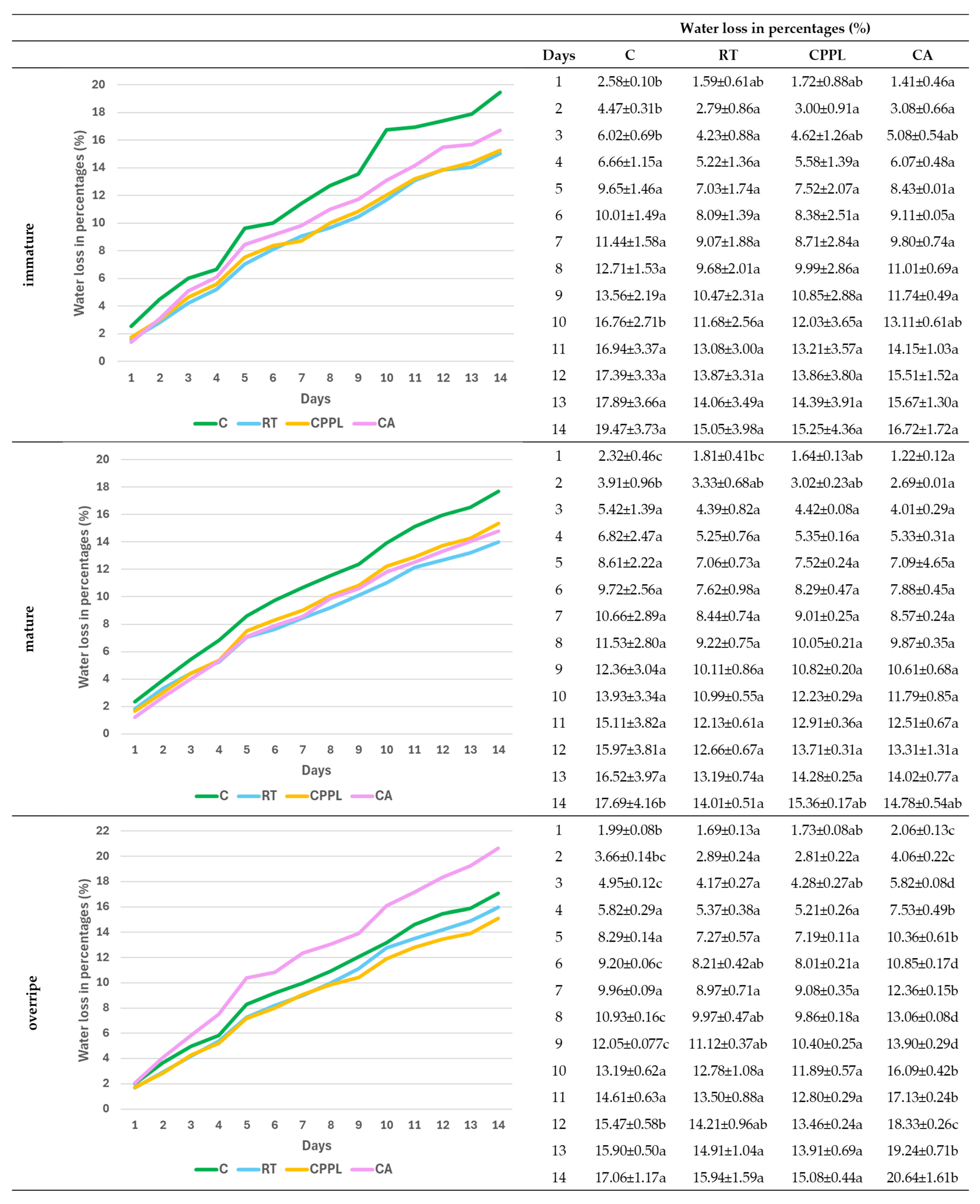
3.3. Effect of Soil Conditioner Treatments and Ripening Stage on Daily Water Loss Rates
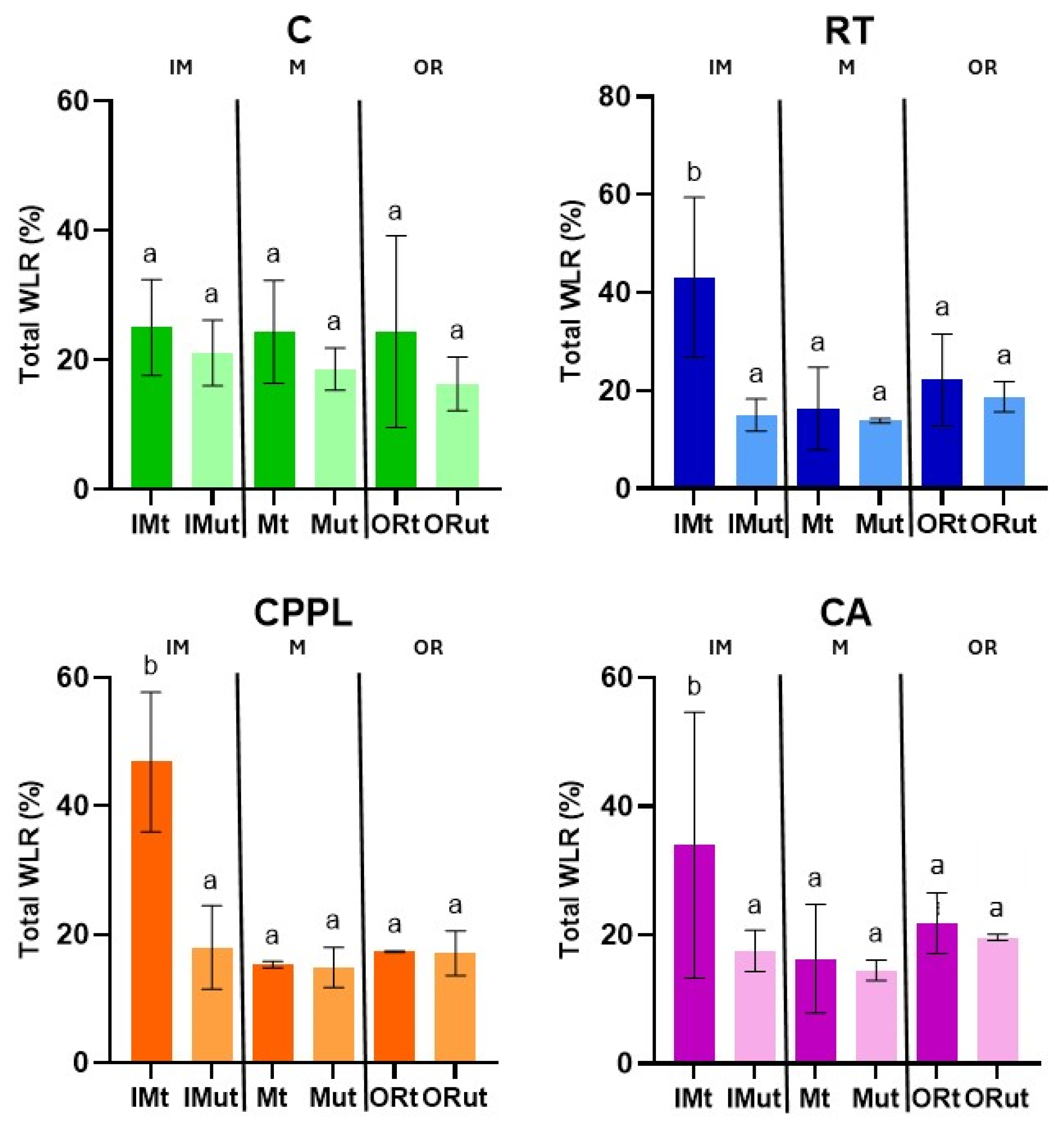
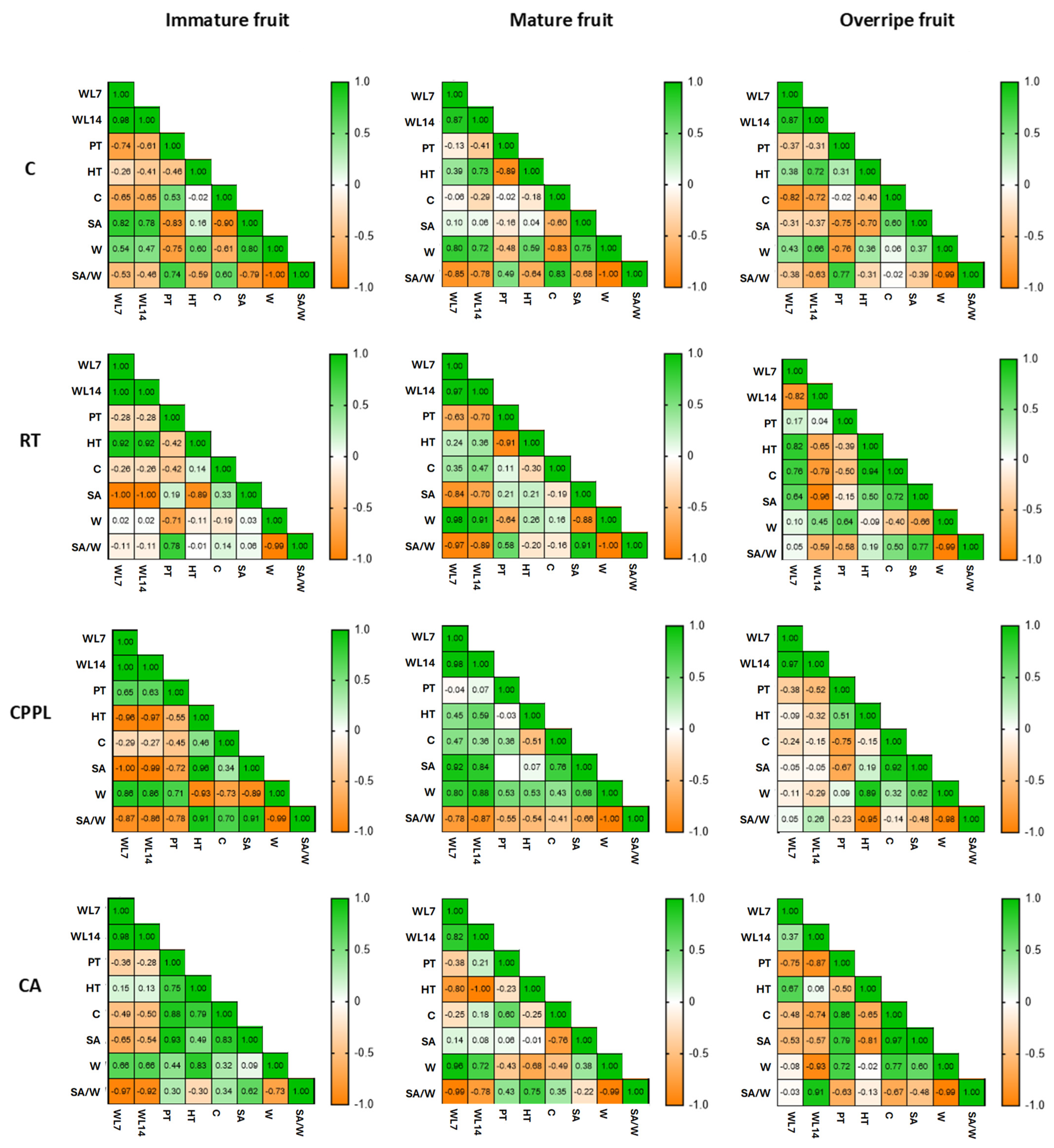
3.4. Ripening Stage and Treatment-Dependent Changes in Water Loss Rate and Micromorphometric Parameters of Fruits of the ‘Tizenegyes’ Pepper Cultivar
4. Discussion
4.1. Micromorphometrical Changes During Ripening
4.2. Role of the Cuticle in Water Loss of Pepper Fruit
4.3. Effects of the Nutrient Supply on the Micromorphometric Parameters of Pericarp
4.4. Surface-Area-to-Weight Ratio and Pepper Fruit Water Loss
4.5. Effect of Rhyolite Tuff as an Alternative Nutrient Supplement on Water Loss in Pepper Fruit
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velázquez-Ventura, J.C.; Márquez-Quiroz, C.; De la Cruz-Lázaro, E.; Osorio-Osorio, R.; Preciado-Rangel, P. Morphological variation of wild peppers (Capsicum spp.) from the state of Tabasco. Mex. Emir. J. Food. Agri. 2018, 30, 115–121. [Google Scholar]
- Lućić, M.; Potkonjak, N.; Sredović Ignjatović, I.; Lević, S.; Dajić-Stevanović, Z.; Kolašinac, S.; Belović, M.; Torbica, A.; Zlatanović, I.; Pavlović, V.; et al. Influence of Ultrasonic and Chemical Pretreatments on Quality Attributes of Dried Pepper (Capsicum annuum). Foods 2023, 12, 2468. [Google Scholar] [CrossRef] [PubMed]
- Tafolla-Arellano, J.C.; Báez-Sañudo, R.; Tiznado-Hernández, M.E. The cuticle as a key factor in the quality of horticultural crops. Sci. Hortic. 2018, 232, 145–152. [Google Scholar] [CrossRef]
- Lara, I.; Heredia, A.; Domínguez, E. Shelf life potential and the fruit cuticle: The unexpected player. Front. Plant Sci. 2019, 10, 770. [Google Scholar] [CrossRef]
- García-Coronado, H.; Tafolla-Arellano, J.C.; Hernández-Oñate, M.Á.; Burgara-Estrella, A.J.; Robles-Parra, J.M.; Tiznado-Hernández, M.E. Molecular biology, composition and physiological functions of cuticle lipids in fleshy fruits. Plants 2022, 11, 1133. [Google Scholar] [CrossRef]
- Fernández-Muñoz, R.; Heredia, A.; Domínguez, E. The role of cuticle in fruit shelf-life. Curr. Opin. Biotechnol. 2022, 78, 102802. [Google Scholar] [CrossRef]
- Banaras, M.; Lownds, N.K.; Bosland, P.W. Relationship of physical properties to postharvest water loss in pepper fruits (Capsicum annuum L.). Pak. J. Bot. 1994, 26, 321–326. [Google Scholar]
- Konishi, A.; Terabayashi, S.; Itai, A. Relationship of cuticle development with water loss and texture of pepper fruit. Can. J. Plant Sci. 2021, 102, 103–111. [Google Scholar] [CrossRef]
- Pessoa, A.M.S.; Rêgo, E.R.; Silva, B.R.S.; Rêgo, M.M. Performance of pepper cultivars, for fresh market, based on fruit traits. Hortic. Bras. 2023, 41, e2495. [Google Scholar] [CrossRef]
- Popovsky-Sarid, S.; Borovsky, Y.; Faigenboim, A.; Parsons, E.P.; Lohrey, G.T.; Alkalai-Tuvia, S.; Fallik, E.; Jenks, M.A.; Paran, I. Genetic and biochemical analysis reveals linked QTLs determining natural variation for fruit postharvest water loss in pepper (Capsicum). Theor. Appl. Genet. 2017, 130, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, Q.; Mao, L.Z.; Yuan, Q.L.; Huang, Y.; Chen, M.; Fu, C.F.; Zhao, X.H.; Li, Z.Y.; Dai, Y.H.; et al. Investigating the Molecular Mechanisms of Pepper Fruit Tolerance to Storage via Transcriptomics and Metabolomics. Horticulturae 2021, 7, 242. [Google Scholar] [CrossRef]
- Arya, G.C.; Sarkar, S.; Manasherova, E.; Aharoni, A.; Cohen, H. The plant cuticle: An ancient guardian barrier set against long-standing rivals. Front. Plant Sci. 2021, 12, 663165. [Google Scholar] [CrossRef]
- Jetter, R.; Riederer, M. Localization of the transpiration barrier in the epi- and intracuticular waxes of eight plant species: Water transport resistances are associated with fatty acyl rather than alicyclic components. Plant Physiol. 2016, 170, 921–934. [Google Scholar] [CrossRef]
- Philippe, G.; De Bellis, D.; Rose, J.K.C.; Nawrath, C. Trafficking processes and secretion pathways underlying the formation of plant cuticles. Front. Plant Sci. 2022, 12, 786874. [Google Scholar] [CrossRef] [PubMed]
- Parsons, E.P.; Popopvsky, S.; Lohrey, G.T.; Alkalai-Tuvia, S.; Perzelan, Y.; Bosland, P.; Bebeli, P.J.; Paran, I.; Fallik, E.; Jenks, M.A. Fruit cuticle lipid composition and water loss in a diverse collection of pepper (Capsicum). Physiol. Plant. 2013, 149, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.; Bres, C.; Reynoud, N.; Lahaye, M.; Marion, D.; Bakan, B.; Rothan, C. Unraveling cuticle formation, structure, and properties by using tomato genetic diversity. Front. Plant Sci. 2021, 12, 778131. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C.; Muy-Rangel, M.; Mascorro, A.G. Fruit size and stage of ripeness affect postharvest water loss in bell pepper fruit (Capsicum annuum L.). J. Sci. Food Agric. 2007, 87, 68–73. [Google Scholar] [CrossRef]
- O’Donoghue, E.M.; Brummell, D.A.; McKenzie, M.J.; Hunter, D.A.; Lill, R.E. Sweet capsicum: Postharvest physiology and technologies. New Zealand J. Crop Hortic. Sci. 2018, 46, 269–297. [Google Scholar] [CrossRef]
- Bojórquez-Gálvez, A.; Vega-García, M.; Caro-Corrales, J.; Carrillo-López, A.; López-Valenzuela, J.A. Effect of gradual cooling storage on chilling injury and phenylalanine ammonia-lyase activity in tomato fruit. J. Food Biochem. 2010, 34, 295–307. [Google Scholar] [CrossRef]
- Fallik, E.; Perzelan, Y.; Alkalai-Tuvia, S.; Nemny-Lavy, E.; Nestel, D. Development of cold quarantine protocols to arrest the development of the Mediterranean fruit fly (Ceratitis capitata) in pepper (Capsicum annuum L.) fruit after harvest. Postharvest Biol. Technol. 2012, 70, 7–12. [Google Scholar] [CrossRef]
- Sudhakar Rao, D.V.; Hebbar, S.S.; Narayana, C.K. CFB box wrapping: A new shrink wrapping technology for extension of storage life of colour capsicum (cv. Bachata). J. Food Sci. Technol. 2021, 58, 3039–3048. [Google Scholar] [CrossRef]
- Kehila, S.; Alkalai-Tuvia, S.; Chalupowicz, D.; Poverenov, E.; Fallik, E. Can Edible Coatings Maintain Sweet Pepper Quality after Prolonged Storage at Sub-Optimal Temperatures? Horticulturae 2021, 7, 387. [Google Scholar] [CrossRef]
- Hanaei, S.; Bodaghi, H.; Ghasimi Hagh, Z. Alleviation of postharvest chilling injury in sweet pepper using Salicylic acid foliar spraying incorporated with caraway oil coating under cold storage. Front. Plant Sci. 2022, 13, 999518. [Google Scholar] [CrossRef]
- Rêgo, E.R.; Rêgo, M.M. Genetics and Breeding of Chili Pepper Capsicum spp. In Production and Breeding of Chilli Peppers (Capsicum spp.); Rêgo, E.R., Rêgo, M.M., Finger, F.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–129. [Google Scholar]
- Rêgo, E.R.d.; Finger, F.L.; Pessoa, A.M.d.S.; da Silva, A.R.; Azevedo, A.A.; Meira, R.M.S.A.; da Silva, A.L.B.R.; Silva, R.d.S.; Rêgo, M.M.d. Exploring Gene Action Underlying Post-Harvest Water Loss in Fresh Market Peppers. Agronomy 2024, 14, 1351. [Google Scholar] [CrossRef]
- Weryszko-Chmielewska, E.; Michałojć, Z. Anatomical traits of sweet pepper (Capsicum annuum L.) fruit. Acta Agrobot. 2011, 64, 181–188. [Google Scholar]
- Kissinger, M.; Tuvia-Alkalai, S.; Shalom, Y.; Fallik, E.; Elkind, Y.; Jenks, M.A.; Goodwin, M.S. Characterization of physiological and biochemical factors associated with postharvest water loss in ripe pepper fruit during storage. J. Am. Soc. Hortic. Sci. 2005, 130, 735–741. [Google Scholar] [CrossRef]
- Elibox, W.; Meynard, C.; Umaharan, P. Fruit volume and width at harvest can be used to predict shelf life in pepper (Capsicum chinense Jacq.). Trop. Agric. 2017, 94, 122–131. [Google Scholar]
- Albert, Z.; Erős-Honti, Z.; Solymossy, G.; Kuznyák, L.; Miskó, A.; Deák, C.; Ladányi, M.; Terbe, I.; Papp, I. Epidermal and exodermal tissue structures are characteristic for the long shelf-life ‘Kápia’ pepper cultivar. Acta Aliment. 2012, 41, 1–11. [Google Scholar] [CrossRef]
- Byeon, S.E.; Jeong, S.; Lwin, H.P.; Lee, J.; Latt, T.T.; Park, H.; Yun, Y.E.; Lee, J.S.; Lee, J. Seasonal Difference of Fruit Quality Attributes and Physiological Disorders in Paprika Cultivars under a Simulated Export System. Hortic. Sci. Technol. 2023, 41, 414–428. [Google Scholar] [CrossRef]
- Parsons, E.P.; Popopvsky, S.; Lohrey, G.T.; Lü, S.; Alkalai-Tuvia, S.; Perzelan, Y.; Paran, I.; Fallik, E.; Jenks, M.A. Fruit cuticle lipid composition and fruit post-harvest water loss in an advanced backcross generation of pepper (Capsicum sp.). Physiol. Plant. 2012, 146, 15–25. [Google Scholar] [CrossRef]
- O’Donoghue, E.M.; Somerfield, S.D.; Chen, R.K.Y.; Tiffin, H.R.; Hunter, D.A.; Brummell, D.A. Cell wall composition during expansion, ripening and postharvest water loss of red bell peppers (Capsicum annuum L.). Postharvest Biol. Technol. 2020, 168, 111225. [Google Scholar] [CrossRef]
- Berova, M.; Karanatsidis, G.; Sapundzhieva, K.; Nikolova, V. Effect of organic fertilization on growth and yield of pepper plants (Capsicum annuum L.). Folia Hortic. 2010, 22, 3–7. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Bowen, P.A. The effect of preharvest foliar sprays of calcium on quality and shelf life of two cultivars of sweet bell peppers (Capsicum annuum L.) grown in plasticulture. Can. J. Plant Sci. 1999, 79, 411–416. [Google Scholar] [CrossRef]
- Khandaker, M.M.; Rohani, F.; Dalorima, T.; Mat, N. Effects of Different Organic Fertilizers on Growth, Yield and Quality of Capsicum annuum L. var. Kulai (Red Chilli Kulai). Biosci. Biotech. Res. Asia 2017, 14, 185–192. [Google Scholar] [CrossRef]
- Hammam, K.A.; Eisa, E.A.; Dewidar, A.A. Effect of Organic Fertilization and Amino Acids on Growth, Chemical Composition and Capsaicin Content of Hot Pepper (Capsicum annum L. var. Minimum) Plant. Asian Plant Res. J. 2020, 6, 40–52. [Google Scholar] [CrossRef]
- Zamljen, T.; Veberič, R.; Slatnar, A. Fertilization with Humic Acids in Production Changes the Quality of Fresh and Stored Sweet Pepper Fruits (Capsicum annuum L.). J. Plant Growth Regul. 2025, 44, 3965–3973. [Google Scholar] [CrossRef]
- Nyamah, E.Y.; Maalekuu, B.K.; Oppong-Sekyere, D. Influence of different soil amendments on postharvest performance of tomato (Lycopersicon esculentum). J. Stored Prod. Postharvest Res. 2011, 3, 11–13. [Google Scholar]
- Santhosh, S.; Chitdeshwari, T.; Chinnasamy, K. Calcium influences biochemical and antioxidant enzymatic activities in tomato fruits during storage. J. Appl. Biol. Biotechnol. 2022, 11, 1–7. [Google Scholar] [CrossRef]
- Addo, I.; Santo, K.G.; Khalid, A.A.; Ackah, E. Effects of different soil amendments and age of transplant on the post-harvest quality and shelf life of sweet pepper (Capsicum annuum) fruits. Bull. Natl. Res. Cent. 2021, 45, 170. [Google Scholar] [CrossRef]
- Guilherme, R.; Reboredo, F.; Guerra, M.; Ressurreição, S.; Alvarenga, N. Elemental Composition and Some Nutritional Parameters of Sweet Pepper from Organic and Conventional Agriculture. Plants 2020, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Basay, S.; Cimen, A.; Baba, Y.; Yildirim, A.B.; Turker, A.U. Organic and conventional solanaceous vegetables: Comparison of phenolic constituents, antioxidant and antibacterial potentials. Acta Aliment. 2021, 50, 442–452. [Google Scholar] [CrossRef]
- Szafirowska, A.; Elkner, K. The comparison of yielding and nutritive value of organic and conventional pepper fruits. Veg. Crops Res. Bull. 2009, 71, 111–121. [Google Scholar] [CrossRef]
- Mukherjee, A.; Omondi, E.C.; Hepperly, P.R.; Seidel, R.; Heller, W.P. Impacts of Organic and Conventional Management on the Nutritional Level of Vegetables. Sustainability 2020, 12, 8965. [Google Scholar] [CrossRef]
- Ceglie, F.G.; Amodio, M.L.; Colelli, G. Effect of Organic Production Systems on Quality and Postharvest Performance of Horticultural Produce. Horticulturae 2016, 2, 4. [Google Scholar] [CrossRef]
- Kiss, N.É.; Tamás, J.; Szőllősi, N.; Gorliczay, E.; Nagy, A. Assessment of Composted Pelletized Poultry Litter as an Alternative to Chemical Fertilizers Based on the Environmental Impact of Their Production. Agriculture 2021, 11, 1130. [Google Scholar] [CrossRef]
- Al-Ajlouni, M.G.; Ayad, J.Y.; Othman, Y.A. Particle Size of Volcanic Tuff Improves Shoot Growth and Flower Quality of Asiatic Hybrid Lily Using Soilless Culture. Hort. Technol. 2017, 27, 223–227. [Google Scholar] [CrossRef]
- El-Desoky, A.; Hassan, A.; Mahmoud, A. Volcanic ash as a material for soil conditioner and fertility. J. Soil Sci. Agric. Eng. 2018, 9, 491–495. [Google Scholar] [CrossRef]
- Al-Ajlouni, M.G.; Othman, Y.A.; Abu-Shanab, N.S.; Alzyoud, L.F. Evaluating the Performance of Cocopeat and Volcanic Tuff in Soilless Cultivation of Roses. Plants 2024, 13, 2293. [Google Scholar] [CrossRef]
- Simon, L.; Makádi, M.; Vincze, G.; Szabó, B.; Szabó, M.; Aranyos, T. Impact of ammonium nitrate and rhyolite tuff soil application on the photosynthesis and growth of energy willow. In International Multidisciplinary Conference, 10th ed.; 22–24 May 2013. Baia Mare, Romania—Nyíregyháza Hungary. Scientific Bulletin, Serie C, Fascicle: Mechanics, Tribology, Machine Manufacturing Technology; Ungureanu, N., Cotetiu, R., Sikolya, L., Páy, G., Eds.; Bessenyei Publishing House: Nyíregyháza, Hungary, 2013; pp. 143–146. ISBN 978-615-5097-66-9. [Google Scholar]
- Al-Zboon, K.K.; Al-Tabbal, J.A.; Al-Kharabsheh, N.M.; Al-Mefleh, N.K. Natural volcanic tuff as a soil mulching: Effect on plant growth and soil chemistry under water stress. Appl. Water Sci. 2019, 9, 123. [Google Scholar] [CrossRef]
- Xue, D.; Wang, Y.; Sun, H.; Fu, L.; Zhu, L.; Liu, J.; Zhi, Z.; He, J.; Wang, W.; Wu, C. Effects of Soil Conditioner (Volcanic Ash) on Yield Quality and Rhizosphere Soil Characteristics of Melon. Plants 2024, 13, 1787. [Google Scholar] [CrossRef]
- Mihai, R.A.; Rodríguez Valencia, K.E.; Sivizaca Flores, N.G.; Ramiro Fernando, V.G.; Nelson Santiago, C.I.; Catana, R.D. Consequences of Volcanic Ash on Antioxidants, Nutrient Composition, Heavy Metal Accumulation, and Secondary Metabolites in Key Crops of Cotopaxi Province, Ecuador. Toxics 2025, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Mihai, R.A.; Vivanco Gonzaga, R.F.; Romero Balladares, N.R.; Catana, R.D. Characterization of Volcanic Ash Influence on the Nutritional Quality and Biological Traits in Potato Crops of the Cotopaxi Region. Toxics 2025, 13, 453. [Google Scholar] [CrossRef]
- Hao, X.; He, Z. Pelletizing animal manures for on-and off-farm use. In Animal Manure: Production, Characteristics, Environmental Concerns, and Management; Waldrip, H.M., Pagliari, P.H., He, Z., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 2020; pp. 323–344. [Google Scholar] [CrossRef]
- Olaniyi, J.O.; Ajibola, A.T. Effects of inorganic and organic fertilizers application on the growth, fruit yield and quality of tomato (Lycopersicon lycopersicum). J. Appl. Biosci. 2008, 8, 236–242. Available online: https://m.elewa.org/JABS/2008/8(1)/2.pdf (accessed on 5 May 2025).
- Sahin, O.; Taskin, M.B.; Kadıoglu, Y.K.; Inal, A.; Pilbeam, D.J.; Gunes, A. Elemental composition of pepper plants fertilizered with pelletized poultry manure. J. Plant Nutr. 2014, 37, 458–468. [Google Scholar] [CrossRef]
- Sovarel, G.; Scurtu, I.; Hogea, S.S.; Sbîrciog, G. Influence of different doses of organic fertilizer from poultry fertilizer pellets on melon and cucumber crops. Acta Hortic. 2023, 1375, 373–380. [Google Scholar] [CrossRef]
- Buzatu, M.A.; Scurtu, I.; Sbîrciog, G.; Costache, I.; Dorobanțu, A. The influence of different doses of pelletized organic fertilizer from poultry farms on round pepper crop. Acta Hortic. 2024, 1391, 443–448. [Google Scholar] [CrossRef]
- Nikolić, I.; Mijić, K.; Mitrović, I. Characteristics of Food Industry Wastewaters and Their Potential Application in Biotechnological Production. Processes 2025, 13, 2401. [Google Scholar] [CrossRef]
- Baker, F.S.; Miller, C.E.; Repik, A.J.; Tolles, E.D. Activated Carbon. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000; ISBN 978-0-471-23896-6. [Google Scholar]
- Murray, H.H. Chapter 6 Bentonite Applications. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 111–130. [Google Scholar]
- Borah, D.; Nath, H.; Saikia, H. Modification of bentonite clay & its applications: A review. Rev. Inorg. Chem. 2022, 42, 265–282. [Google Scholar] [CrossRef]
- Mi, J.; Gregorich, E.G.; Xu, S.; McLaughlin, N.B.; Ma, B.; Liu, J. Changes in soil biochemical properties following application of bentonite as a soil amendment. Eur. J. Soil Biol. 2021, 102, 103251. [Google Scholar] [CrossRef]
- Valizadeh Ghale Beig, A.; Neamat, S.H.; Tehranifar, A.; Emami, H. Effects of A200 superabsorbent, bentonite and water stress on physiological traits and vitamin C of lettuce under greenhouse cultivation. J. Soil Plant Interact. 2015, 6, 157–168. [Google Scholar][Green Version]
- Mohammadifard, F.; Tarakemeh, A.; Moghaddam, M.; Zim, M. Bentonite Mitigates the Adverse Effects of Drought Stress in Fenugreek (Trigonella foenum-graecum L.). J. Soil Sci. Plant Nutr. 2022, 22, 1098–1111. [Google Scholar] [CrossRef]
- Arias-Estévez, M.; López-Periago, E.; Nóvoa-Muñoz, J.C.; Torrado-Agrasar, A.; Simal-Gándara, J. Treatment of an acid soil with bentonite used for wine fining: Effects on soil properties and the growth of Lolium multiflorum. J. Agric. Food Chem. 2007, 55, 7541–7546. [Google Scholar] [CrossRef] [PubMed]
- Bahloul, H.H.E.; Shafshak, N.S.A.; El Nagar, M.M.; Zaki, M.E.A. Effect of Some Soil Applications and Irrigation Levels on Growth and Productivity of Sweet Pepper Plants for Increasing Drought Tolerance under Plastic Greenhouse Conditions. Sci. J. Agric. Sci. 2024, 6, 23–42. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Rodríguez-Salgado, I.; Pérez-Rodríguez, P.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M. Time evolution of the general characteristics and Cu retention capacity in an acid soil amended with a bentonite winery waste. J. Environ. Manag. 2015, 150, 435–443. [Google Scholar] [CrossRef]
- Rodríguez-Salgado, I.; Paradelo-Pérez, M.; Pérez-Rodríguez, P.; Cutillas-Barreiro, L.; Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M. Cyprodinil retention on mixtures of soil and solid wastes from wineries. Effects of waste dose and ageing. Environ. Sci. Pollut. Res. 2014, 21, 9785–9795. [Google Scholar] [CrossRef]
- Csorba, P. Magyarország Kistájai; Meridián Táj-és Környezetföldrajzi Alapítvány: Debrecen, Hungary, 2021; pp. 72–73. ISBN 978-963-89712-4-1. [Google Scholar]
- Bihari, Z.; Babolcsai, G.; Bartholy, J.; Ferenczi, Z.; Gerhátné Kerényi, J.; Haszpra, L.; Homokiné Ujváry, K.; Kovács, T.; Lakatos, M.; Németh, Á.; et al. Magyarország éghajlata. In Magyarország Nemzeti Atlasza: Természeti Környezet; Kocsis, K., Ed.; Magyar Tudományos Akadémia, Csillagászati és Földtudományi Kutatóközpont, Földrajztudományi Intézet: Budapest, Hungary, 2018; pp. 58–69. [Google Scholar]
- Kormány, G. Nyíregyháza éghajlata. In Nyíregyháza; Előadások a város újratelepítésének 250., Évfordulóján; Frisnyák, S., Ed.; Nyíregyházi Főiskola Földrajz Tanszéke: Nyíregyháza, Hungary, 2003. [Google Scholar]
- Zatykó, L. Étkezési paprika. In Zöldségtermesztők Kézikönyve; Balázs, S., Ed.; Mezőgazada Kiadó: Budapest, Hungary, 1994; pp. 226–256. [Google Scholar]
- MSZ 08-0206/2:1978; Determination of the pH of Soil. Hungarian Standard Association: Budapest, Hungary, 1978. (In Hungarian)
- MSZ-08-0210:1977; Testing Organic Carbon Content in Soils. Hungarian Standard Association: Budapest, Hungary, 1977. (In Hungarian)
- MSZ-08-0458:1980; Determination of Total Nitrogen Content in Soils. Hungarian Standard Association: Budapest, Hungary, 1980. (In Hungarian)
- MSZ 20135:1999; Determination of the Soluble Nutrient Element Content of the Soil. Hungarian Standard Association: Budapest, Hungary, 1999. (In Hungarian)
- Nemzeti Élelmiszerlánc-Biztonsági Hivatal. Zöldségnövények—Paprika. In Nemzeti Fajtajegyzék; Nemzeti Élelmiszerlánc-Biztonsági Hivatal: Budapest, Hungary, 2023; pp. 11–17. Available online: https://portal.nebih.gov.hu/documents/10182/81819/NFJ_z%C3%B6lds%C3%A9g+gy%C3%B3gy-%C3%A9s+f%C5%B1szern%C3%B6v%C3%A9nyek_2023_v_1_1.pdf/29be7792-f5fd-ae74-2644-73fb13de41ff?t=1696321639176 (accessed on 10 May 2025).
- Köhler, M.; Nemes, G. Bodrogkeresztúri Riolittufa. A Növénytermesztés és A Talajjavítás szolgálatában. Őstermelő 2014, 14, 18–19. [Google Scholar]
- Gorliczay, E.; Pecsmán, D.; Tamás, J. Testing laboratory parameters of compost tea. Acta Agrar. Debreceniensis 2018, 75, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.toro-ag.it/public/prod_schede/Toro_Aqua-Traxx_ENG.pdf (accessed on 1 June 2025).
- Albert, Z. Molecular Biological Characterization of Exocarps of Apple and Bell Pepper in Different Developmental Stages and During Storage. Ph.D. Thesis, Corvinus University of Budapest Department of Plant Physiology and Plant Biochemistry, Budapest, Hungary, 2014. [Google Scholar]
- Tiwari, A.; Vivian-Smith, A.; Ljung, K.; Offringa, R.; Heuvelink, E. Physiological and morphological changes during early and later stages of fruit growth in Capsicum annuum. Physiol. Plant. 2013, 147, 396–406. [Google Scholar] [CrossRef]
- Anwar, R.; Mattoo, A.K.; Handa, A.K. Ripening and Senescence of Fleshy Fruits. In Postharvest Biology and Nanotechnology; Paliyath, G., Subramanian, J., Lim, L.-T., Subramanian, K.S., Handa, A.K., Mattoo, A.K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 15–51. [Google Scholar]
- Seymour, G.B.; Ostergaard, L.; Chapman, N.H.; Knapp, S.; Martin, C. Fruit development and ripening. Annu. Rev. Plant Biol. 2013, 64, 219–241. [Google Scholar] [CrossRef]
- Hou, X.; Li, H.; Zhang, W.; Yao, Z.; Wang, Y.; Du, T. Water transport in fleshy fruits: Research advances, methodologies, and future directions. Physiol. Plant 2021, 172, 2203–2216. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Rose, J.K.C. A relationship between tomato fruit softening, cuticle properties and water availability. Food Chem. 2019, 295, 300–310. [Google Scholar] [CrossRef]
- Trivedi, P.; Nguyen, N.; Hykkerud, A.L.; Haggman, H.; Martinussen, I.; Jaakola, L.; Karppinen, K. Developmental and Environmental Regulation of Cuticular Wax Biosynthesis in Fleshy Fruits. Front. Plant Sci. 2019, 10, 431. [Google Scholar] [CrossRef] [PubMed]
- Bertin, N. Analysis of the tomato fruit growth response to temperature and plant fruit load in relation to cell division, cell expansion and DNA endoreduplication. Ann. Bot. 2004, 95, 439–447. [Google Scholar] [CrossRef]
- Priya Sethu, K.M.; Prabha, T.N.; Tharanathan, R.N. Post-harvest biochemical changes associated with the softening phenomenon in Capsicum annuum fruits. Phytochemistry 1990, 42, 961–966. [Google Scholar] [CrossRef]
- Weryszko-Chmielewska, E.; Michałojć, Z. Anatomical features of leaves of sweet pepper (Capsicum annuum L.) fed with calcium using foliar nutrition. Acta Agrobot. 2009, 62, 155–164. [Google Scholar] [CrossRef][Green Version]
- Gross, K.C.; Sams, C.E. Changes in cell wall neutral sugar composition during fruit ripening: A species survey. Phytochemistry 1984, 23, 2457–2461. [Google Scholar] [CrossRef]
- Goulao, L.F.; Oliveira, C.M. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- Serrani, J.C.; Ruiz-Rivero, O.; Fos, M.; García-Martínez, J.L. Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J. 2008, 56, 922–934. [Google Scholar] [CrossRef]
- Adewoyin, O.; Famaye, A.; Ipinmoroti, R.; Ibidapo, A.; Fayose, F. Postharvest Handling Methods, Processes and Practices for Pepper. In Capsicum–Current Trends and Perspectives; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Smith, D.L.; Stommel, J.R.; Fung, R.W.M.; Wang, C.Y.; Whitaker, B.D. Influence of cultivar and harvest method on postharvest storage quality of pepper (Capsicum anuum L.) fruit. Postharvest Biol. Technol. 2006, 42, 243–247. [Google Scholar] [CrossRef]
- Russo, V.; Biles, C. Fertilizer rate and β-galactosidase and peroxidase activity in pepper fruit at different stages and years of harvest. Plant Foods Hum. Nutr. 2003, 58, 231–239. [Google Scholar] [CrossRef]
- Colombari, L.F.; Silva, G.F.d.; Chamma, L.; Chaves, P.P.N.; Martins, B.N.M.; Jorge, L.G.; de Lima Silva, P.N.; Putti, F.F.; Cardoso, A.I.I. Maturation and resting of sweet pepper fruits on physiological quality and biochemical response of seeds. Braz. Arch. Biol. Technol. 2021, 64, e21200733. [Google Scholar] [CrossRef]
- Tsegay, D.; Tesfaye, B.; Mohammed, A.; Yirga, H.; Bayleyegn, A. Effects of harvesting stage and storage duration on postharvest quality and shelf life of sweet bell pepper (Capsicum annuum L.) varieties under passive refrigeration system. Int. J. Biotechnol. Mol. Biol. Res. 2013, 4, 98–104. [Google Scholar] [CrossRef]
- Ciccarese, A.; Stellacci, M.; Gentilesco, G.; Rubino, P. Effectiveness of pre- and post-veraison calcium applications to control decay and maintain table grape fruit quality during storage. J. Postharvest Biol. Technol. 2013, 75, 135–141. [Google Scholar] [CrossRef]
- Bayoumi, Y. Improvement of postharvest keeping quality of white pepper fruits (Capsicum annuum L.) by hydrogen peroxide treatment under storage conditions. Acta Biol. Szeged. 2008, 52, 7–15. [Google Scholar]
- Moneruzzaman, M.; Hossain, S.; Sani, W.; Saifuddin, M.; Alenazi, M. Effect of harvesting and storage conditions on the postharvest quality of tomato (Lycopersicon esculentum Mill) cv. Roma VF. Aust. J. Crop Sci. 2009, 3, 113–121. [Google Scholar]
- Martí, M.C.; Camejo, D.; Vallejo, F.; Romojaro, F.; Bacarizo, S.; Palma, J.M.; Sevilla, F.; Jiménez, A. Influence of fruit ripening stage and harvest period on the antioxidant content of sweet pepper cultivars. Plant Foods Hum. Nutr. 2011, 66, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Aizat, W.M.; Dias, D.A.; Stangoulis, J.C.R.; Able, J.A.; Roessner, U.; Able, A.J. Metabolomics of capsicum ripening reveals modification of the ethylene related-pathway and carbon metabolism. Postharvest Biol. Technol. 2014, 89, 19–31. [Google Scholar] [CrossRef]
- Kosma, D.K.; Parsons, E.P.; Isaacson, T.; Lü, S.; Rose, J.K.; Jenks, M.A. Fruit cuticle lipid composition during development in tomato ripening mutants. Physiol. Plant. 2010, 139, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Lownds, N.K.; Banaras, M.; Bosland, P.W. Postharvest water loss and storage quality of nine pepper (Capsicum) cultivars. Hort. Sci. 1994, 29, 191–193. [Google Scholar] [CrossRef]
- Maalekuu, K.; Elkind, Y.; Tuvia-Alkalai, S.; Shalom, Y.; Fallik, E. The influence of harvest season and cultivar type on several quality traits and quality stability in three commercial sweet bell peppers during the harvest period. Adv. Hortic. Sci. 2004, 18, 21–25. [Google Scholar]

| Year | Data | Jan. | Feb. | Mar. | Apr. | May | June | July | Aug. | Sept. | Oct. | Nov. | Dec. | Year |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MYA | Temperature (°C) | −2.4 | −0.1 | 4.6 | 10.7 | 15.9 | 19.0 | 20.6 | 19.8 | 15.5 | 9.9 | 4.2 | −0.4 | 9.77 |
| 2023 | 4.4 | 2.6 | 7 | 9.8 | 16.5 | 19.7 | 22.4 | 22.8 | 19.5 | 13.7 | 5.8 | 2.6 | 12.23 | |
| MYA | Precipitation (mm) | 29.5 | 30 | 30 | 39.5 | 54 | 76 | 66.5 | 65 | 43 | 44 | 46.5 | 40.5 | 564.5 |
| 2023 | 65.9 | 8.5 | 48.7 | 46.4 | 47.8 | 99.4 | 53.6 | 59.1 | 37.5 | 61.3 | 122.5 | 65.1 | 715.8 |
| C | ||||
| Immature Fruit | Mature Fruit | Overripe Fruit | ||
| exocarp | Cuticle (μm) | 11.51 ± 1.09a | 12.32 ± 1.01a | 12.68 ± 2.33a |
| Epidermis (μm) | 15.01 ± 0.2a | 16.65 ± 0.28c | 15.92 ± 0.14b | |
| mesocarp | Collenchyma thickness (μm) | 51.98 ± 5.05b | 58.23 ± 6.18c | 41.87 ± 5.54a |
| Collenchyma cell walls (μm) | 4.19 ± 1.02a | 5.18 ± 1.14b | 4.09 ± 1.02a | |
| Parenchyma (μm) | 4150.38 ± 420.5b | 4510.22 ± 400.26c | 3550.28 ± 370.21a | |
| endocarp | Giant cells (μm) | 343.28 ± 69.18a | 408.09 ± 65.95b | 352.56 ± 59.87a |
| Inner epidermis (μm) | 36.77 ± 4.92a | 50.04 ± 5.12b | 38.02 ± 4.32a | |
| RT | ||||
| Immature fruit | Mature fruit | Overripe fruit | ||
| exocarp | Cuticle (μm) | 10.67 ± 1.15a | 10.61 ± 1.27a | 12.18 ± 1.59b |
| Epidermis (μm) | 13.87 ± 2.04a | 14.38 ± 1.75a | 16.08 ± 1.82b | |
| mesocarp | Collenchyma thickness (μm) | 72.38 ± 2.09c | 63.62 ± 1.71b | 26.44 ± 4.55a |
| Collenchyma cell walls (μm) | 6.47 ± 1.08c | 5.27 ± 1.02b | 3.89 ± 0.98a | |
| Parenchyma (μm) | 5040 ± 730ab | 5300 ± 730b | 4500 ± 190a | |
| endocarp | Giant cells (μm) | 367.87 ± 37.86a | 459.87 ± 67.98b | 382.12 ± 58.99a |
| Inner epidermis (μm) | 40.67 ± 5.02a | 54.98 ± 4.67b | 42.12 ± 3.98a | |
| CPPL | ||||
| Immature fruit | Mature fruit | Overripe fruit | ||
| exocarp | Cuticle (μm) | 11.34 ± 1.89a | 12.52 ± 1.08b | 10.39 ± 1.15a |
| Epidermis (μm) | 9.58 ± 1.98b | 8.46 ± 0.97a | 8.79 ± 0.76ab | |
| mesocarp | Collenchyma thickness (μm) | 68.51 ± 3.89c | 45.59 ± 6.48b | 35.21 ± 5.32a |
| Collenchyma cell walls (μm) | 5.18 ± 1.12b | 4.23 ± 0.98a | 3.99 ± 0.87a | |
| Parenchyma (μm) | 4000 ± 770a | 4840 ± 480b | 3970 ± 141a | |
| endocarp | Giant cells (μm) | 332.17 ± 24.13a | 354.18 ± 42.68a | 312.98 ± 22.89a |
| Inner epidermis (μm) | 42.12 ± 3.89a | 54.68 ± 4.36b | 46.89 ± 4.42a | |
| CA | ||||
| Immature fruit | Mature fruit | Overripe fruit | ||
| exocarp | Cuticle (μm) | 11.46 ± 1.23ab | 12.20 ± 1.95b | 11.05 ± 0.81a |
| Epidermis (μm) | 11.34 ± 1.02b | 12.12 ± 1.12b | 10.02 ± 0.78a | |
| mesocarp | Collenchyma thickness (μm) | 71.4 ± 6.72c | 37.75 ± 3.83b | 29.67 ± 3.59a |
| Collenchyma cell walls (μm) | 4.89 ± 0.97b | 3.89 ± 0.56a | 3.04 ± 0.71a | |
| Parenchyma (μm) | 4220 ± 950b | 4390 ± 70b | 3380 ± 440a | |
| endocarp | Giant cells (μm) | 372.01 ± 12.54b | 381.11 ± 42.32b | 314.67 ± 23.11a |
| Inner epidermis (μm) | 33.56 ± 3.23b | 36.98 ± 3.36b | 30.06 ± 2.24a | |
| Water Loss (WLR) 7th day | Water Loss (WLR) 14th day | Cuticle Thickness | Hypoderm Thickness | Pericarp Thickness | Fruit Diameter | Fruit Length | Fruit Surface Area | Fruit Weight | Surface Area/Fruit Weight | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ripening Stages | Soil Treatments | % | % | μm | μm | mm | mm | mm | mm2 | g | |
| immature | C | 10.61 ± 3.51a | 20.73 ± 6.51b | 11.51 ± 1.09b | 70.88 ± 6.18a | 4.15 ± 0.42ab | 32.65 ± 2.55a | 76.12 ± 9.15a | 3903.14 ± 23.46c | 26.43 ± 4.75a | 155.94 ± 30.05a |
| RT | 9.07 ± 1.54a | 15.05 ± 3.25a | 10.67 ± 1.15a | 86.19 ± 2.09c | 5.04 ± 0.73bc | 36.87 ± 6.67a | 75.24 ± 9.6a | 4354.15 ± 98.96d | 25.25 ± 5.28a | 177.94 ± 35.92a | |
| CPPL | 9.56 ± 3.32a | 17.97 ± 6.52a | 11.34 ± 1.89ab | 78.09 ± 3.89b | 4.00 ± 0.77a | 32.43 ± 4.19a | 67.87 ± 17.60a | 3454.54 ± 112.05b | 23.79 ± 5.76a | 175.55 ± 32.47a | |
| CA | 10.13 ± 1.59a | 17.53 ± 3.22a | 11.46 ± 1.23b | 82.74 ± 6.72bc | 4.22 ± 0.95ab | 31.92 ± 4.12a | 61.36 ± 15.69a | 3075.02 ± 100.84a | 30.64 ± 3.48a | 115.19 ± 19.52a | |
| mature | C | 10.72 ± 3.13a | 18.59 ± 3.27b | 12.32 ± 1.01b | 62.99 ± 5.05b | 4.51 ± 0.40ab | 40.40 ± 5.63a | 81.10 ± 2.41a | 5144.01 ± 41.16a | 42.18 ± 9.16a | 107.53 ± 10.55ab |
| RT | 8.15 ± 0.69a | 13.81 ± 0.48a | 10.61 ± 1.27a | 78.00 ± 1.71c | 5.30 ± 0.73b | 39.97 ± 4.20a | 82.13 ± 4.29ab | 5152.61 ± 24.63a | 38.38 ± 7.03a | 150.3 ± 12.69c | |
| CPPL | 9.19 ± 1.68a | 15.79 ± 3.13ab | 12.52 ± 1.08b | 54.05 ± 6.48a | 4.84 ± 0.48ab | 40.66 ± 5.01a | 81.30 ± 4.74a | 5189.88 ± 37.82a | 46.06 ± 8.91a | 97.96 ± 6.52a | |
| CA | 8.69 ± 0.98a | 14.49 ± 1.57ab | 12.20 ± 1.95b | 49.87 ± 3.83a | 4.39 ± 0.07a | 40.09 ± 8.59a | 85.61 ± 6.20b | 5530.03 ± 87.69b | 41.34 ± 12.43a | 152.55 ± 16.87c | |
| overripe | C | 9.78 ± 1.79a | 16.31 ± 4.15a | 12.68 ± 2.33c | 53.80 ± 5.55b | 3.55 ± 0.37a | 36.20 ± 3.49ab | 85.30 ± 4.49a | 4847.94 ± 25.87a | 31.78 ± 6.92a | 168.08 ± 29.84b |
| RT | 8.76 ± 0.81a | 18.72 ± 3.78a | 12.18 ± 1.59bc | 42.52 ± 4.55a | 4.50 ± 1.9a | 39.00 ± 6.39b | 87.64 ± 13.81a | 5366.2 ± 136.42c | 34.16 ± 4.55a | 151.52 ± 18.32ab | |
| CPPL | 12.54 ± 5.01a | 19.35 ± 5.35a | 10.39 ± 1.15a | 44.00 ± 5.32a | 3.97 ± 1.41a | 32.40 ± 3.92a | 90.62 ± 10.92a | 4609.66 ± 65.89a | 41.80 ± 7.43a | 134.76 ± 20.01ab | |
| CA | 12.84 ± 2.3a | 21.81 ± 4.69a | 11.05 ± 0.81b | 39.69 ± 3.59a | 3.38 ± 0.44a | 37.30 ± 5.89ab | 88.43 ± 11.22a | 5178.54 ± 102.12b | 41.66 ± 6.65a | 113.16 ± 17.76a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, C.; Pilik, G.G.; Oláh, K.I.; Tóth, B. The Effect of Alternative Nutrient Supplements on Histological Traits and Postharvest Water Loss in Pepper Fruit. Horticulturae 2025, 11, 1113. https://doi.org/10.3390/horticulturae11091113
Tóth C, Pilik GG, Oláh KI, Tóth B. The Effect of Alternative Nutrient Supplements on Histological Traits and Postharvest Water Loss in Pepper Fruit. Horticulturae. 2025; 11(9):1113. https://doi.org/10.3390/horticulturae11091113
Chicago/Turabian StyleTóth, Csilla, Gábor Gergő Pilik, Katalin Irinyi Oláh, and Brigitta Tóth. 2025. "The Effect of Alternative Nutrient Supplements on Histological Traits and Postharvest Water Loss in Pepper Fruit" Horticulturae 11, no. 9: 1113. https://doi.org/10.3390/horticulturae11091113
APA StyleTóth, C., Pilik, G. G., Oláh, K. I., & Tóth, B. (2025). The Effect of Alternative Nutrient Supplements on Histological Traits and Postharvest Water Loss in Pepper Fruit. Horticulturae, 11(9), 1113. https://doi.org/10.3390/horticulturae11091113








